Various Organ Damages in Rats with Fetal Growth Restriction and Their Slight Attenuation by Bifidobacterium breve Supplementation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Surgery
2.2. Group Allocation and Oral Supplementation
2.3. Analysis of Organs and Their Histology
2.4. Statistics
3. Results
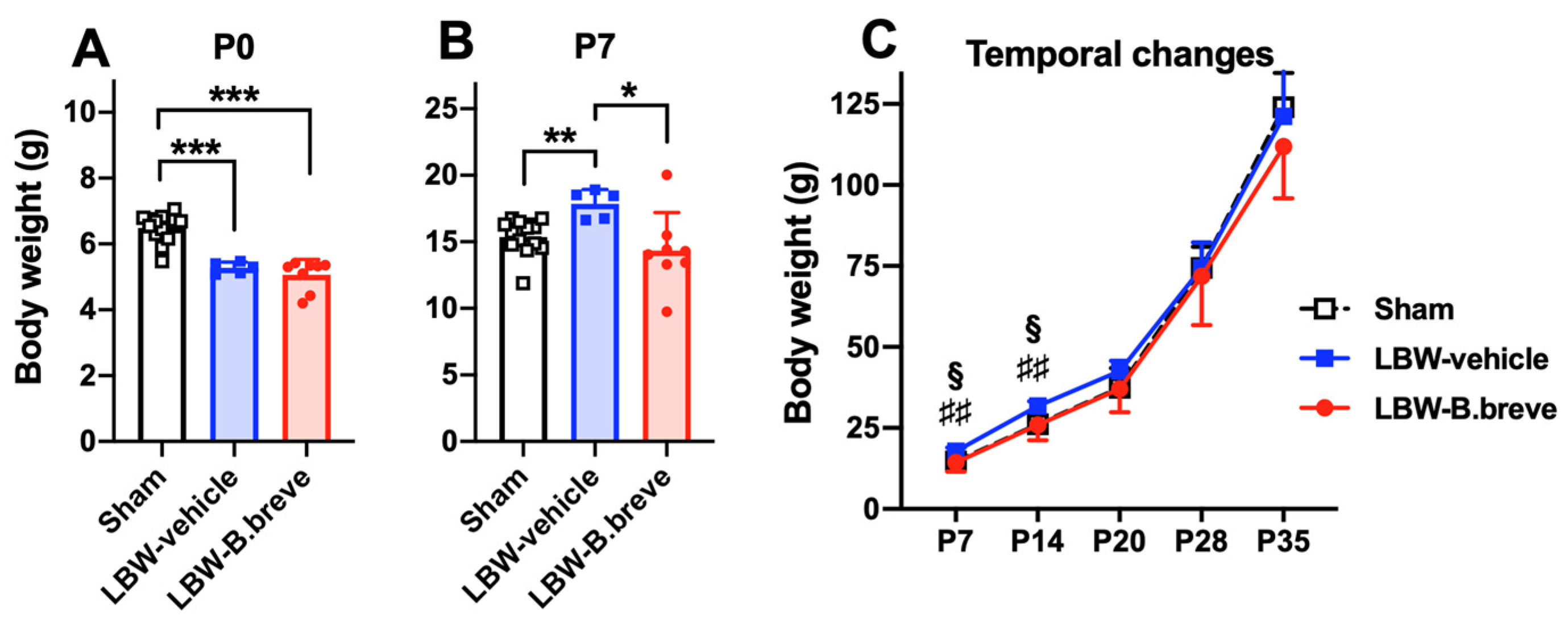
3.1. Temporal Changes in Body Weight
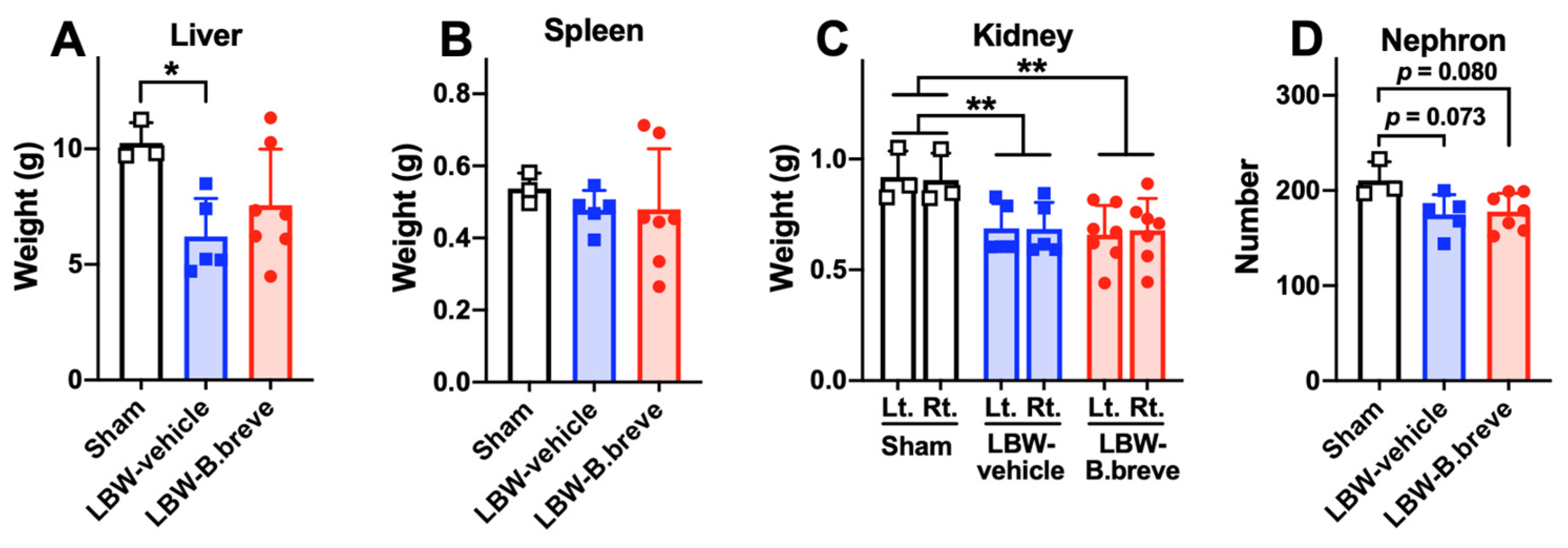
3.2. Solid Organs in the Abdomen
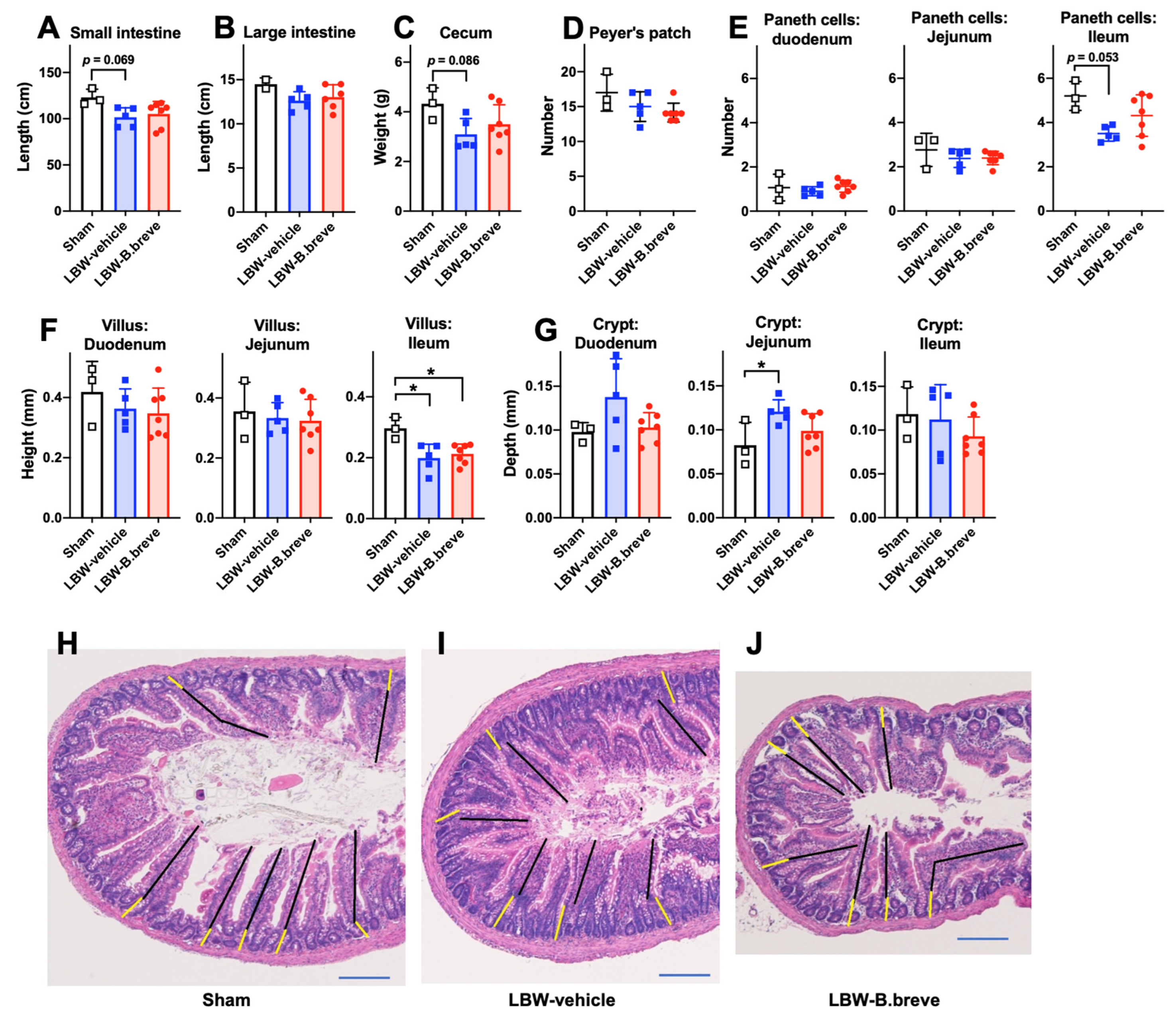
3.3. The Small and Large Intestines
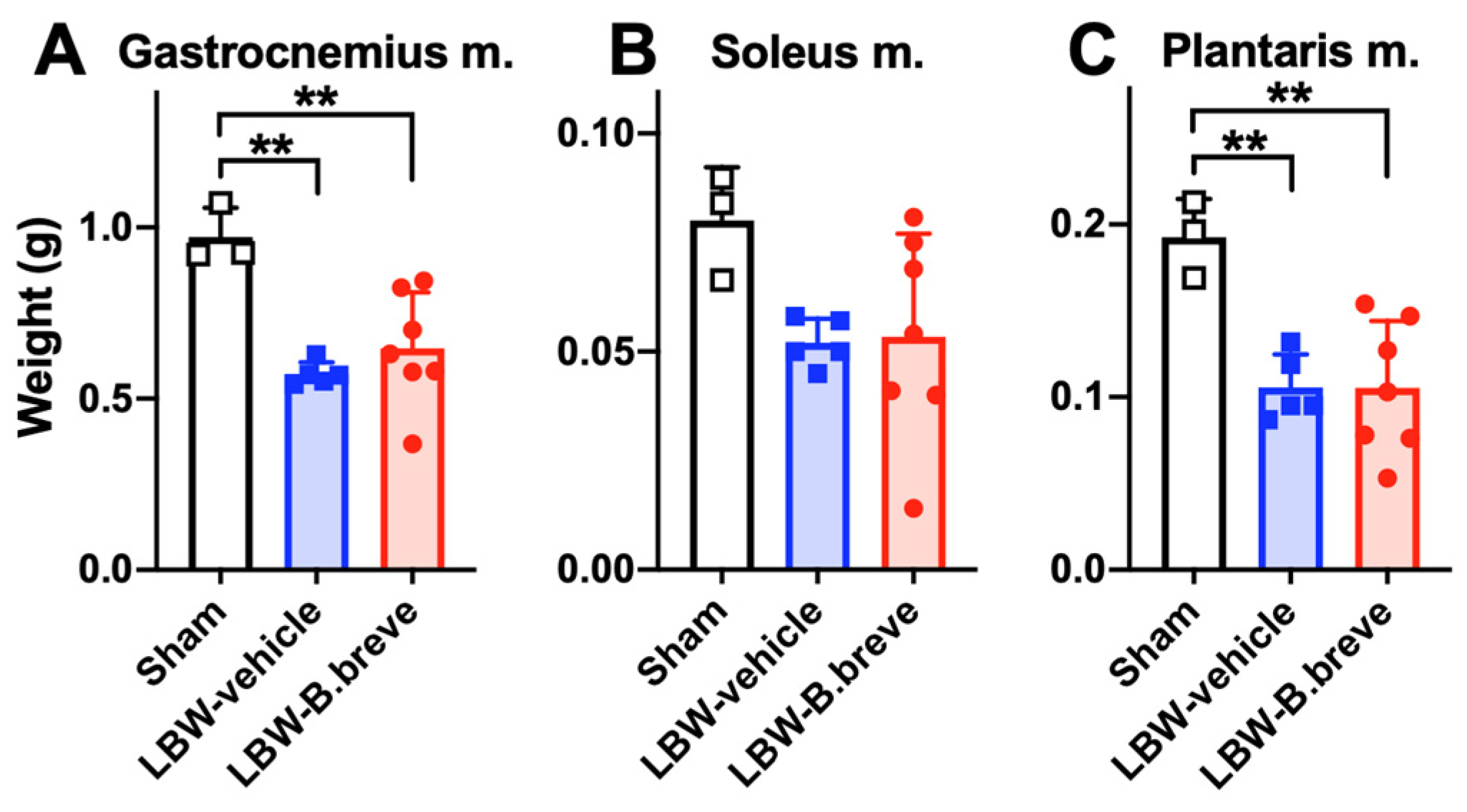
3.4. The Skeletal Muscles
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffman, D.J.; Powell, T.L.; Barrett, E.S.; Hardy, D.B. Developmental origins of metabolic diseases. Physiol. Rev. 2021, 101, 739–795. [Google Scholar] [CrossRef]
- Benz, K.; Amann, K. Maternal nutrition, low nephron number and arterial hypertension in later life. Biochim. Biophys. Acta 2010, 1802, 1309–1317. [Google Scholar] [CrossRef][Green Version]
- Petermann-Rocha, F.; Chen, M.; Gray, S.R.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Factors associated with sarcopenia: A cross-sectional analysis using UK Biobank. Maturitas 2020, 133, 60–67. [Google Scholar] [CrossRef]
- Gagnon, R. Placental insufficiency and its consequences. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110 (Suppl. S1), S99–S107. [Google Scholar] [CrossRef]
- Neitzke, U.; Harder, T.; Plagemann, A. Intrauterine growth restriction and developmental programming of the metabolic syndrome: A critical appraisal. Microcirculation 2011, 18, 304–311. [Google Scholar] [CrossRef]
- Basilious, A.; Yager, J.; Fehlings, M.G. Neurological outcomes of animal models of uterine artery ligation and relevance to human intrauterine growth restriction: A systematic review. Dev. Med. Child Neurol. 2015, 57, 420–430. [Google Scholar] [CrossRef]
- Ohshima, M.; Coq, J.O.; Otani, K.; Hattori, Y.; Ogawa, Y.; Sato, Y.; Harada-Shiba, M.; Ihara, M.; Tsuji, M. Mild intrauterine hypoperfusion reproduces neurodevelopmental disorders observed in prematurity. Sci. Rep. 2016, 6, 39377. [Google Scholar] [CrossRef]
- Coq, J.O.; Delcour, M.; Ogawa, Y.; Peyronnet, J.; Castets, F.; Turle-Lorenzo, N.; Montel, V.; Bodineau, L.; Cardot, P.; Brocard, C.; et al. Mild Intrauterine Hypoperfusion Leads to Lumbar and Cortical Hyperexcitability, Spasticity, and Muscle Dysfunctions in Rats: Implications for Prematurity. Front. Neurol. 2018, 9, 423. [Google Scholar] [CrossRef]
- Tsuji, M.; Coq, J.O.; Ogawa, Y.; Yamamoto, Y.; Ohshima, M. A Rat Model of Mild Intrauterine Hypoperfusion with Microcoil Stenosis. J. Vis. Exp. 2018, 7, 56723. [Google Scholar] [CrossRef]
- Salmaso, N.; Jablonska, B.; Scafidi, J.; Vaccarino, F.M.; Gallo, V. Neurobiology of premature brain injury. Nat. Neurosci. 2014, 17, 341–346. [Google Scholar] [CrossRef]
- Shanklin, D.R.; Cooke, R.J. Effects of intrauterine growth on intestinal length in the human fetus. Biol. Neonate 1993, 64, 76–81. [Google Scholar] [CrossRef]
- Woodall, S.M.; Breier, B.H.; Johnston, B.M.; Gluckman, P.D. A model of intrauterine growth retardation caused by chronic maternal undernutrition in the rat: Effects on the somatotrophic axis and postnatal growth. J. Endocrinol. 1996, 150, 231–242. [Google Scholar] [CrossRef]
- Cha, C.J.; Gelardi, N.L.; Oh, W. Growth and cellular composition in rats with intrauterine growth retardation: Effects of postnatal nutrition. J. Nutr. 1987, 117, 1463–1468. [Google Scholar] [CrossRef]
- Qiu, X.S.; Huang, T.T.; Shen, Z.Y.; Deng, H.Y.; Ke, Z.Y. Effect of early nutrition on intestine development of intrauterine growth retardation in rats and its correlation to leptin. World J. Gastroenterol. 2005, 11, 4419–4422. [Google Scholar] [CrossRef]
- Lalles, J.P.; Orozco-Solis, R.; Bolanos-Jimenez, F.; de Coppet, P.; Le Drean, G.; Segain, J.P. Perinatal undernutrition alters intestinal alkaline phosphatase and its main transcription factors KLF4 and Cdx1 in adult offspring fed a high-fat diet. J. Nutr. Biochem 2012, 23, 1490–1497. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Quinn, R.A.; Debelius, J.; Xu, Z.Z.; Morton, J.; Garg, N.; Jansson, J.K.; Dorrestein, P.C.; Knight, R. Microbiome-wide association studies link dynamic microbial consortia to disease. Nature 2016, 535, 94–103. [Google Scholar] [CrossRef]
- Chi, C.; Xue, Y.; Lv, N.; Hao, Y.; Liu, R.; Wang, Y.; Ding, X.; Zeng, H.; Li, G.; Shen, Q.; et al. Longitudinal Gut Bacterial Colonization and Its Influencing Factors of Low Birth Weight Infants During the First 3 Months of Life. Front. Microbiol. 2019, 10, 1105. [Google Scholar] [CrossRef]
- Tsuji, H.; Oozeer, R.; Matsuda, K.; Matsuki, T.; Ohta, T.; Nomoto, K.; Tanaka, R.; Kawashima, M.; Kawashima, K.; Nagata, S.; et al. Molecular monitoring of the development of intestinal microbiota in Japanese infants. Benef. Microb. 2012, 3, 113–125. [Google Scholar] [CrossRef]
- Arboleya, S.; Binetti, A.; Salazar, N.; Fernandez, N.; Solis, G.; Hernandez-Barranco, A.; Margolles, A.; de Los Reyes-Gavilan, C.G.; Gueimonde, M. Establishment and development of intestinal microbiota in preterm neonates. FEMS Microbiol. Ecol. 2012, 79, 763–772. [Google Scholar] [CrossRef]
- Barrett, E.; Deshpandey, A.K.; Ryan, C.A.; Dempsey, E.M.; Murphy, B.; O’Sullivan, L.; Watkins, C.; Ross, R.P.; O’Toole, P.W.; Fitzgerald, G.F.; et al. The neonatal gut harbours distinct bifidobacterial strains. Arch. Dis. Childhood. Fetal Neonatal Ed. 2015, 100, F405–F410. [Google Scholar] [CrossRef]
- Patole, S.; Keil, A.D.; Chang, A.; Nathan, E.; Doherty, D.; Simmer, K.; Esvaran, M.; Conway, P. Effect of Bifidobacterium breve M-16V supplementation on fecal bifidobacteria in preterm neonates--a randomised double blind placebo controlled trial. PLoS ONE 2014, 9, e89511. [Google Scholar] [CrossRef]
- Horigome, A.; Hisata, K.; Odamaki, T.; Iwabuchi, N.; Xiao, J.Z.; Shimizu, T. Colonization of Supplemented Bifidobacterium breve M-16V in Low Birth Weight Infants and Its Effects on Their Gut Microbiota Weeks Post-administration. Front. Microbiol. 2021, 12, 610080. [Google Scholar] [CrossRef]
- Itoh, A.; Tanaka, N.; Fukunaga, S.; Nakano-Doi, A.; Matsuyama, T.; Nakagomi, T.; Tsuji, M. Bifidobacterium breve during infancy attenuates mobility in low birthweight rats. Pediatr. Int. 2022, 64, e15209. [Google Scholar] [CrossRef]
- Athalye-Jape, G.; Rao, S.; Simmer, K.; Patole, S. Bifidobacterium breve M-16V as a Probiotic for Preterm Infants: A Strain-Specific Systematic Review. JPEN J. Parenter. Enteral. Nutr. 2018, 42, 677–688. [Google Scholar] [CrossRef]
- Wong, C.B.; Iwabuchi, N.; Xiao, J.Z. Exploring the Science behind Bifidobacterium breve M-16V in Infant Health. Nutrients 2019, 11, 1724. [Google Scholar] [CrossRef]
- Inoue, Y.; Iwabuchi, N.; Xiao, J.Z.; Yaeshima, T.; Iwatsuki, K. Suppressive effects of bifidobacterium breve strain M-16V on T-helper type 2 immune responses in a murine model. Biol. Pharm. Bull. 2009, 32, 760–763. [Google Scholar] [CrossRef]
- Enomoto, T.; Sowa, M.; Nishimori, K.; Shimazu, S.; Yoshida, A.; Yamada, K.; Furukawa, F.; Nakagawa, T.; Yanagisawa, N.; Iwabuchi, N.; et al. Effects of bifidobacterial supplementation to pregnant women and infants in the prevention of allergy development in infants and on fecal microbiota. Allergol. Int. 2014, 63, 575–585. [Google Scholar] [CrossRef]
- Ehara, T.; Izumi, H.; Tsuda, M.; Nakazato, Y.; Iwamoto, H.; Namba, K.; Takeda, Y. Combinational effects of prebiotic oligosaccharides on bifidobacterial growth and host gene expression in a simplified mixed culture model and neonatal mice. Br. J. Nutr. 2016, 116, 270–278. [Google Scholar] [CrossRef]
- Luyckx, V.A.; Brenner, B.M. Birth weight, malnutrition and kidney-associated outcomes—A global concern. Nat. Rev. Nephrol. 2015, 11, 135–149. [Google Scholar] [CrossRef]
- Jornayvaz, F.R.; Vollenweider, P.; Bochud, M.; Mooser, V.; Waeber, G.; Marques-Vidal, P. Low birth weight leads to obesity, diabetes and increased leptin levels in adults: The CoLaus study. Cardiovasc. Diabetol. 2016, 15, 73. [Google Scholar] [CrossRef]
- Barker, D.J.; Osmond, C.; Forsen, T.J.; Kajantie, E.; Eriksson, J.G. Trajectories of growth among children who have coronary events as adults. N. Engl. J. Med. 2005, 353, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, R.; Zhou, L.; He, J.; Huang, Q.; Siyal, F.A.; Zhang, L.; Zhong, X.; Wang, T. Intrauterine growth retardation promotes fetal intestinal autophagy in rats via the mechanistic target of rapamycin pathway. J. Reprod. Dev. 2017, 63, 547–554. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Alexander, B.T. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension 2003, 41, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Tarry-Adkins, J.L.; Fernandez-Twinn, D.S.; Hargreaves, I.P.; Neergheen, V.; Aiken, C.E.; Martin-Gronert, M.S.; McConnell, J.M.; Ozanne, S.E. Coenzyme Q10 prevents hepatic fibrosis, inflammation, and oxidative stress in a male rat model of poor maternal nutrition and accelerated postnatal growth. Am. J. Clin. Nutr. 2016, 103, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, V.A.; Perico, N.; Somaschini, M.; Manfellotto, D.; Valensise, H.; Cetin, I.; Simeoni, U.; Allegaert, K.; Vikse, B.E.; Steegers, E.A.; et al. A developmental approach to the prevention of hypertension and kidney disease: A report from the Low Birth Weight and Nephron Number Working Group. Lancet 2017, 390, 424–428. [Google Scholar] [CrossRef]
- Yuasa, K.; Kondo, T.; Nagai, H.; Mino, M.; Takeshita, A.; Okada, T. Maternal protein restriction that does not have an influence on the birthweight of the offspring induces morphological changes in kidneys reminiscent of phenotypes exhibited by intrauterine growth retardation rats. Congenit. Anom. 2016, 56, 79–85. [Google Scholar] [CrossRef]
- Merlet-Benichou, C.; Gilbert, T.; Muffat-Joly, M.; Lelievre-Pegorier, M.; Leroy, B. Intrauterine growth retardation leads to a permanent nephron deficit in the rat. Pediatr. Nephrol. 1994, 8, 175–180. [Google Scholar] [CrossRef]
- Wlodek, M.E.; Westcott, K.; Siebel, A.L.; Owens, J.A.; Moritz, K.M. Growth restriction before or after birth reduces nephron number and increases blood pressure in male rats. Kidney Int. 2008, 74, 187–195. [Google Scholar] [CrossRef]
- Murano, Y.; Nishizaki, N.; Endo, A.; Ikeda, N.; Someya, T.; Nakagawa, M.; Hara, T.; Sakuraya, K.; Hara, S.; Hirano, D.; et al. Evaluation of kidney dysfunction and angiotensinogen as an early novel biomarker of intrauterine growth restricted offspring rats. Pediatr. Res. 2015, 78, 678–682. [Google Scholar] [CrossRef]
- Yliharsila, H.; Kajantie, E.; Osmond, C.; Forsen, T.; Barker, D.J.; Eriksson, J.G. Birth size, adult body composition and muscle strength in later life. Int. J. Obes. 2007, 31, 1392–1399. [Google Scholar] [CrossRef]
- Patel, H.P.; Jameson, K.A.; Syddall, H.E.; Martin, H.J.; Stewart, C.E.; Cooper, C.; Sayer, A.A. Developmental influences, muscle morphology, and sarcopenia in community-dwelling older men. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.B.; Storgaard, H.; Madsbad, S.; Richter, E.A.; Vaag, A.A. Altered skeletal muscle fiber composition and size precede whole-body insulin resistance in young men with low birth weight. J. Clin. Endocrinol. Metab. 2007, 92, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Maltin, C.A.; Delday, M.I.; Sinclair, K.D.; Steven, J.; Sneddon, A.A. Impact of manipulations of myogenesis in utero on the performance of adult skeletal muscle. Reproduction 2001, 122, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Delcour, M.; Russier, M.; Xin, D.L.; Massicotte, V.S.; Barbe, M.F.; Coq, J.O. Mild musculoskeletal and locomotor alterations in adult rats with white matter injury following prenatal ischemia. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2011, 29, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Tarry-Adkins, J.L.; Fernandez-Twinn, D.S.; Chen, J.H.; Hargreaves, I.P.; Neergheen, V.; Aiken, C.E.; Ozanne, S.E. Poor maternal nutrition and accelerated postnatal growth induces an accelerated aging phenotype and oxidative stress in skeletal muscle of male rats. Dis. Models Mech. 2016, 9, 1221–1229. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef]
- Tomosada, Y.; Villena, J.; Murata, K.; Chiba, E.; Shimazu, T.; Aso, H.; Iwabuchi, N.; Xiao, J.Z.; Saito, T.; Kitazawa, H. Immunoregulatory effect of bifidobacteria strains in porcine intestinal epithelial cells through modulation of ubiquitin-editing enzyme A20 expression. PLoS ONE 2013, 8, e59259. [Google Scholar] [CrossRef]
- Indrio, F.; Martini, S.; Francavilla, R.; Corvaglia, L.; Cristofori, F.; Mastrolia, S.A.; Neu, J.; Rautava, S.; Russo Spena, G.; Raimondi, F.; et al. Epigenetic Matters: The Link between Early Nutrition, Microbiome, and Long-term Health Development. Front. Pediatr. 2017, 5, 178. [Google Scholar] [CrossRef]
- Kiu, R.; Treveil, A.; Harnisch, L.C.; Caim, S.; Leclaire, C.; van Sinderen, D.; Korcsmaros, T.; Hall, L.J. Bifidobacterium breve UCC2003 Induces a Distinct Global Transcriptomic Program in Neonatal Murine Intestinal Epithelial Cells. iScience 2020, 23, 101336. [Google Scholar] [CrossRef]
- Izumi, H.; Minegishi, M.; Sato, Y.; Shimizu, T.; Sekine, K.; Takase, M. Bifidobacterium breve alters immune function and ameliorates DSS-induced inflammation in weanling rats. Pediatr. Res. 2015, 78, 407–416. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsuji, M.; Tanaka, N.; Koike, H.; Sato, Y.; Shimoyama, Y.; Itoh, A. Various Organ Damages in Rats with Fetal Growth Restriction and Their Slight Attenuation by Bifidobacterium breve Supplementation. Life 2023, 13, 2005. https://doi.org/10.3390/life13102005
Tsuji M, Tanaka N, Koike H, Sato Y, Shimoyama Y, Itoh A. Various Organ Damages in Rats with Fetal Growth Restriction and Their Slight Attenuation by Bifidobacterium breve Supplementation. Life. 2023; 13(10):2005. https://doi.org/10.3390/life13102005
Chicago/Turabian StyleTsuji, Masahiro, Nao Tanaka, Hitomi Koike, Yoshiaki Sato, Yoshie Shimoyama, and Ayaka Itoh. 2023. "Various Organ Damages in Rats with Fetal Growth Restriction and Their Slight Attenuation by Bifidobacterium breve Supplementation" Life 13, no. 10: 2005. https://doi.org/10.3390/life13102005
APA StyleTsuji, M., Tanaka, N., Koike, H., Sato, Y., Shimoyama, Y., & Itoh, A. (2023). Various Organ Damages in Rats with Fetal Growth Restriction and Their Slight Attenuation by Bifidobacterium breve Supplementation. Life, 13(10), 2005. https://doi.org/10.3390/life13102005





