Cellular Behaviors of Periodontal Ligament Stem Cells in the Presence of Bone Grafting Biomaterials, In-Vitro Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Employed
2.2. Culture and Proliferation of Periodontal Ligament Stem Cells
2.3. Assessment of Cellular Attachment and Proliferation via the MTS Assay
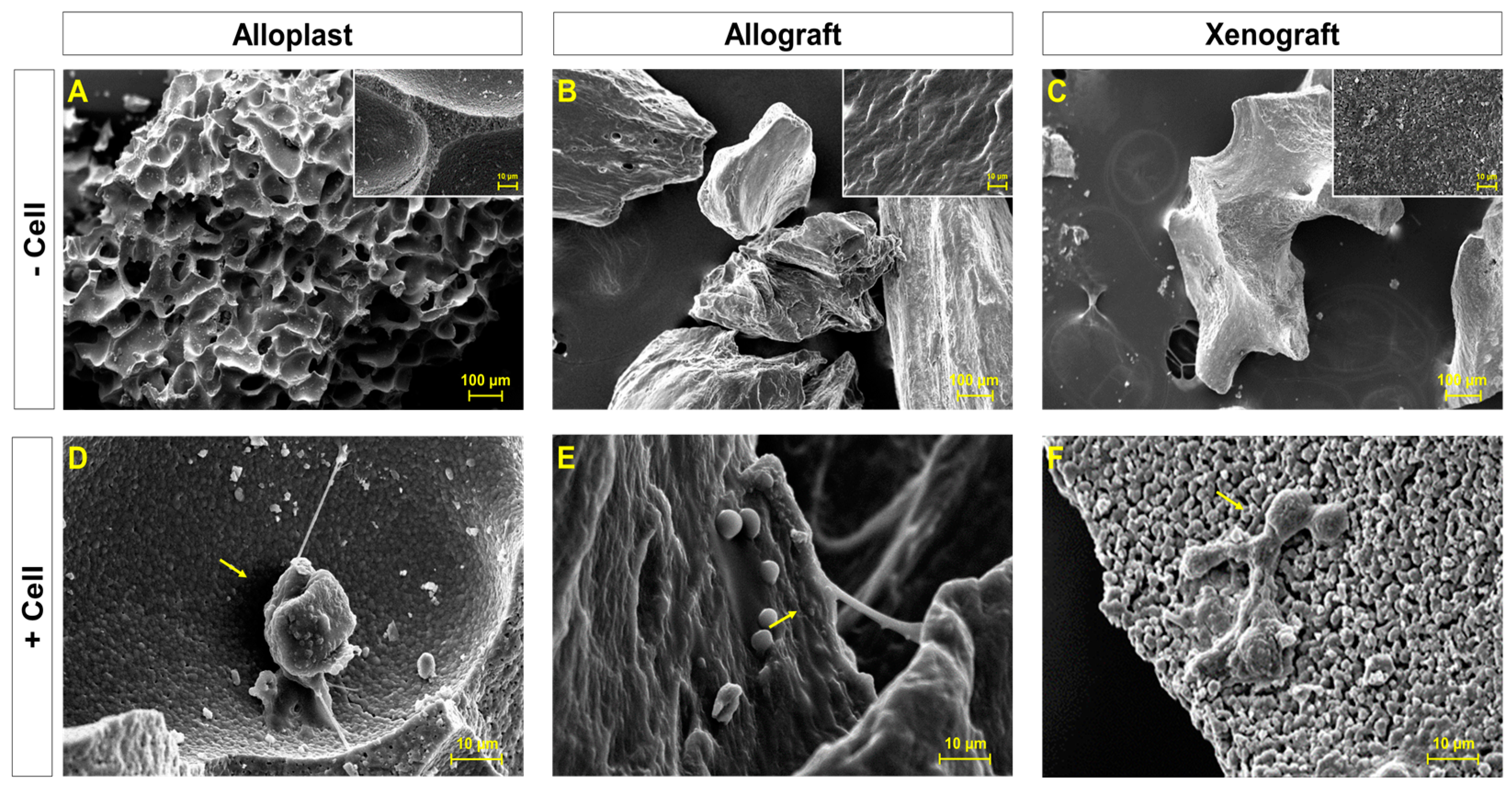
2.4. Assessment of Cellular Morphology with SEM
2.5. Osteoblastic Differentiation
2.6. Study of the Osteoblastic Differentiation Rate by qRT-PCR
2.7. Statistical Studies
3. Results
3.1. Surface Morphology and Attachment of Cells on Bone Grafts Substitutes
3.2. Assessment of the Attachment Capacity of PDLSCs on Bone Graft Substitutes
3.3. Assessment of PDLSCs Proliferation Rate on Bone Graft Substitutes
3.4. Evaluation of PDLSCs Proliferation on Bone Graft Substitutes over Time
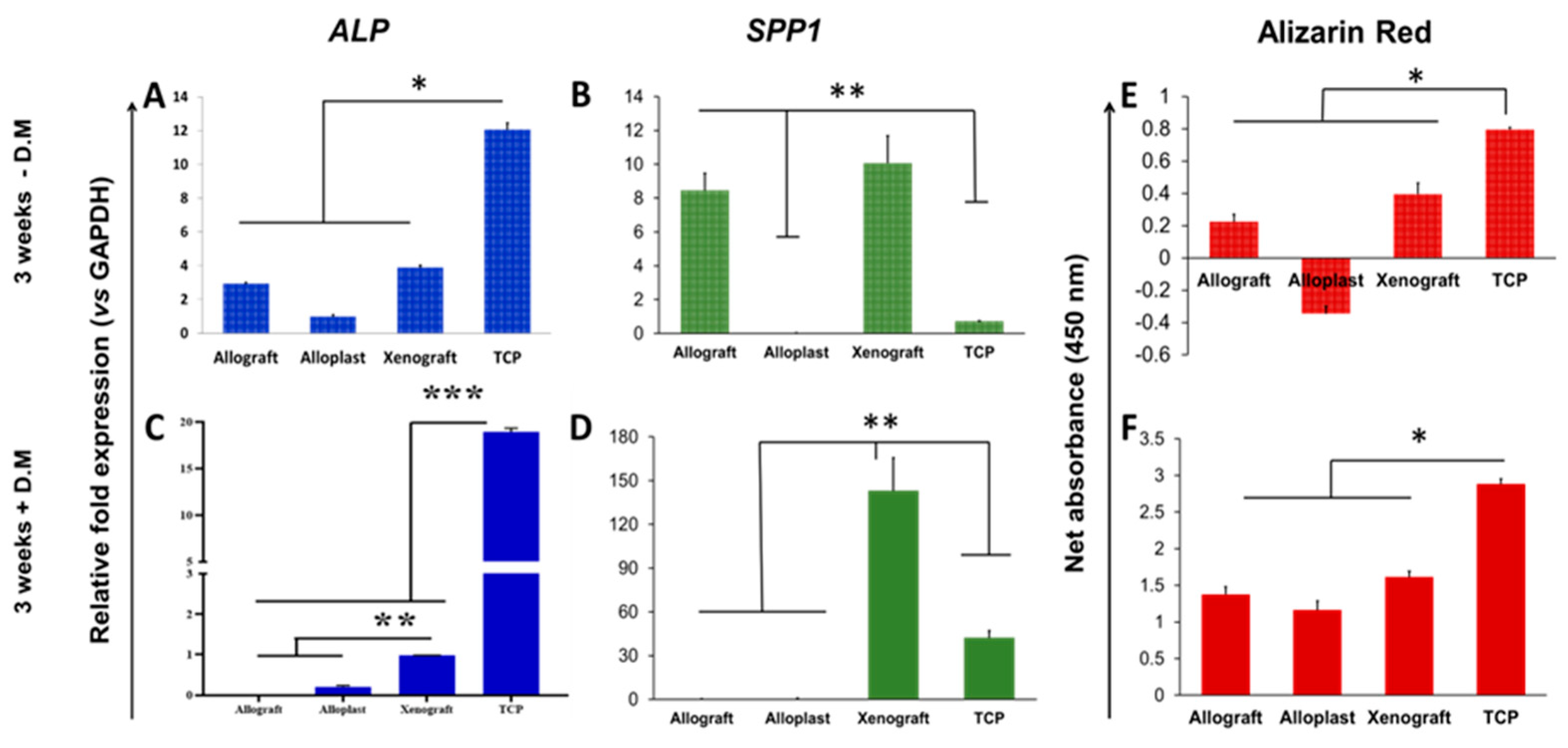
3.5. Assessment of PDLSCs Bone Differentiation Potential on Bone Graft Substitutes
4. Discussion
4.1. Comparison of Osteoblastic Differentiation Rate between Allografts, Xenografts, and Alloplasts
4.2. Comparison of the Osteoblastic Differentiation Rate in the Presence or Absence of the Differentiation Medium
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cochran, D.L.; Cobb, C.M.; Bashutski, J.D.; Chun, Y.-H.P.; Lin, Z.; Mandelaris, G.A.; McAllister, B.S.; Murakami, S.; Rios, H.F. Emerging regenerative approaches for periodontal reconstruction: A consensus report from the AAP Regeneration Workshop. J. Periodontol. 2015, 86 (Suppl. S2), S153–S156. [Google Scholar] [CrossRef] [PubMed]
- Melcher, A.H. On the repair potential of periodontal tissues. J. Periodontol. 1976, 47, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Hitti, R.A.; Kerns, D.G. Guided bone regeneration in the oral cavity: A review. Open Pathol. J. 2011, 5, 33–45. [Google Scholar] [CrossRef]
- Karring, T.; Nyman, S.; Gottlow, J.; Laurell, L. Development of the biological concept of guided tissue regeneration—Animal and human studies. Periodontology 1993, 1, 26–35. [Google Scholar] [CrossRef]
- Parlar, A.; Bosshardt, D.D.; Unsal, B.; Cetiner, D.; Haytaç, C.; Lang, N.P. New formation of periodontal tissues around titanium implants in a novel dentin chamber model. Clin. Oral. Implant. Res. 2005, 16, 259–267. [Google Scholar] [CrossRef]
- Manescu, A.; Giuliani, A.; Mohammadi, S.; Tromba, G.; Mazzoni, S.; Diomede, F.; Zini, N.; Piattelli, A.; Trubiani, O. Osteogenic potential of dualblocks cultured with human periodontal ligament stem cells: In vitro and synchrotron microtomography study. J. Periodontal. Res. 2016, 51, 112–124. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, S.Y.; Ju, Y.M.; Yoo, J.J.; Smith, T.L.; Khang, G.; Lee, S.J.; Atala, A. In Vitro osteogenic differentiation of human amniotic fluid-derived stem cells on a poly(lactide-co-glycolide) (PLGA)-bladder submucosa matrix (BSM) composite scaffold for bone tissue engineering. Biomed. Mater. 2013, 8, 014107. [Google Scholar] [CrossRef]
- Bosshardt, D.D. Biological mediators and periodontal regeneration: A review of enamel matrix proteins at the cellular and molecular levels. J. Clin. Periodontol. 2008, 35 (Suppl. S8), 87–105. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, Y.; Wu, X.; Zhao, B.; Ji, Y.; Xu, X. A comprehensive study on donor-matched comparisons of three types of mesenchymal stem cells-containing cells from human dental tissue. J. Periodontal. Res. 2019, 54, 286–299. [Google Scholar] [CrossRef]
- Lynch, S.E.; Williams, R.C.; Polson, A.M.; Howell, T.H.; Reddy, M.S.; Zappa, U.E.; Antoniades, H.N. A combination of platelet-derived and insulin-like growth factors enhances periodontal regeneration. J. Clin. Periodontol. 1989, 16, 545–548. [Google Scholar] [CrossRef]
- Bartold, P.M.; McCulloch, C.A.; Narayanan, A.S.; Pitaru, S. Tissue engineering: A new paradigm for periodontal regeneration based on molecular and cell biology. Periodontol. 2000 2000, 24, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.A.; Aichelmann-Reidy, M.E.; Branch-Mays, G.L.; Gunsolley, J.C. The efficacy of bone replacement grafts in the treatment of periodontal osseous defects. A systematic review. Ann. Periodontol. Am. Acad. Periodontol. 2003, 8, 227–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Greenwell, H.; Fiorellini, J.; Giannobile, W.; Offenbacher, S.; Salkin, L.; Townsend, C.; Sheridan, P.; Genco, R.J. Periodontal regeneration. J. Periodontol. 2005, 76, 1601–1622. [Google Scholar]
- Miron, R.J.; Bosshardt, D.D.; Hedbom, E.; Zhang, Y.; Haenni, B.; Buser, D.; Sculean, A. Adsorption of enamel matrix proteins to a bovine-derived bone grafting material and its regulation of cell adhesion, proliferation, and differentiation. J. Periodontol. 2012, 83, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Bosshardt, D.D.; Laugisch, O.; Dard, M.; Gemperli, A.C.; Buser, D.; Gruber, R.; Sculean, A. In Vitro evaluation of demineralized freeze-dried bone allograft in combination with enamel matrix derivative. J. Periodontol. 2013, 84, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Vandana, K.L.; Rajendra, D.; Priyanka, J.D. Autologous Stem Cell Application in Periodontal Regeneration Technique (SAI-PRT) Using PDLSCs Directly from an Extracted Tooth an Insight. Int. J. Stem Cells 2015, 8, 235. [Google Scholar] [CrossRef]
- Su, F.; Liu, S.S.; Ma, J.L.; Wang, D.S.; Liu, H.C. Enhancement of periodontal tissue regeneration by transplantation of osteoprotegerin-engineered periodontal ligament stem cells. Stem Cell Res. Ther. 2015, 6, 22. [Google Scholar] [CrossRef]
- Chen, F.M.; Gao, L.N.; Tian, B.M.; Zhang, X.Y.; Zhang, Y.J.; Dong, G.Y. Treatment of periodontal intrabony defects using autologous periodontal ligament stem cells: A randomized clinical trial. Stem Cell Res. Ther. 2016, 7, 33. [Google Scholar] [CrossRef]
- Bernhardt, A.; Lode, A.; Peters, F.; Gelinsky, M. Comparative evaluation of different calcium phosphate-based bone graft granules—An in vitro study with osteoblast-like cells. Clin. Oral Implant. Res. 2013, 24, 441–449. [Google Scholar] [CrossRef]
- Payer, M.; Lohberger, B.; Stadelmeyer, E.; Bartmann, C.; Windhager, R.; Jakse, N. Behaviour of multipotent maxillary bone-derived cells on beta-tricalcium phosphate and highly porous bovine bone mineral. Clin. Oral Implant. Res. 2010, 21, 699–708. [Google Scholar] [CrossRef]
- Miron, R.J.; Zhang, Y.F. Osteoinduction: A review of old concepts with new standards. J. Dent. Res. 2012, 91, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yi, G.S.; Lee, J.W.; Kim, D.J. Physicochemical characterization of porcine bone-derived grafting material and comparison with bovine xenografts for dental applications. J. Periodontal. Implant. Sci. 2017, 47, 388–401. [Google Scholar] [CrossRef] [PubMed]
- Trajkovski, B.; Jaunich, M.; Müller, W.D.; Beuer, F.; Zafiropoulos, G.G.; Houshmand, A. Hydrophilicity, Viscoelastic, and Physicochemical Properties Variations in Dental Bone Grafting Substitutes. Materials 2018, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Lafzi, A.; Vahabi, S.; Ghods, S.; Torshabi, M. In vitro effect of mineralized and demineralized bone allografts on proliferation and differentiation of MG-63 osteoblast-like cells. Cell Tissue Bank 2016, 17, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Caluseru, O.M.; Guillemette, V.; Zhang, Y.; Buser, D.; Chandad, F.; Sculean, A. Effect of bone graft density on in vitro cell behavior with enamel matrix derivative. Clin. Oral Investig. 2015, 19, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, M.; He, J. A review of biomimetic scaffolds for bone regeneration: Toward a cell-free strategy. Bioeng. Transl. Med. 2021, 6, e10206. [Google Scholar] [CrossRef] [PubMed]
- Motamedian, S.R.; Tabatabaei, F.S.; Akhlaghi, F.; Torshabi, M.; Gholamin, P.; Khojasteh, A. Response of Dental Pulp Stem Cells to Synthetic, Allograft, and Xenograft Bone Scaffolds. Int. J. Periodontics Restor. Dent. 2017, 37, 49–59. [Google Scholar] [CrossRef]
- Franchimont, N.; Durant, D.; Rydziel, S.; Canalis, E. Platelet-derived growth factor induces interleukin-6 transcription in osteoblasts through the activator protein-1 complex and activating transcription factor-2. J. Biol. Chem. 1999, 274, 6783–6789. [Google Scholar] [CrossRef]
- Yu, X.; Hsieh, S.C.; Bao, W.; Graves, D.T. Temporal expression of PDGF receptors and PDGF regulatory effects on osteoblastic cells in mineralizing cultures. Am. J. Physiol. 1997, 272, C1709–C1716. [Google Scholar] [CrossRef]
- Choi, M.H.; Noh, W.C.; Park, J.W.; Lee, J.M.; Suh, J.Y. Gene expression pattern during osteogenic differentiation of human periodontal ligament cells in vitro. J. Periodontal. Implant. Sci. 2011, 41, 167–175. [Google Scholar] [CrossRef]
- Kusuyama, J.; Bandow, K.; Ohnishi, T.; Hisadome, M.; Shima, K.; Semba, I.; Matsuguchi, T. Osteopontin inhibits osteoblast responsiveness through the down-regulation of focal adhesion kinase mediated by the induction of low-molecular weight protein tyrosine phosphatase. Mol. Biol. Cell. 2017, 28, 1326–1336. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Egusa, H. Current bone substitutes for implant dentistry. J. Prosthodont. Res. 2018, 62, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Li, D.C.; Huang, Z.S.; Yuan, Z. Effects of sodium hydroxide, sodium hypochlorite, and gaseous hydrogen peroxide on the natural properties of cancellous bone. Artif. Organs. 2013, 37, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Lei, P.; Sun, R.; Wang, L.; Zhou, J.; Wan, L.; Zhou, T.; Hu, Y. A new method for xenogeneic bone graft deproteinization: Comparative study of radius defects in a rabbit model. PLoS ONE 2015, 10, e0146005. [Google Scholar] [CrossRef]
- Pilipchuk, S.P.; Plonka, A.B.; Monje, A.; Taut, A.D.; Lanis, A.; Kang, B.; Giannobile, W.V. Tissue engineering for bone regeneration and osseointegration in the oral cavity. Dent. Mater. 2015, 31, 317–338. [Google Scholar] [CrossRef]
- Miron, R.J.; Bosshardt, D.D.; Gemperli, A.C.; Dard, M.; Buser, D.; Gruber, R.; Sculean, A. In Vitro characterization of a synthetic calcium phosphate bone graft on periodontal ligament cell and osteoblast behavior and its combination with an enamel matrix derivative. Clin. Oral Investig. 2014, 18, 443–451. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esfahanian, V.; Ejeian, F.; Mohebinia, H.; Zojaji Nejad, Z.S.; Yazdchi, M.; Ebrahimi Dastgerdi, M.; Ebrahimi Dastgerdi, M.; Nasr-Esfahani, M.H. Cellular Behaviors of Periodontal Ligament Stem Cells in the Presence of Bone Grafting Biomaterials, In-Vitro Study. Life 2023, 13, 89. https://doi.org/10.3390/life13010089
Esfahanian V, Ejeian F, Mohebinia H, Zojaji Nejad ZS, Yazdchi M, Ebrahimi Dastgerdi M, Ebrahimi Dastgerdi M, Nasr-Esfahani MH. Cellular Behaviors of Periodontal Ligament Stem Cells in the Presence of Bone Grafting Biomaterials, In-Vitro Study. Life. 2023; 13(1):89. https://doi.org/10.3390/life13010089
Chicago/Turabian StyleEsfahanian, Vahid, Fatemeh Ejeian, Hajar Mohebinia, Zahra Sadat Zojaji Nejad, Maryam Yazdchi, Maziar Ebrahimi Dastgerdi, Mehrnoush Ebrahimi Dastgerdi, and Mohammad Hossein Nasr-Esfahani. 2023. "Cellular Behaviors of Periodontal Ligament Stem Cells in the Presence of Bone Grafting Biomaterials, In-Vitro Study" Life 13, no. 1: 89. https://doi.org/10.3390/life13010089
APA StyleEsfahanian, V., Ejeian, F., Mohebinia, H., Zojaji Nejad, Z. S., Yazdchi, M., Ebrahimi Dastgerdi, M., Ebrahimi Dastgerdi, M., & Nasr-Esfahani, M. H. (2023). Cellular Behaviors of Periodontal Ligament Stem Cells in the Presence of Bone Grafting Biomaterials, In-Vitro Study. Life, 13(1), 89. https://doi.org/10.3390/life13010089






