Biomarker Alteration after Neoadjuvant Endocrine Therapy or Chemotherapy in Estrogen Receptor-Positive Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Pathological Evaluation
2.3. Statistical Analysis
3. Results
3.1. Patient Selection and Baseline Clinicopathological Information
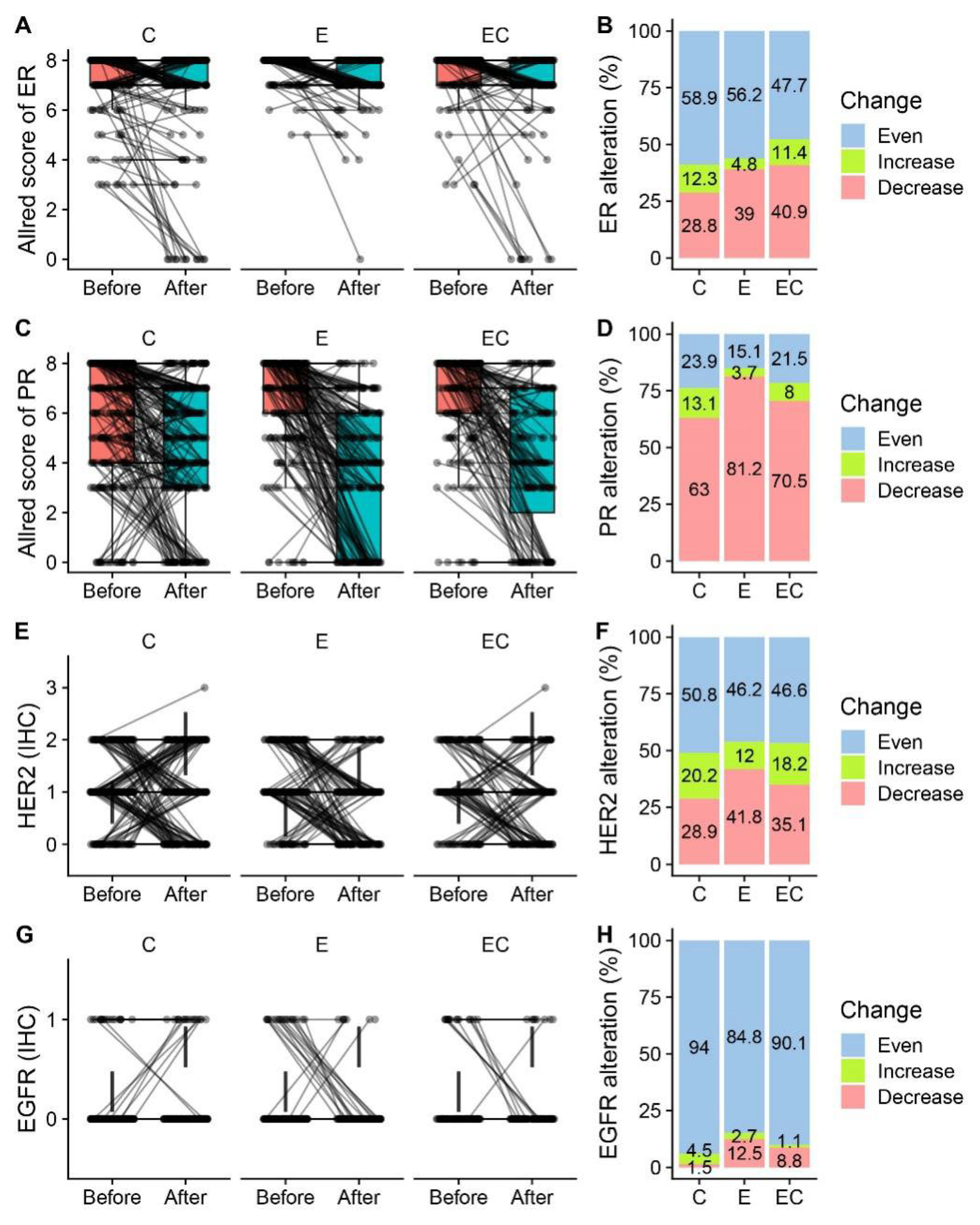
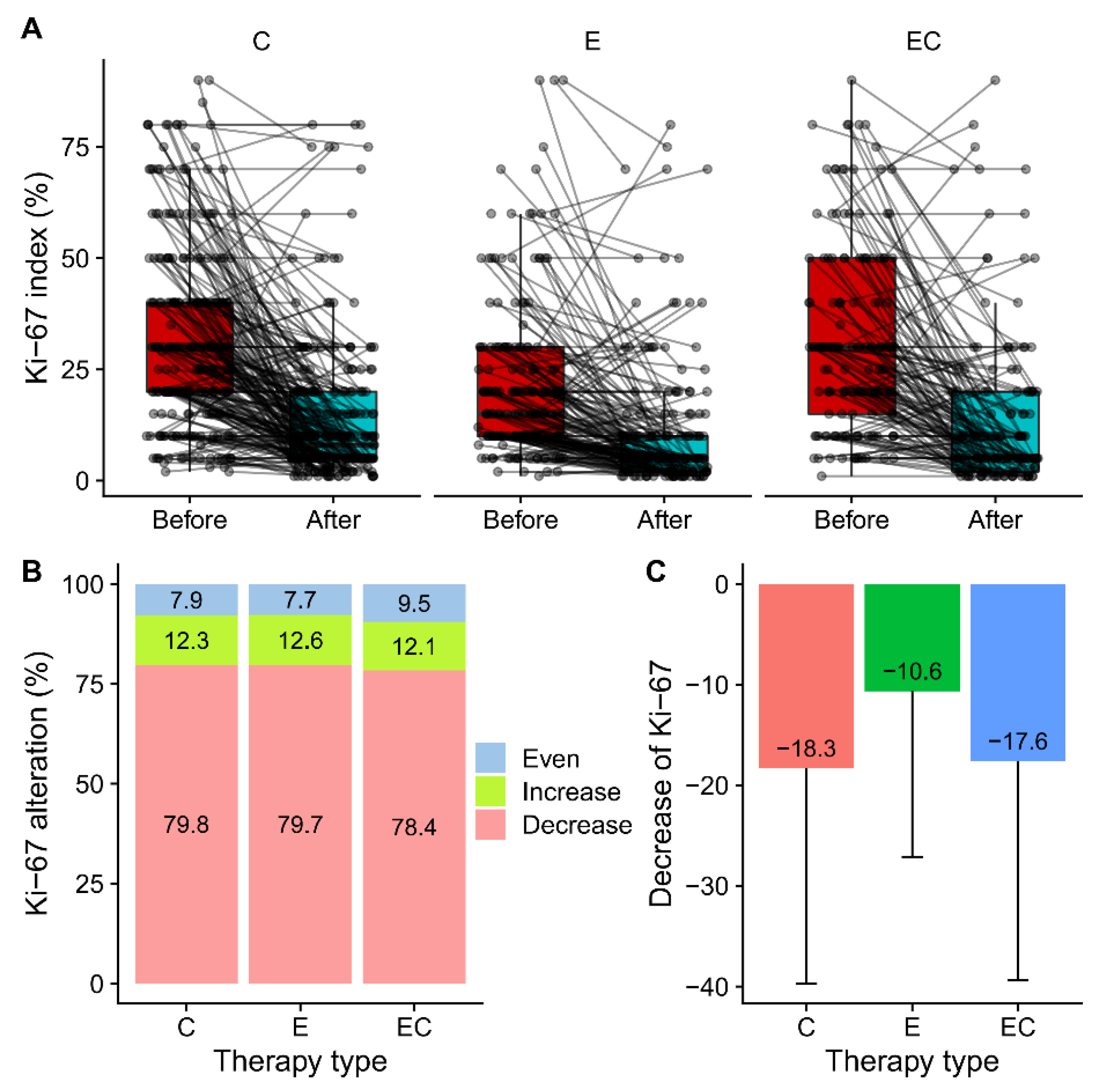
3.2. Alteration of ER, PR, and Ki-67 Expression after Neoadjuvant Therapy
3.3. PR Loss Was Associated with Baseline PR Score but Not with Ki-67 Alteration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnston, S.J.; Cheung, K.L. Endocrine Therapy for Breast Cancer: A Model of Hormonal Manipulation. Oncol. Ther. 2018, 6, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Marti, C.; Sanchez-Mendez, J.I. The Present and Future of Neoadjuvant Endocrine Therapy for Breast Cancer Treatment. Cancers 2021, 13, 2538. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.J.; Suman, V.J.; Hoog, J.; Lin, L.; Snider, J.; Prat, A.; Parker, J.S.; Luo, J.; DeSchryver, K.; Allred, D.C.; et al. Randomized phase II neoadjuvant comparison between letrozole, anastrozole, and exemestane for postmenopausal women with estrogen receptor-rich stage 2 to 3 breast cancer: Clinical and biomarker outcomes and predictive value of the baseline PAM50-based intrinsic subtype—ACOSOG Z1031. J. Clin. Oncol. 2011, 29, 2342–2349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, C.Y.; Chen, H.M.; Wu, S.Y. Neoadjuvant Chemotherapy or Endocrine Therapy for Invasive Ductal Carcinoma of the Breast with High Hormone Receptor Positivity and Human Epidermal Growth Factor Receptor 2 Negativity. JAMA Netw. Open 2021, 4, e211785. [Google Scholar] [CrossRef] [PubMed]
- Sugiu, K.; Iwamoto, T.; Kelly, C.M.; Watanabe, N.; Motoki, T.; Ito, M.; Ohtani, S.; Higaki, K.; Imada, T.; Yuasa, T.; et al. Neoadjuvant Chemotherapy with or without Concurrent Hormone Therapy in Estrogen Receptor-Positive Breast Cancer: NACED-Randomized Multicenter Phase II Trial. Acta Med. Okayama 2015, 69, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.D.; Wu, S.Y.; Liu, G.Y.; Wu, J.; Di, G.H.; Hu, Z.; Hou, Y.F.; Chen, C.M.; Fan, L.; Tang, L.C.; et al. Concurrent neoadjuvant chemotherapy and estrogen deprivation in patients with estrogen receptor-positive, human epidermal growth factor receptor 2-negative breast cancer (CBCSG-036): A randomized, controlled, multicenter trial. Cancer 2019, 125, 2185–2193. [Google Scholar] [CrossRef]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.H.; Zhang, X.; Song, J.L.; Ran, L.; Luo, R.; Li, H.Y.; Wang, Y.H. Neoadjuvant chemotherapy reduces the expression rates of ER, PR, HER2, Ki67, and P53 of invasive ductal carcinoma. Medicine 2019, 98, e13554. [Google Scholar] [CrossRef] [PubMed]
- Rey-Vargas, L.; Mejia-Henao, J.C.; Sanabria-Salas, M.C.; Serrano-Gomez, S.J. Effect of neoadjuvant therapy on breast cancer biomarker profile. BMC Cancer 2020, 20, 675. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.E.; Dowsett, M.; Ebbs, S.R.; Dixon, J.M.; Skene, A.; Blohmer, J.U.; Ashley, S.E.; Francis, S.; Boeddinghaus, I.; Walsh, G.; et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: The Immediate Preoperative Anastrozole, Tamoxifen, or Combined with Tamoxifen (IMPACT) multicenter double-blind randomized trial. J. Clin. Oncol. 2005, 23, 5108–5116. [Google Scholar] [CrossRef]
- Eiermann, W.; Paepke, S.; Appfelstaedt, J.; Llombart-Cussac, A.; Eremin, J.; Vinholes, J.; Mauriac, L.; Ellis, M.; Lassus, M.; Chaudri-Ross, H.A.; et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double-blind multicenter study. Ann. Oncol. 2001, 12, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Niikura, N.; Tomotaki, A.; Miyata, H.; Iwamoto, T.; Kawai, M.; Anan, K.; Hayashi, N.; Aogi, K.; Ishida, T.; Masuoka, H.; et al. Changes in tumor expression of HER2 and hormone receptors status after neoadjuvant chemotherapy in 21,755 patients from the Japanese breast cancer registry. Ann. Oncol. 2016, 27, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.J.; Tao, Y.; Luo, J.; A’Hern, R.; Evans, D.B.; Bhatnagar, A.S.; Chaudri Ross, H.A.; von Kameke, A.; Miller, W.R.; Smith, I.; et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J. Natl. Cancer Inst. 2008, 100, 1380–1388. [Google Scholar] [CrossRef]
- Wang, X.; Fan, Z.; Wang, X.; He, Y.; Liu, Y.; Wang, X.; Zhang, B.; Jiang, Z.; Wang, T.; Yu, Z.; et al. Neoadjuvant endocrine therapy for strongly hormone receptor-positive and HER2-negative early breast cancer: Results of a prospective multi-center study. Breast Cancer Res. Treat. 2022, 195, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Liu, Y.; Wu, J.; Hu, X.L.; Zhao, G.; Liang, B.; Wang, S.; Long, M. Triple-Negative Apocrine Breast Carcinoma Has Better Prognosis despite Poor Response to Neoadjuvant Chemotherapy. J. Clin. Med. 2022, 11, 1607. [Google Scholar] [CrossRef]
- Allred, D.C.; Harvey, J.M.; Berardo, M.; Clark, G.M. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod. Pathol. 1998, 11, 155–168. [Google Scholar]
- Harvey, J.M.; Clark, G.M.; Osborne, C.K.; Allred, D.C. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J. Clin. Oncol. 1999, 17, 1474–1481. [Google Scholar] [CrossRef]
- Fontein, D.B.; Charehbili, A.; Nortier, J.W.; Meershoek-Klein Kranenbarg, E.; Kroep, J.R.; Putter, H.; van Riet, Y.; Nieuwenhuijzen, G.A.; de Valk, B.; Terwogt, J.M.; et al. Efficacy of six month neoadjuvant endocrine therapy in postmenopausal, hormone receptor-positive breast cancer patients—A phase II trial. Eur. J. Cancer 2014, 50, 2190–2200. [Google Scholar] [CrossRef]
- Dixon, J.M.; Renshaw, L.; Macaskill, E.J.; Young, O.; Murray, J.; Cameron, D.; Kerr, G.R.; Evans, D.B.; Miller, W.R. Increase in response rate by prolonged treatment with neoadjuvant letrozole. Breast Cancer Res. Treat. 2009, 113, 145–151. [Google Scholar] [CrossRef]
- Ahn, S.; Kim, H.J.; Kim, M.; Chung, Y.R.; Kang, E.; Kim, E.K.; Kim, S.H.; Kim, Y.J.; Kim, J.H.; Kim, I.A.; et al. Negative Conversion of Progesterone Receptor Status after Primary Systemic Therapy Is Associated with Poor Clinical Outcome in Patients with Breast Cancer. Cancer Res. Treat. 2018, 50, 1418–1432. [Google Scholar] [CrossRef]
- Kurozumi, S.; Matsumoto, H.; Inoue, K.; Tozuka, K.; Hayashi, Y.; Kurosumi, M.; Oyama, T.; Fujii, T.; Horiguchi, J.; Kuwano, H. Impact of combining the progesterone receptor and preoperative endocrine prognostic index (PEPI) as a prognostic factor after neoadjuvant endocrine therapy using aromatase inhibitors in postmenopausal ER positive and HER2 negative breast cancer. PLoS ONE 2018, 13, e0201846. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Chen, Y.; Liu, Y.; Zhang, D.; Pan, J.; Long, M. Classification of PR-positive and PR-negative subtypes in ER-positive and HER2-negative breast cancers based on pathway scores. BMC Med. Res. Methodol. 2021, 21, 108. [Google Scholar] [CrossRef]
- Yao, N.; Song, Z.; Wang, X.; Yang, S.; Song, H. Prognostic Impact of Progesterone Receptor Status in Chinese Estrogen Receptor Positive Invasive Breast Cancer Patients. J. Breast Cancer 2017, 20, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, D.; Yin, X.; Zhang, X.; Huang, J.; Wu, Y.; Wang, M.; Yi, Z.; Li, H.; Li, H.; et al. Clinicopathological Characteristics and Breast Cancer-Specific Survival of Patients with Single Hormone Receptor-Positive Breast Cancer. JAMA Netw. Open 2020, 3, e1918160. [Google Scholar] [CrossRef] [PubMed]
- Schrijver, W.; Suijkerbuijk, K.P.M.; van Gils, C.H.; van der Wall, E.; Moelans, C.B.; van Diest, P.J. Receptor Conversion in Distant Breast Cancer Metastases: A Systematic Review and Meta-analysis. J. Natl. Cancer Inst. 2018, 110, 568–580. [Google Scholar] [CrossRef]
- Muti, P. Is progesterone a neutral or protective factor for breast cancer? Nat. Rev. Cancer 2014, 14, 146. [Google Scholar] [CrossRef]
- Brisken, C. Progesterone signalling in breast cancer: A neglected hormone coming into the limelight. Nat. Rev. Cancer 2013, 13, 385–396. [Google Scholar] [CrossRef]
- Tanos, T.; Sflomos, G.; Echeverria, P.C.; Ayyanan, A.; Gutierrez, M.; Delaloye, J.F.; Raffoul, W.; Fiche, M.; Dougall, W.; Schneider, P.; et al. Progesterone/RANKL is a major regulatory axis in the human breast. Sci. Transl. Med. 2013, 5, 182ra155. [Google Scholar] [CrossRef]
- Alkhalaf, M.; El-Mowafy, A.; Karam, S. Growth inhibition of MCF-7 human breast cancer cells by progesterone is associated with cell differentiation and phosphorylation of Akt protein. Eur. J. Cancer Prev. 2002, 11, 481–488. [Google Scholar] [CrossRef]
- Lin, V.C.; Eng, A.S.; Hen, N.E.; Ng, E.H.; Chowdhury, S.H. Effect of progesterone on the invasive properties and tumor growth of progesterone receptor-transfected breast cancer cells MDA-MB-231. Clin. Cancer Res. 2001, 7, 2880–2886. [Google Scholar]
- Schernhammer, E.S.; Sperati, F.; Razavi, P.; Agnoli, C.; Sieri, S.; Berrino, F.; Krogh, V.; Abbagnato, C.; Grioni, S.; Blandino, G.; et al. Endogenous sex steroids in premenopausal women and risk of breast cancer: The ORDET cohort. Breast Cancer Res. 2013, 15, R46. [Google Scholar] [CrossRef] [PubMed]
- Inda, M.A.; Blok, E.J.; Kuppen, P.J.K.; Charehbili, A.; den Biezen-Timmermans, E.C.; van Brussel, A.; Fruytier, S.E.; Meershoek-Klein Kranenbarg, E.; Kloet, S.; van der Burg, B.; et al. Estrogen Receptor Pathway Activity Score to Predict Clinical Response or Resistance to Neoadjuvant Endocrine Therapy in Primary Breast Cancer. Mol. Cancer Ther. 2020, 19, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Chic, N.; Schettini, F.; Braso-Maristany, F.; Sanfeliu, E.; Adamo, B.; Vidal, M.; Martinez, D.; Galvan, P.; Gonzalez-Farre, B.; Cortes, J.; et al. Oestrogen receptor activity in hormone-dependent breast cancer during chemotherapy. EBioMedicine 2021, 69, 103451. [Google Scholar] [CrossRef] [PubMed]
- Brechbuhl, H.M.; Xie, M.; Kopin, E.G.; Han, A.L.; Vinod-Paul, K.; Hagen, J.; Edgerton, S.; Owens, P.; Sams, S.; Elias, A.; et al. Neoadjuvant endocrine therapy expands stromal populations that predict poor prognosis in estrogen receptor-positive breast cancer. Mol. Carcinog. 2021, 61, 359–371. [Google Scholar] [CrossRef]

| Cohort-C | Cohort-E | Cohort-EC | p-Value | |
|---|---|---|---|---|
| Total patients | 633 | 314 | 247 | |
| Age (years) 1 | 48.9 (9.9) | 57.8 (10.5) | 51.3 (10.3) | <0.001 |
| Histology 2 | 0.492 | |||
| IDC-NST | 587 (92.7) | 288 (91.7) | 233 (94.3) | |
| Special subtype | 46 (7.3) | 26 (8.3) | 14 (5.7) | |
| Histological grade 2 | <0.001 | |||
| I | 88 (13.9) | 62 (19.7) | 35 (14.2) | |
| II | 472 (74.6) | 244 (77.7) | 192 (77.7) | |
| III | 73 (11.5) | 8 (2.5) | 20 (8.1) | |
| Surgery type 2 | <0.001 | |||
| BCS | 242 (38.2) | 197 (62.7) | 138 (55.9) | |
| Mastectomy | 391 (61.8) | 117 (37.3) | 109 (44.1) | |
| ER Allred score 2 | <0.001 | |||
| 3/4/5 | 61 (9.6) | 2 (0.6) | 17 (6.9) | |
| 6/7/8 | 572 (90.4) | 312 (99.4) | 230 (93.1) | |
| PR Allred score 2 | 0.001 | |||
| 0/2 | 63 (10.0) | 13 (4.1) | 15 (6.1) | |
| 3/4/5 | 140 (22.1) | 51 (16.2) | 49 (19.8) | |
| 6/7/8 | 430 (67.9) | 250 (79.6) | 183 (74.1) | |
| Ki-67 (%) 1 | 34.3 (22.5) | 21.9 (16.9) | 33.7 (21.8) | <0.001 |
| NET duration (months) 1 | N/A | 9.9 (8.3) | 8.5 (7.5) | 0.043 |
| Use of aromatase inhibitor 2 | <0.001 | |||
| No | 633 (100.0) | 29 (9.2) | 40 (16.2) | |
| Yes | 0 (0.0) | 285 (90.8) | 207 (83.8) |
| Cohort-C | Cohort-E | Cohort-EC | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | ||||
| ER Allred score 1 | p < 0.001 | p < 0.001 | p < 0.001 | ||||||
| 0/2 | 13 (5.3) | 1 (0.5) | 8 (5.4) | ||||||
| 3/4/5 | 17 (7.0) | 14 (5.8) | 2 (1.1) | 6 (3.2) | 7 (4.7) | 6 (4.0) | |||
| 6/7/8 | 226 (93.0) | 216 (88.9) | 185 (98.9) | 180 (96.3) | 142 (95.3) | 135 (90.6) | |||
| PR Allred score | p < 0.001 | p < 0.001 | p < 0.001 | ||||||
| 0/2 | 20 (8.2) | 47 (19.3) | 9 (4.8) | 60 (32.1) | 7 (4.7) | 38 (25.5) | |||
| 3/4/5 | 64 (26.3) | 81 (33.3) | 35 (18.7) | 72 (38.5) | 26 (17.4) | 49 (32.9) | |||
| 6/7/8 | 159 (65.4) | 115 (47.3) | 143 (76.5) | 55 (29.4) | 116 (77.9) | 62 (41.6) | |||
| Ki-67(%), mean (SD) | 32.4 (21.0) | 14.0 (16.3) | p < 0.001 | 21.5 (17.7) | 10.8 (14.7) | p < 0.001 | 31.3 (21.1) | 13.7 (17.9) | p < 0.001 |
| HER2 | p < 0.001 | p < 0.001 | p < 0.001 | ||||||
| 0 | 48 (19.8) | 74 (30.5) | 29 (15.5) | 65 (34.8) | 32 (21.5) | 51 (34.2) | |||
| 1 | 107 (44.0) | 80 (32.9) | 95 (50.8) | 88 (47.1) | 66 (44.3) | 59 (39.6) | |||
| 2 | 88 (36.2) | 87 (35.8) | 63 (33.7) | 31 (16.6) | 51 (34.2) | 37 (24.8) | |||
| 3 | 1 (0.4) | 1 (0.7) | |||||||
| Unknown | 1 (0.4) | 3 (1.6) | 1 (0.7) | ||||||
| Age ≤ 60 Years (n = 106) | Age > 60 Years (n = 81) | p-Value | NET Length ≤6 Months (n = 92) | NET Length >6 Months (n = 95) | p-Value | |
|---|---|---|---|---|---|---|
| ER | 0.68 | 0.2 | ||||
| Decrease (%) | 40.6 | 37.0 | 30.9 | 45.3 | ||
| Even (%) | 53.8 | 59.3 | 58.8 | 50.5 | ||
| Decrease (%) | 5.7 | 3.7 | 5.2 | 4.2 | ||
| PR | 0.42 | 0.53 | ||||
| Decrease (%) | 80.2 | 84.0 | 79.4 | 80.0 | ||
| Even (%) | 17.0 | 11.1 | 11.3 | 16.8 | ||
| Decrease (%) | 2.8 | 4.9 | 4.1 | 3.2 | ||
| Ki-67 | 0.87 | 0.37 | ||||
| Decrease (%) | 80.4 | 78.8 | 83.1 | 76.3 | ||
| Even (%) | 6.9 | 8.8 | 4.5 | 10.8 | ||
| Decrease (%) | 12.7 | 12.5 | 12.4 | 12.9 |
| PR-Loss | PR-Preserved | p-Value | |
|---|---|---|---|
| Total patients | 53 | 125 | |
| Age (years, mean (SD)) 1 | 58.6 (9.0) | 57.4 (11.5) | 0.503 |
| Histology 2 | 0.028 | ||
| IDC-NST | 46 (86.8) | 121 (96.8) | |
| Special subtype | 7 (13.2) | 4 (3.2) | |
| Histological grade 2 | 0.604 | ||
| I | 10 (18.9) | 28 (22.4) | |
| II | 41 (77.4) | 95 (76.0) | |
| III | 2 (3.8) | 2 (1.6) | |
| Surgery type 2 | 0.285 | ||
| BCS | 37 (69.8) | 75 (60.0) | |
| Mastectomy | 16 (30.2) | 50 (40.0) | |
| ER Allred score (pre) 2 | 1.000 | ||
| 3/4/5 | 1 (1.9) | 1 (0.8) | |
| 6/7/8 | 52 (98.1) | 124 (99.2) | |
| ER Allred score (post) 2 | 0.274 | ||
| 0/2 | 1 (1.9) | ||
| 3/4/5 | 1 (1.9) | 4 (3.2) | |
| 6/7/8 | 51 (96.2) | 121 (96.8) | |
| PR Allred score (pre) 2 | 0.001 | ||
| 3/4/5 | 19 (35.8) | 16 (12.8) | |
| 6/7/8 | 34 (64.2) | 109 (87.2) | |
| PR Allred score (post) 2 | < 0.001 | ||
| 0/2 | 53 (100.0) | ||
| 3/4/5 | 70 (56.0) | ||
| 6/7/8 | 55 (44.0) | ||
| Ki-67 (%, pre) 1 | 22.9 (18.5) | 20.6 (17.4) | 0.428 |
| Ki-67 (%, post) 1 | 8.4 (13.2) | 10.4 (12.8) | 0.370 |
| EGFR (pre) 2 | 0.498 | ||
| 0 | 38 (71.7) | 80 (64.0) | |
| 1 | 3 (5.7) | 13 (10.4) | |
| Unknown | 12 (22.6) | 32 (25.6) | |
| EGFR (post) 2 | 0.236 | ||
| 0 | 41 (77.4) | 96 (76.8) | |
| 1 | 12 (22.6) | 23 (18.4) | |
| Unknown | 6 (4.8) | ||
| HER2 (pre) 2 | 0.439 | ||
| 0 | 7 (13.2) | 20 (16.0) | |
| 1 | 31 (58.5) | 60 (48.0) | |
| 2 | 15 (28.3) | 45 (36.0) | |
| HER2 (post) 2 | 0.780 | ||
| 0 | 15 (28.3) | 45 (36.0) | |
| 1 | 27 (50.9) | 59 (47.2) | |
| 2 | 10 (18.9) | 19 (15.2) | |
| Unknown | 1 (1.9) | 2 (1.6) | |
| NET duration (months) 1 | 10.1 (9.2) | 9.1 (6.6) | 0.378 |
| Use of aromatase inhibitor 2 | 0.377 | ||
| No | 1 (1.9) | 8 (6.4) | |
| Yes | 52 (98.1) | 117 (93.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Long, M.; You, C.; Song, Q.; Hu, L.X.J.; Guo, Z.; Yao, Q.; Hou, W.; Sun, W.; Liang, B.; Zhou, X.-H.; et al. Biomarker Alteration after Neoadjuvant Endocrine Therapy or Chemotherapy in Estrogen Receptor-Positive Breast Cancer. Life 2023, 13, 74. https://doi.org/10.3390/life13010074
Long M, You C, Song Q, Hu LXJ, Guo Z, Yao Q, Hou W, Sun W, Liang B, Zhou X-H, et al. Biomarker Alteration after Neoadjuvant Endocrine Therapy or Chemotherapy in Estrogen Receptor-Positive Breast Cancer. Life. 2023; 13(1):74. https://doi.org/10.3390/life13010074
Chicago/Turabian StyleLong, Mengping, Chong You, Qianqian Song, Lina X. J. Hu, Zhaorong Guo, Qian Yao, Wei Hou, Wei Sun, Baosheng Liang, Xiao-Hua Zhou, and et al. 2023. "Biomarker Alteration after Neoadjuvant Endocrine Therapy or Chemotherapy in Estrogen Receptor-Positive Breast Cancer" Life 13, no. 1: 74. https://doi.org/10.3390/life13010074
APA StyleLong, M., You, C., Song, Q., Hu, L. X. J., Guo, Z., Yao, Q., Hou, W., Sun, W., Liang, B., Zhou, X.-H., Liu, Y., & Hu, T. (2023). Biomarker Alteration after Neoadjuvant Endocrine Therapy or Chemotherapy in Estrogen Receptor-Positive Breast Cancer. Life, 13(1), 74. https://doi.org/10.3390/life13010074






