Role of Transient Receptor Potential Vanilloid 1 in Sonic Hedgehog-Dependent Taste Bud Differentiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Protocol
2.3. Chemicals and Antibodies
2.4. Live Imaging of Mouse Tongue by Two-Photon Microscope
2.5. Histological Analysis
2.6. Immunofluorescence Analysis
2.7. Statistical Analysis
3. Results
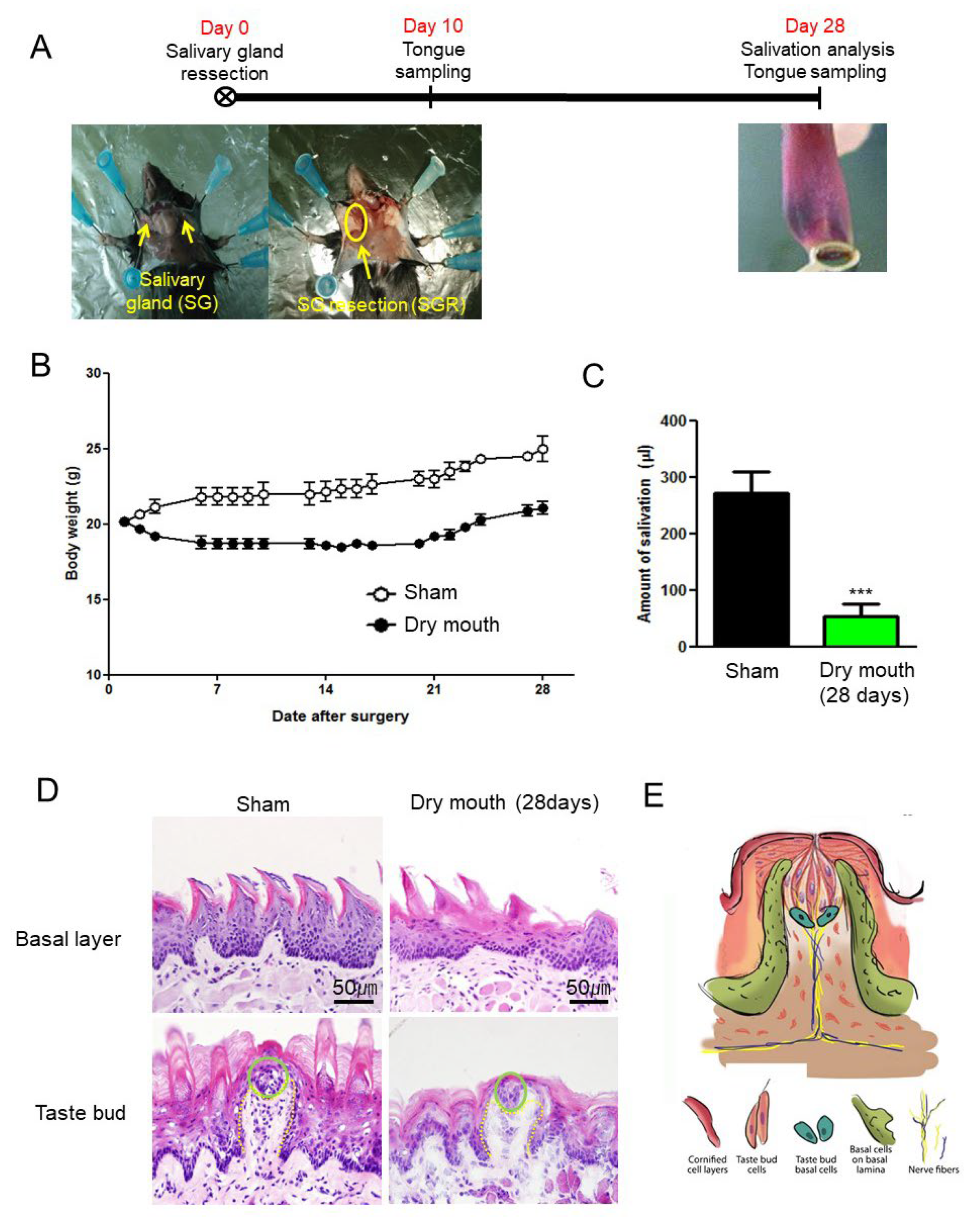
3.1. SGR Induced Body Weight Decrease and Dry Mouth
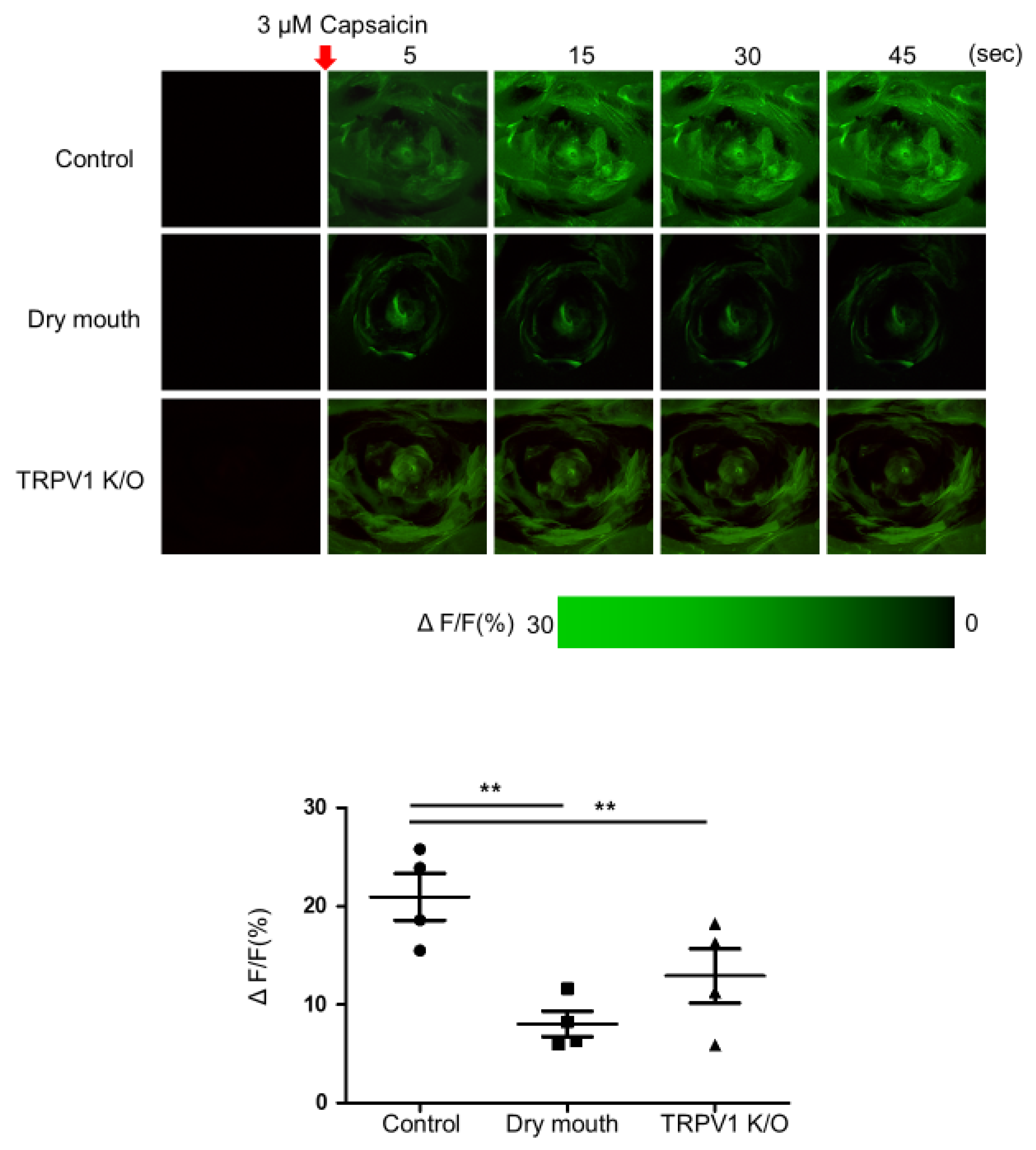
3.2. Comparison of Calcium Influx in Taste Bud Stimulation
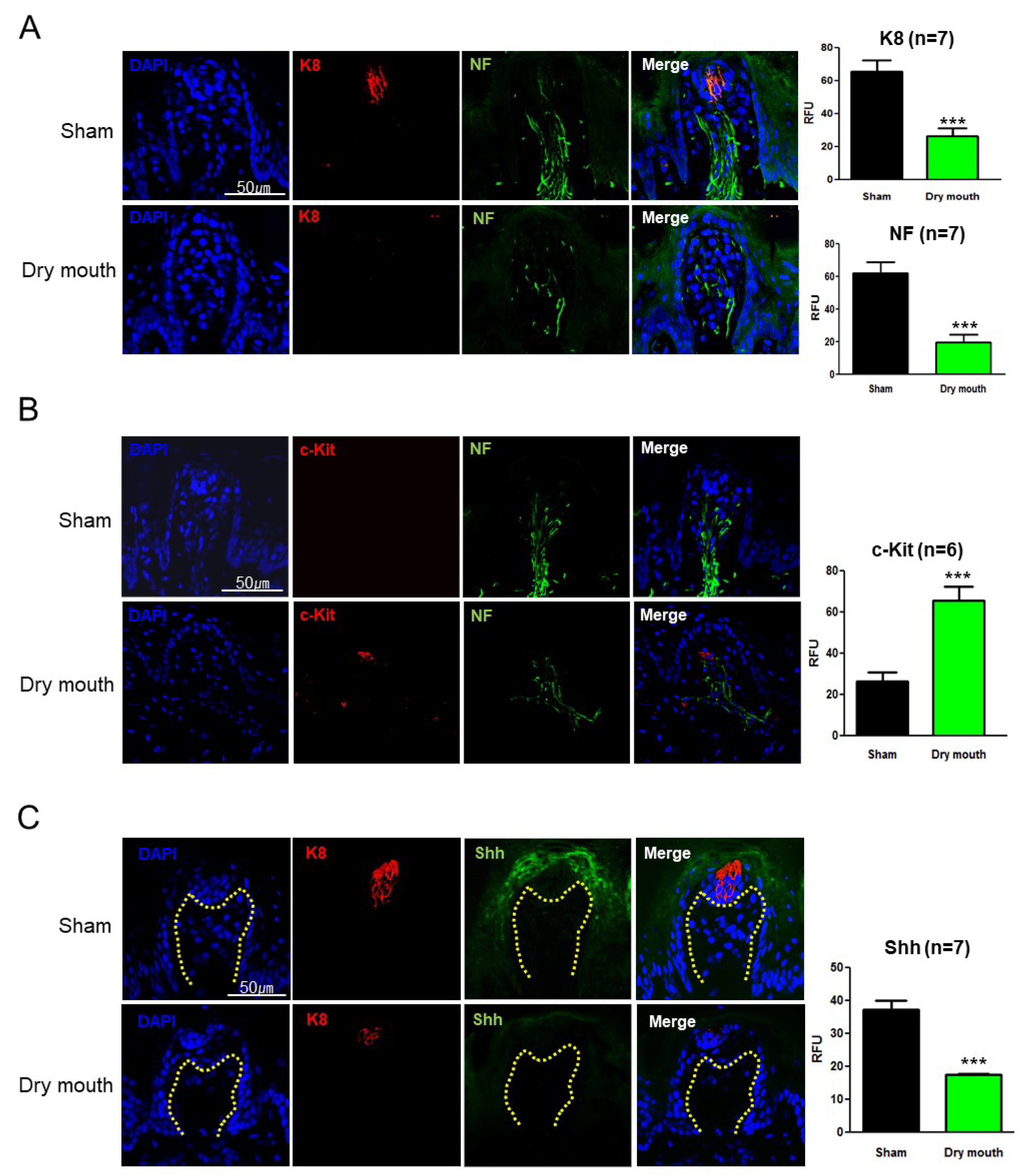
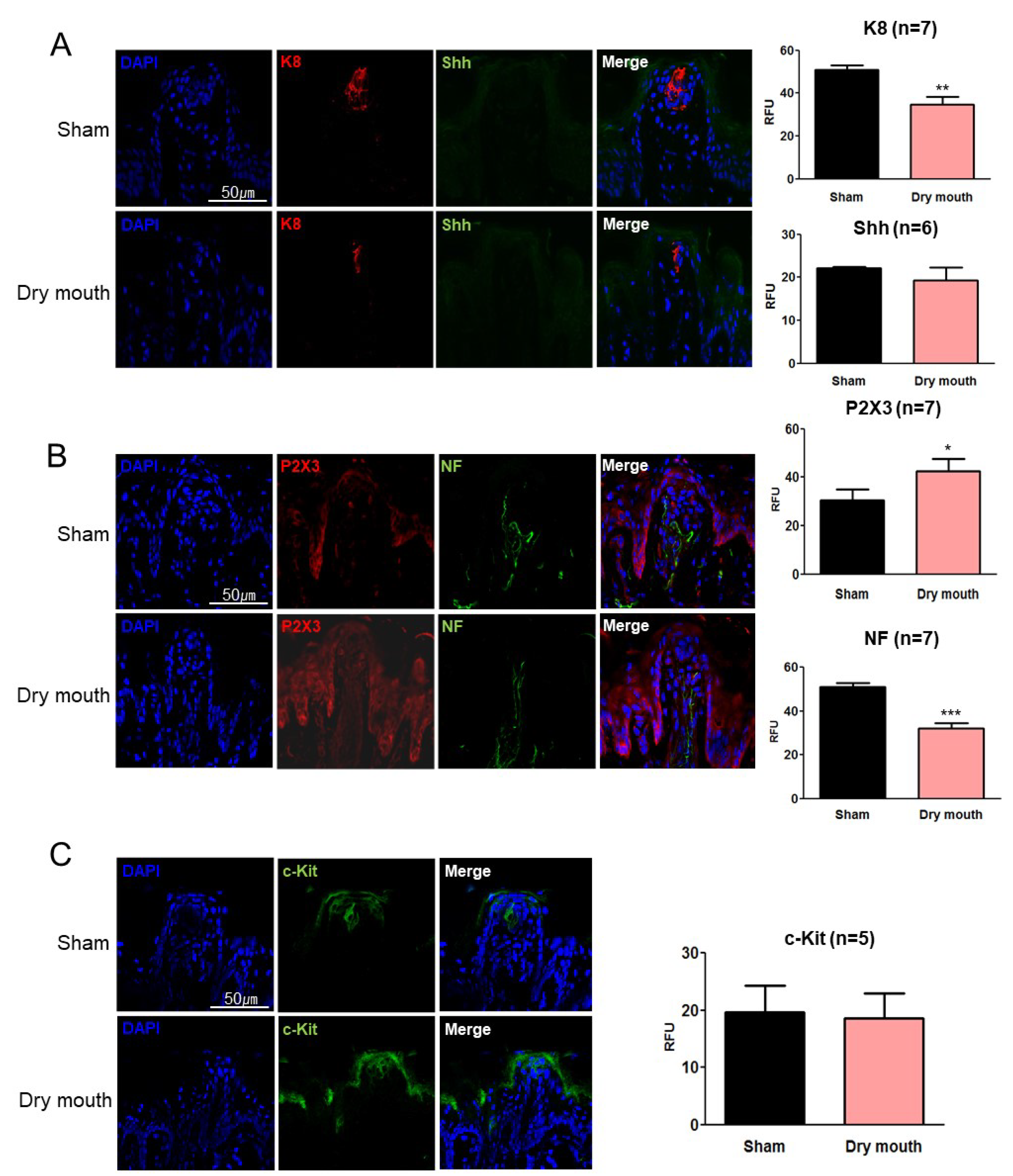
3.3. Dry Mouth-Altered Keratin and NF Expression
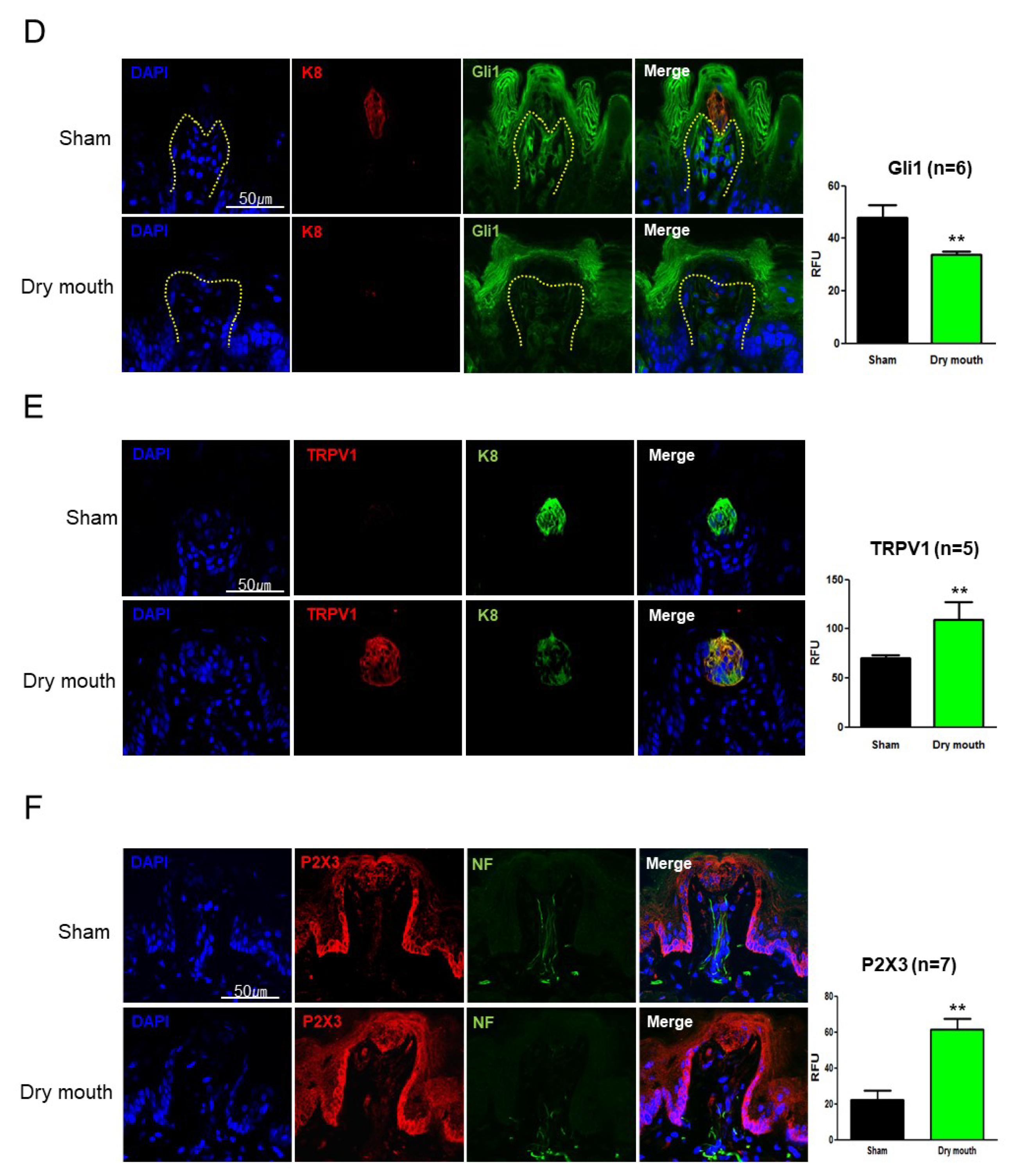
3.4. Dry Mouth-Induced NF Retraction Downregulated Shh and Gli1
3.5. TRPV1 Contributed to Taste Bud Development
3.6. TRPV1 Affected Shh and c-Kit Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Barlow, L.A. Progress and renewal in gustation: New insights into taste bud development. Development 2015, 142, 3620–3629. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.K.; Cong, W.N.; Cai, H.; Kim, W.; Maudsley, S.; Egan, J.M.; Martin, B. Age-related changes in mouse taste bud morphology, hormone expression, and taste responsivity. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Kumarhia, D.; He, L.; McCluskey, L.P. Inflammatory stimuli acutely modulate peripheral taste function. J. Neurophysiol. 2016, 115, 2964–2975. [Google Scholar] [CrossRef]
- Streelman, J.T.; Bloomquist, R.F.; Fowler, T.E. Developmental Plasticity of Patterned and Regenerating Oral Organs. Curr. Top. Dev. Biol. 2015, 115, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Takeda, N.; Jain, R.; Li, D.; Li, L.; Lu, M.M.; Epstein, J.A. Lgr5 Identifies Progenitor Cells Capable of Taste Bud Regeneration after Injury. PLoS ONE 2013, 8, e66314. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.K.; Atwal, K.; Bakaj, I.; Carlucci-Derbyshire, S.; Buber, M.T.; Cerne, R.; Cortes, R.Y.; Devantier, H.R.; Jorgensen, V.; Pawlyk, A.; et al. Triphenylphosphine oxide is a potent and selective inhibitor of the transient receptor potential melastatin-5 ion channel. Assay Drug Dev. Technol. 2010, 8, 703–713. [Google Scholar] [CrossRef]
- Henkin, R.I.; Knoppel, A.B.; Abdelmeguid, M.; Stateman, W.A.; Hosein, S. Theophylline increases saliva sonic hedgehog and improves taste dysfunction. Arch. Oral Biol. 2017, 82, 263–270. [Google Scholar] [CrossRef]
- Haas, A.J.; Le Page, Y.; Zhadobov, M.; Boriskin, A.; Sauleau, R.; Le Drean, Y. Impact of 60-GHz millimeter waves on stress and pain-related protein expression in differentiating neuron-like cells. Bioelectromagnetics 2016, 37, 444–454. [Google Scholar] [CrossRef]
- Houghton, J.W.; Hans, J.; Pesaro, M.; Ley, J.P.; Carpenter, G.H.; Proctor, G. Sensory effects of transient receptor potential channel agonists on whole mouth saliva extensional rheology. J. Texture Stud. 2017, 48, 313–317. [Google Scholar] [CrossRef]
- El Andaloussi-Lilja, J.; Lundqvist, J.; Forsby, A. TRPV1 expression and activity during retinoic acid-induced neuronal differentiation. Neurochem. Int. 2009, 55, 768–774. [Google Scholar] [CrossRef]
- Lu, W.J.; Mann, R.K.; Nguyen, A.; Bi, T.; Silverstein, M.; Tang, J.Y.; Chen, X.; Beachy, P.A. Neuronal delivery of Hedgehog directs spatial patterning of taste organ regeneration. Proc. Natl. Acad. Sci. USA 2018, 115, E200–E209. [Google Scholar] [CrossRef]
- Mistretta, C.M.; Kumari, A. Tongue and Taste Organ Biology and Function: Homeostasis Maintained by Hedgehog Signaling. Annu. Rev. Physiol. 2017, 79, 335–356. [Google Scholar] [CrossRef]
- Miura, H.; Kusakabe, Y.; Harada, S. Cell lineage and differentiation in taste buds. Arch. Histol. Cytol. 2006, 69, 209–225. [Google Scholar] [CrossRef]
- Prochazkova, M.; Hakkinen, T.J.; Prochazka, J.; Spoutil, F.; Jheon, A.H.; Ahn, Y.; Krumlauf, R.; Jernvall, J.; Klein, O.D. FGF signaling refines Wnt gradients to regulate the patterning of taste papillae. Development 2017, 144, 2212–2221. [Google Scholar] [CrossRef]
- Che, H.; Xiao, G.S.; Sun, H.Y.; Wang, Y.; Li, G.R. Functional TRPV2 and TRPV4 channels in human cardiac c-kit(+) progenitor cells. J. Cell. Mol. Med. 2016, 20, 1118–1127. [Google Scholar] [CrossRef]
- Abashev, T.M.; Metzler, M.A.; Wright, D.M.; Sandell, L.L. Retinoic acid signaling regulates Krt5 and Krt14 independently of stem cell markers in submandibular salivary gland epithelium. Dev. Dyn. 2017, 246, 135–147. [Google Scholar] [CrossRef]
- Qin, Y.; Sukumaran, S.K.; Jyotaki, M.; Redding, K.; Jiang, P.; Margolskee, R.F. Gli3 is a negative regulator of Tas1r3-expressing taste cells. PLoS Genet. 2018, 14, e1007058. [Google Scholar] [CrossRef]
- Kumari, A.; Ermilov, A.N.; Allen, B.L.; Bradley, R.M.; Dlugosz, A.A.; Mistretta, C.M. Hedgehog pathway blockade with the cancer drug LDE225 disrupts taste organs and taste sensation. J. Neurophysiol. 2015, 113, 1034–1040. [Google Scholar] [CrossRef]
- Miura, H.; Kusakabe, Y.; Sugiyama, C.; Kawamatsu, M.; Ninomiya, Y.; Motoyama, J.; Hino, A. Shh and Ptc are associated with taste bud maintenance in the adult mouse. Mech. Dev. 2001, 106, 143–145. [Google Scholar] [CrossRef]
- Miura, H.; Scott, J.K.; Harada, S.; Barlow, L.A. Sonic hedgehog-expressing basal cells are general post-mitotic precursors of functional taste receptor cells. Dev. Dyn. 2014, 243, 1286–1297. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ong, H.L.; Ambudkar, I. TRP Channel Involvement in Salivary Glands-Some Good, Some Bad. Cells 2018, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Shuba, Y.M. Beyond Neuronal Heat Sensing: Diversity of TRPV1 Heat-Capsaicin Receptor-Channel Functions. Front. Cell. Neurosci. 2020, 14, 612480. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, R.; Sato, T.; Yajima, T.; Kano, M.; Suzuki, T.; Ichikawa, H. The distribution of TRPV1 and TRPV2 in the rat pharynx. Cell. Mol. Neurobiol. 2013, 33, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.Z.; Ando, H.; Unno, S.; Masuda, Y.; Kitagawa, J. Activation of TRPV1 and TRPM8 Channels in the Larynx and Associated Laryngopharyngeal Regions Facilitates the Swallowing Reflex. Int. J. Mol. Sci. 2018, 19, 4113. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Zimmermann, F. Tribromoethanol (Avertin) as an anaesthetic in mice. Lab. Anim. 1999, 33, 192–193. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.H.; Lee, W.M.; Yun, S.H. Intravital Microscopic Interrogation of Peripheral Taste Sensation. Sci. Rep. 2015, 5, 1–6. [Google Scholar] [CrossRef]
- Grienberger, C.; Konnerth, A. Imaging calcium in neurons. Neuron 2012, 73, 862–885. [Google Scholar] [CrossRef]
- Hai, B.; Qin, L.; Yang, Z.; Zhao, Q.; Shangguan, L.; Ti, X.; Zhao, Y.; Kim, S.; Rangaraj, D.; Liu, F. Transient activation of hedgehog pathway rescued irradiation-induced hyposalivation by preserving salivary stem/progenitor cells and parasympathetic innervation. Clin. Cancer Res. 2014, 20, 140–150. [Google Scholar] [CrossRef]
- Lee, M.J.; Kim, J.Y.; Lee, S.I.; Sasaki, H.; Lunny, D.P.; Lane, E.B.; Jung, H.S. Association of Shh and Ptc with keratin localization in the initiation of the formation of circumvallate papilla and von Ebner’s gland. Cell Tissue Res. 2006, 325, 253–261. [Google Scholar] [CrossRef]
- Mistretta, C.M.; Kumari, A. Hedgehog Signaling Regulates Taste Organs and Oral Sensation: Distinctive Roles in the Epithelium, Stroma, and Innervation. Int. J. Mol. Sci. 2019, 20, 1341. [Google Scholar] [CrossRef]
- Haara, O.; Fujimori, S.; Schmidt-Ullrich, R.; Hartmann, C.; Thesleff, I.; Mikkola, M.L. Ectodysplasin and Wnt pathways are required for salivary gland branching morphogenesis. Development 2011, 138, 2681–2691. [Google Scholar] [CrossRef]
- Hall, J.M.; Bell, M.L.; Finger, T.E. Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev. Biol. 2003, 255, 263–277. [Google Scholar] [CrossRef]
- Ermilov, A.N.; Kumari, A.; Li, L.; Joiner, A.M.; Grachtchouk, M.A.; Allen, B.L.; Dlugosz, A.A.; Mistretta, C.M. Maintenance of Taste Organs is Strictly Dependent on Epithelial Hedgehog/GLI Signaling. PLoS Genet. 2016, 12, e1006442. [Google Scholar] [CrossRef]
- Kist, R.; Watson, M.; Crosier, M.; Robinson, M.; Fuchs, J.; Reichelt, J.; Peters, H. The formation of endoderm-derived taste sensory organs requires a Pax9-dependent expansion of embryonic taste bud progenitor cells. PLoS Genet. 2014, 10, e1004709. [Google Scholar] [CrossRef]
- Henkin, R.I.; Knoppel, A.B.; Abdelmeguid, M.; Stateman, W.A.; Hosein, S. Sonic hedgehog is present in parotid saliva and is decreased in patients with taste dysfunction. J. Oral Pathol. Med. 2017, 46, 829–833. [Google Scholar] [CrossRef]
- Kurosaka, M.; Ogura, Y.; Funabashi, T.; Akema, T. Involvement of Transient Receptor Potential Cation Channel Vanilloid 1 (TRPV1) in Myoblast Fusion. J. Cell. Physiol. 2016, 231, 2275–2285. [Google Scholar] [CrossRef]
- Duo, L.; Wu, T.; Ke, Z.; Hu, L.; Wang, C.; Teng, G.; Zhang, W.; Wang, W.; Ge, Q.; Yang, Y.; et al. Gain of Function of Ion Channel TRPV1 Exacerbates Experimental Colitis by Promoting Dendritic Cell Activation. Mol. Ther. Nucleic Acids 2020, 22, 924–936. [Google Scholar] [CrossRef]
- Ramirez-Barrantes, R.; Marchant, I.; Olivero, P. TRPV1 may increase the effectiveness of estrogen therapy on neuroprotection and neuroregeneration. Neural Regen. Res. 2016, 11, 1204–1207. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ikeda, K.; Kawakami, K. Regulatory role of Six1 in the development of taste papillae. Cell Tissue Res. 2010, 339, 513–525. [Google Scholar] [CrossRef]
- Castillo-Azofeifa, D.; Losacco, J.T.; Salcedo, E.; Golden, E.J.; Finger, T.E.; Barlow, L.A. Sonic hedgehog from both nerves and epithelium is a key trophic factor for taste bud maintenance. Development 2017, 144, 3054–3065. [Google Scholar] [CrossRef]
- Wang, D.; Lu, J.; Xu, X.; Yuan, Y.; Zhang, Y.; Xu, J.; Chen, H.; Liu, J.; Shen, Y.; Zhang, H. Satellite Glial Cells Give Rise to Nociceptive Sensory Neurons. Stem Cell Rev. Rep. 2021, 17, 999–1013. [Google Scholar] [CrossRef] [PubMed]
- Sulk, M.; Seeliger, S.; Aubert, J.; Schwab, V.D.; Cevikbas, F.; Rivier, M.; Nowak, P.; Voegel, J.J.; Buddenkotte, J.; Steinhoff, M. Distribution and expression of non-neuronal transient receptor potential (TRPV) ion channels in rosacea. J. Investig. Dermatol. 2012, 132, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Sterle, I.; Zupancic, D.; Romih, R. Correlation between urothelial differentiation and sensory proteins P2X3, P2X5, TRPV1, and TRPV4 in normal urothelium and papillary carcinoma of human bladder. Biomed. Res. Int. 2014, 2014, 805236. [Google Scholar] [CrossRef] [PubMed]
- Stock, K.; Garthe, A.; de Almeida Sassi, F.; Glass, R.; Wolf, S.A.; Kettenmann, H. The capsaicin receptor TRPV1 as a novel modulator of neural precursor cell proliferation. Stem Cells 2014, 32, 3183–3195. [Google Scholar] [CrossRef]
- Miura, H.; Kato, H.; Kusakabe, Y.; Tagami, M.; Miura-Ohnuma, J.; Ookura, T.; Shindo, Y.; Ninomiya, Y.; Hino, A. Shh signaling and regulatory gene expression in mouse taste buds. Chem. Senses 2005, 30 (Suppl. 1), i50–i51. [Google Scholar] [CrossRef]
- Davies, A.N.; Thompson, J. Parasympathomimetic drugs for the treatment of salivary gland dysfunction due to radiotherapy. Cochrane Database Syst. Rev. 2015, 10, CD003782. [Google Scholar] [CrossRef]
- Amantini, C.; Farfariello, V.; Cardinali, C.; Morelli, M.B.; Marinelli, O.; Nabissi, M.; Santoni, M.; Bonfili, L.; Cecarini, V.; Eleuteri, A.M.; et al. The TRPV1 ion channel regulates thymocyte differentiation by modulating autophagy and proteasome activity. Oncotarget 2017, 8, 90766–90780. [Google Scholar] [CrossRef]
- Thirumangalathu, S.; Barlow, L.A. beta-Catenin signaling regulates temporally discrete phases of anterior taste bud development. Development 2015, 142, 4309–4317. [Google Scholar] [CrossRef]
- Liu, H.X.; Ermilov, A.; Grachtchouk, M.; Li, L.; Gumucio, D.L.; Dlugosz, A.A.; Mistretta, C.M. Multiple Shh signaling centers participate in fungiform papilla and taste bud formation and maintenance. Dev. Biol. 2013, 382, 82–97. [Google Scholar] [CrossRef]
- Kramer, N.; Chen, G.; Ishan, M.; Cui, X.; Liu, H.X. Early taste buds are from Shh(+) epithelial cells of tongue primordium in distinction from mature taste bud cells which arise from surrounding tissue compartments. Biochem. Biophys. Res. Commun. 2019, 515, 149–155. [Google Scholar] [CrossRef]
- Liu, H.X.; Grosse, A.S.; Iwatsuki, K.; Mishina, Y.; Gumucio, D.L.; Mistretta, C.M. Separate and distinctive roles for Wnt5a in tongue, lingual tissue and taste papilla development. Dev. Biol. 2012, 361, 39–56. [Google Scholar] [CrossRef]
- Gaillard, D.; Barlow, L.A. Taste bud cells of adult mice are responsive to Wnt/beta-catenin signaling: Implications for the renewal of mature taste cells. Genesis 2011, 49, 295–306. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhee, Y.-H.; Choi, Y.-H.; Hu, A.C.; Lee, M.Y.; Ahn, J.-C.; Kim, S.; Mo, J.-H.; Woo, S.H.; Chung, P.-S. Role of Transient Receptor Potential Vanilloid 1 in Sonic Hedgehog-Dependent Taste Bud Differentiation. Life 2023, 13, 75. https://doi.org/10.3390/life13010075
Rhee Y-H, Choi Y-H, Hu AC, Lee MY, Ahn J-C, Kim S, Mo J-H, Woo SH, Chung P-S. Role of Transient Receptor Potential Vanilloid 1 in Sonic Hedgehog-Dependent Taste Bud Differentiation. Life. 2023; 13(1):75. https://doi.org/10.3390/life13010075
Chicago/Turabian StyleRhee, Yun-Hee, Young-Hoon Choi, Allison C. Hu, Min Young Lee, Jin-Chul Ahn, Sehwan Kim, Ji-Hun Mo, Seung Hoon Woo, and Phil-Sang Chung. 2023. "Role of Transient Receptor Potential Vanilloid 1 in Sonic Hedgehog-Dependent Taste Bud Differentiation" Life 13, no. 1: 75. https://doi.org/10.3390/life13010075
APA StyleRhee, Y.-H., Choi, Y.-H., Hu, A. C., Lee, M. Y., Ahn, J.-C., Kim, S., Mo, J.-H., Woo, S. H., & Chung, P.-S. (2023). Role of Transient Receptor Potential Vanilloid 1 in Sonic Hedgehog-Dependent Taste Bud Differentiation. Life, 13(1), 75. https://doi.org/10.3390/life13010075





