The Neurohormonal Overactivity Syndrome in Heart Failure
Abstract
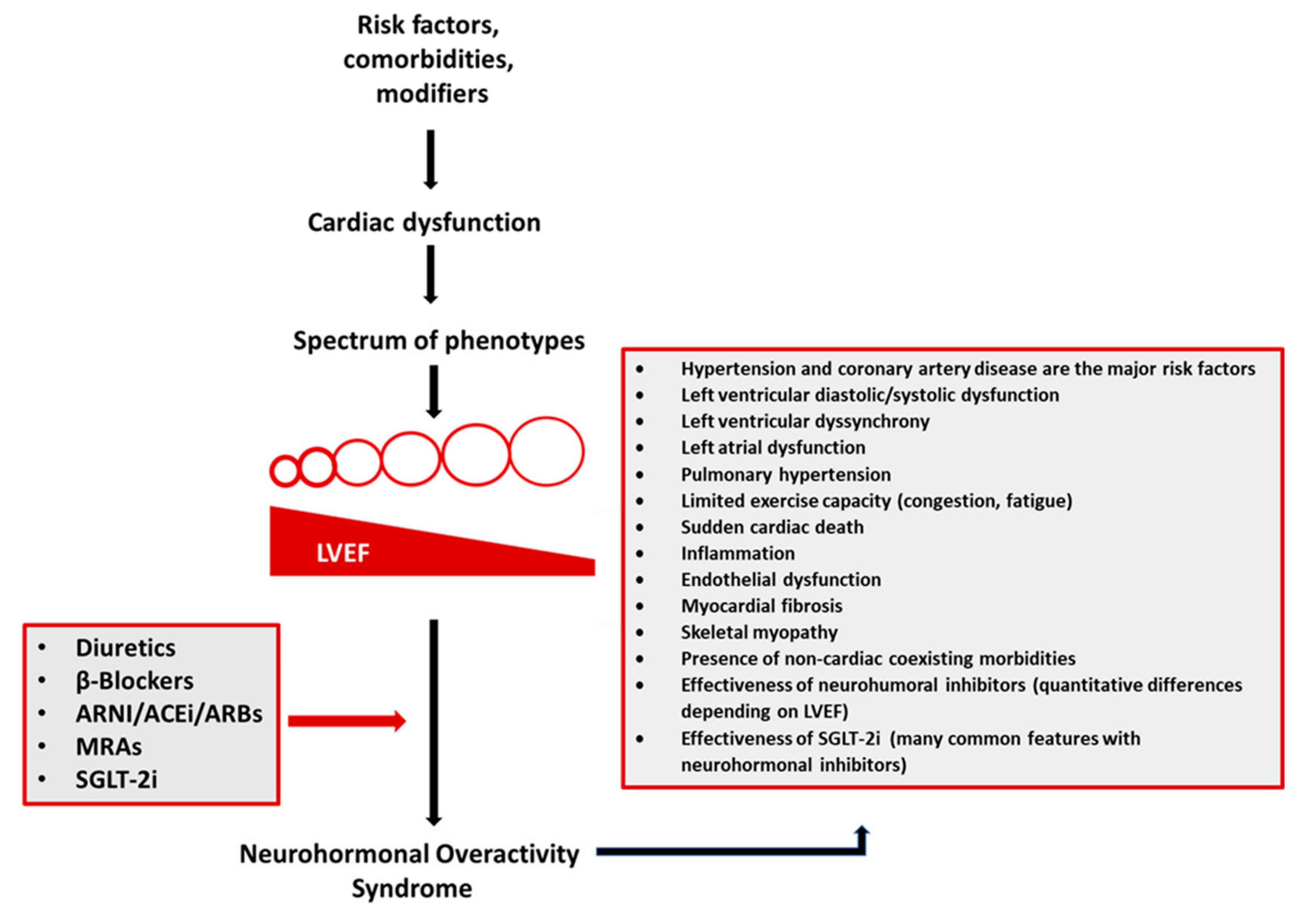
1. Introduction
2. Pathophysiology
3. Clinical Implications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Groenewegen, A.; Rutten, F.H.; Mosterd, A.; Hoes, A.W. Epidemiology of heart failure. Eur. J. Heart Fail. 2020, 22, 1342–1356. [Google Scholar] [CrossRef] [PubMed]
- Triposkiadis, F.; Butler, J.; Abboud, F.M.; Armstrong, P.W.; Adamopoulos, S.; Atherton, J.J.; Backs, J.; Bauersachs, J.; Burkhoff, D.; Bonow, R.O.; et al. The continuous heart failure spectrum: Moving beyond an ejection fraction classification. Eur. Heart J. 2019, 40, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- Triposkiadis, F.; Giamouzis, G.; Kitai, T.; Skoularigis, J.; Starling, R.C.; Xanthopoulos, A. A Holistic View of Advanced Heart Failure. Life 2022, 12, 1298. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Starling, R.C. Chronic Heart Failure: Diagnosis and Management beyond LVEF Classification. J. Clin. Med. 2022, 11, 1718. [Google Scholar] [CrossRef] [PubMed]
- Triposkiadis, F.; Xanthopoulos, A.; Starling, R.C. Medical Treatment of Heart Failure: Ignore the Ejection Fraction and Treat All? J. Card. Fail. 2021, 27, 907–909. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulos, A.; Giamouzis, G.; Skoularigis, J.; Triposkiadis, F. Heart failure with reduced, mildly reduced, or preserved left ventricular ejection fraction: Has reasoning been lost? World J. Cardiol. 2022, 14, 438–445. [Google Scholar] [CrossRef]
- Chioncel, O.; Lainscak, M.; Seferovic, P.M.; Anker, S.D.; Crespo-Leiro, M.G.; Harjola, V.-P.; Parissis, J.; Laroche, C.; Piepoli, M.F.; Fonseca, C.; et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: An analysis of the ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1574–1585. [Google Scholar] [CrossRef]
- Vergaro, G.; Aimo, A.; Prontera, C.; Ghionzoli, N.; Arzilli, C.; Zyw, L.; Taddei, C.; Gabutti, A.; Poletti, R.; Giannoni, A.; et al. Sympathetic and renin-angiotensin-aldosterone system activation in heart failure with preserved, mid-range and reduced ejection fraction. Int. J. Cardiol. 2019, 296, 91–97. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Giamouzis, G.; Parissis, J.; Starling, R.C.; Boudoulas, H.; Skoularigis, J.; Butler, J.; Filippatos, G. Reframing the association and significance of co-morbidities in heart failure. Eur. J. Heart Fail. 2016, 18, 744–758. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Xanthopoulos, A.; Butler, J. Cardiovascular Aging and Heart Failure: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 74, 804–813. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Guazzi, M.; Testani, J.M.; Borlaug, B.A. Altered Hemodynamics and End-Organ Damage in Heart Failure: Impact on the Lung and Kidney. Circulation 2020, 142, 998–1012. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; Melenovsky, V.; Redfield, M.M.; Nishimura, R.A.; Borlaug, B.A. High-Output Heart Failure: A 15-Year Experience. J. Am. Coll. Cardiol. 2016, 68, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Badrov, M.B.; Mak, S.; Floras, J.S. Cardiovascular Autonomic Disturbances in Heart Failure with Preserved Ejection Fraction. Can. J. Cardiol. 2021, 37, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Kaye, D.M.; Nanayakkara, S.; Wang, B.; Shihata, W.; Marques, F.Z.; Esler, M.; Lambert, G.; Mariani, J. Characterization of Cardiac Sympathetic Nervous System and Inflammatory Activation in HFpEF Patients. JACC Basic Transl. Sci. 2022, 7, 116–127. [Google Scholar] [CrossRef]
- Anand, I.S. High-Output Heart Failure Revisited. J. Am. Coll. Cardiol. 2016, 68, 483–486. [Google Scholar] [CrossRef]
- Adamo, L.; Rocha-Resende, C.; Prabhu, S.D.; Mann, D.L. Reappraising the role of inflammation in heart failure. Nat. Rev. Cardiol. 2020, 17, 269–285. [Google Scholar] [CrossRef]
- Paulus, W.J. Cytokines and heart failure. Heart Fail. Monit. 2000, 1, 50–56. [Google Scholar]
- Schwinger, R.H.G. Pathophysiology of heart failure. Cardiovasc. Diagn. Ther. 2021, 11, 263–276. [Google Scholar] [CrossRef]
- Miller, W.L. Fluid Volume Overload and Congestion in Heart Failure: Time to Reconsider Pathophysiology and How Volume Is Assessed. Circ. Heart Fail. 2016, 9, e002922. [Google Scholar] [CrossRef]
- Fudim, M.; Hernandez, A.F.; Felker, G.M. Role of Volume Redistribution in the Congestion of Heart Failure. J. Am. Heart Assoc. 2017, 6, e006817. [Google Scholar] [CrossRef]
- Kuwahara, K. The natriuretic peptide system in heart failure: Diagnostic and therapeutic implications. Pharmacol. Ther. 2021, 227, 107863. [Google Scholar] [CrossRef] [PubMed]
- Baba, M.; Yoshida, K.; Ieda, M. Clinical Applications of Natriuretic Peptides in Heart Failure and Atrial Fibrillation. Int. J. Mol. Sci. 2019, 20, 2824. [Google Scholar] [CrossRef] [PubMed]
- Diez, J. Chronic heart failure as a state of reduced effectiveness of the natriuretic peptide system: Implications for therapy. Eur. J. Heart Fail. 2017, 19, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Havakuk, O.; King, K.S.; Grazette, L.; Yoon, A.J.; Fong, M.; Bregman, N.; Elkayam, U.; Kloner, R.A. Heart Failure-Induced Brain Injury. J. Am. Coll. Cardiol. 2017, 69, 1609–1616. [Google Scholar] [CrossRef]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement from the American Heart Association. Circulation 2019, 139, e840–e878. [Google Scholar] [CrossRef]
- Xanthopoulos, A.; Starling, R.C.; Kitai, T.; Triposkiadis, F. Heart Failure and Liver Disease: Cardiohepatic Interactions. JACC Heart Fail. 2019, 7, 87–97. [Google Scholar] [CrossRef]
- Cho, J.H. Sudden Death and Ventricular Arrhythmias in Heart Failure with Preserved Ejection Fraction. Korean Circ. J. 2022, 52, 251–264. [Google Scholar] [CrossRef]
- Huang, W.A.; Boyle, N.G.; Vaseghi, M. Cardiac Innervation and the Autonomic Nervous System in Sudden Cardiac Death. Card. Electrophysiol. Clin. 2017, 9, 665–679. [Google Scholar] [CrossRef]
- Yang, K.C.; Kyle, J.W.; Makielski, J.C.; Dudley, S.C., Jr. Mechanisms of sudden cardiac death: Oxidants and metabolism. Circ. Res. 2015, 116, 1937–1955. [Google Scholar] [CrossRef]
- Shen, L.; Jhund, P.S.; Petrie, M.C.; Claggett, B.L.; Barlera, S.; Cleland, J.G.; Dargie, H.J.; Granger, C.B.; Kjekshus, J.; Køber, L.; et al. Declining Risk of Sudden Death in Heart Failure. N. Engl. J. Med. 2017, 377, 41–51. [Google Scholar] [CrossRef]
- Li, H.L.; Lip, G.Y.H.; Feng, Q.; Fei, Y.; Tse, Y.K.; Wu, M.Z.; Ren, Q.W.; Tse, H.F.; Cheung, B.Y.; You, K.H. Sodium-glucose cotransporter 2 inhibitors (SGLT2i) and cardiac arrhythmias: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2021, 20, 100. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.C.; Fernandes, A.; Cardoso, R.; Penalver, J.; Knijnik, L.; Mitrani, R.D.; Myerburg, R.J.; Goldberger, J.J. Association of SGLT2 inhibitors with arrhythmias and sudden cardiac death in patients with type 2 diabetes or heart failure: A meta-analysis of 34 randomized controlled trials. Heart Rhythm. 2021, 18, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Sfairopoulos, D.; Zhang, N.; Wang, Y.; Chen, Z.; Letsas, K.P.; Tse, G.; Li, G.; Lip, G.Y.H.; Liu, T.; Korantzopoulos, P. Association between sodium-glucose cotransporter-2 inhibitors and risk of sudden cardiac death or ventricular arrhythmias: A meta-analysis of randomized controlled trials. Europace 2022, 24, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zheng, H.; Guo, Z. Effect of Sodium-Glucose Co-transporter Protein 2 Inhibitors on Arrhythmia in Heart Failure Patients with or without Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Front. Cardiovasc. Med. 2022, 9, 902923. [Google Scholar] [CrossRef] [PubMed]
- Scheen, A.J. Dissecting the reduction in cardiovascular death with SGLT2 inhibitors: Potential contribution of effects on ventricular arrhythmias and sudden cardiac death? Diabetes Epidemiol. Manag. 2022, 8, 100107. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, 1757–1780. [Google Scholar] [CrossRef]
- Pfeffer, M.A.; Claggett, B.; Assmann, S.F.; Boineau, R.; Anand, I.S.; Clausell, N.; Desai, A.S.; Diaz, R.; Fleg, J.L.; Gordeev, I.; et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial. Circulation 2015, 131, 34–42. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Anand, I.S.; Ge, J.; Lam, C.S.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; Pfeffer, M.A.; Pieske, B.; et al. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019, 381, 1609–1620. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Leoncini, G.; Russo, E.; Bussalino, E.; Barnini, C.; Viazzi, F.; Pontremoli, R. SGLT2is and Renal Protection: From Biological Mechanisms to Real-World Clinical Benefits. Int. J. Mol. Sci. 2021, 22, 4441. [Google Scholar] [CrossRef]
- Packer, M. Molecular, Cellular, and Clinical Evidence That Sodium-Glucose Cotransporter 2 Inhibitors Act as Neurohormonal Antagonists When Used for the Treatment of Chronic Heart Failure. J. Am. Heart Assoc. 2020, 9, e016270. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Critical Reanalysis of the Mechanisms Underlying the Cardiorenal Benefits of SGLT2 Inhibitors and Reaffirmation of the Nutrient Deprivation Signaling/Autophagy Hypothesis. Circulation 2022, 146, 1383–1405. [Google Scholar] [CrossRef] [PubMed]
- Verdonschot, J.A.J.; Merlo, M.; Dominguez, F.; Wang, P.; Henkens, M.T.H.M.; Adriaens, M.E.; Hazebroek, M.R.; Masè, M.; Escobar, L.E.; Cobas-Paz, R.; et al. Phenotypic clustering of dilated cardiomyopathy patients highlights important pathophysiological differences. Eur. Heart J. 2021, 42, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Karwath, A.; Bunting, K.V.; Gill, S.K.; Tica, O.; Pendleton, S.; Aziz, F.; Barsky, A.D.; Chernbumroong, S.; Duan, J.; Mobley, A.R.; et al. Redefining beta-blocker response in heart failure patients with sinus rhythm and atrial fibrillation: A machine learning cluster analysis. Lancet 2021, 398, 1427–1435. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xanthopoulos, A.; Skoularigis, J.; Triposkiadis, F. The Neurohormonal Overactivity Syndrome in Heart Failure. Life 2023, 13, 250. https://doi.org/10.3390/life13010250
Xanthopoulos A, Skoularigis J, Triposkiadis F. The Neurohormonal Overactivity Syndrome in Heart Failure. Life. 2023; 13(1):250. https://doi.org/10.3390/life13010250
Chicago/Turabian StyleXanthopoulos, Andrew, John Skoularigis, and Filippos Triposkiadis. 2023. "The Neurohormonal Overactivity Syndrome in Heart Failure" Life 13, no. 1: 250. https://doi.org/10.3390/life13010250
APA StyleXanthopoulos, A., Skoularigis, J., & Triposkiadis, F. (2023). The Neurohormonal Overactivity Syndrome in Heart Failure. Life, 13(1), 250. https://doi.org/10.3390/life13010250





