Abstract
Soybean cyst nematode Heterodera glycines (SCN) is a major threat to global soybean production. Effective management of this disease is dependent on the development of resistant cultivars. Two SCN HG Types, 7 and 1.3.4.7. were previously identified as prevalent H. glycines populations in Northeast China. In order to evaluate soybean cultivars resistant to local SCN populations, 110 domestic commercial soybeans from different regions of Northeast China were assessed in the greenhouse to determine their potential as novel sources of resistance. The results suggested that cultivars responded differently to the two HG types. Of the 110 soybean cultivars evaluated, 24 accessions were classified as resistant or moderately resistant to HG Type 7, and five cultivars were classified as resistant or moderately resistant to HG Type 1.3.4.7. Among the tested cultivars, Kangxian 12 and Qingdou 13 had resistance response to both HG types 7 and 1.3.4.7. Thus, these broad-based SCN cultivars will be the valuable materials in the SCN resistance breeding program.
1. Introduction
Soybean cyst nematode continues to be the most crucial thread to soybean (Glycine max (L.) Merr.) production worldwide. In 1899, China reported the first occurrence of “fire-burned seedlings” caused by SCN in western Heilongjiang Province [1]. It was classified as Heterodera glycines by Ichinohe until 1952 [2]. Nowadays, it spread to most soybean planting-areas around 22 provinces of China [3,4,5,6], which caused an annual yield loss of more than 120 million dollars [7].
SCN has been managed through rotation with nonhost crops, SCN-resistant soybean cultivars, bio-control and nematicide applications, and so on [8,9]. Currently, genetic resistance is the most economical, effective, and environmentally sustainable management means to control this nematode [10,11]. For soybean growers, the development of SCN resistant cultivars has been a significant achievement. Pickett is the first SCN-resistant cultivar through three backcrosses between Peking and the susceptible cultivar Lee [12]. Usually, the yield of resistant cultivars was substantially higher than that of susceptible cultivars in fields with SCN infestation [13,14].
Heilongjiang Province is the major soybean producing region in China. The northern part of this province is the largest planting area of soybeans. More than one-third of total soybean production in China is located in this area, where SCN caused significant yield reductions in soybean producing regions [15]. Eleven H. glycines (HG) Types 0, 1.2.3.5.7, 1.2.3.7, 1.3.4.7, 1.3.7, 2, 2.5.7, 2.7, 6, 6.7, and 7, were reported in Heilongjiang province [16]. However, a big challenge for the management of SCN in the field is the presence of multiple SCN HG types [17]. In northeastern China, the majority of the resistant cultivars are derived from Peking via cultivar Franklin and/or its derived cultivars, whereas long-term, ongoing use of the same resistant source (Peking) caused the adaption of SCN populations in Heilongjiang [18].
Currently, HG type 7 is the most common SCN populations in Heilongjiang [19]. The predominant SCN virulence type of Jilin Province in Northeast China was also HG type 7 [20], while significant efforts have been made in Northeast China to develop soybean cultivars resistant to SCN HG type 0 [21]. In recent years, there has been an increase of SCN virulence, and a virulence shift of SCN populations has been reported in northeastern China [18,22]. HG type 1.3.4.7 were previously identified under continuous cropping in the Anda area of the Suihua region in Heilongjiang Province [16]. In addition, it is unclear which soybean cultivars are resistant to HG type 1.3.4.7. Once HG type 1.3.4.7 becomes a popular virulence phenotype, it will be a serious threat to soybean production. Although the reaction of soybean genotypes to SCN were reported in Northeast China [23], it was more important to test the response of soybean cultivar to the main or likely to be prevalent virulence phenotypes of SCN.
For the commercial soybean cultivars, no information is available on their effectiveness against the common HG type 7 and HG type 1.3.4.7. The objective of this research was to evaluate soybean cultivars for reaction to two nematode populations HG types 7 and 1.3.4.7 that are currently more prevalent or likely to be prevalent in the Heilongjiang than other populations to SCN.
2. Materials and Methods
2.1. Plant Materials
First, 110 domestic soybean cultivars including 25 SCN resistant soybean cultivars, 85 local high-yielding cultivars from Northeast China were evaluated for SCN resistance in the greenhouse. The 25 SCN resistant cultivars had SCN resistance to HG type 0. In addition, in these 25 SCN resistant soybean cultivars, 3 cultivars are from Jilin, and other 22 cultivars are all from Heilongjiang. Eighty-five representative commercial cultivars are from different ecological regions, such as a series of ‘Hefeng’, ‘Suinong’, ‘Dongnong’, ‘Heihe’, ‘Jiyu’, ‘Tiedou’, etc. (Table S1) These cultivars were obtained from Soybean Research Institute, Daqing Branch of the Heilongjiang Academy of Agricultural Sciences. PI 548402, PI 88788, PI 90763, PI 437654, PI 209332, PI 89772, PI 548316, Pickett, and the susceptible standard check Lee74 were also added to the test in order to confirm the virulence phenotypes of SCN [24].
2.2. Nematode Populations
The SCN populations included two near-homogeneous inbred lines that corresponded to HG types 7 and 1.3.4.7. The soil samples of HG types 7 and 1.3.4.7 were collected from soybean fields in Daqing region. These nematode populations had been maintained by Nematology Institute of Northern China of Shenyang Agricultural University for over 30 generations [25]. According to the previous inoculation method, the SCN was used for the following inoculation assays with 2000 eggs of SCN per plant [26]. There were five plants in each repetition, and the experiment was repeated twice. All plants were grown in greenhouses at 26–28 °C with an 16 h light/8 h darkness light cycle.
For inoculation assay, two seeds of each indicator were planted in pots (3.8 cm diameter and 14 cm length) with sandy loam soil (50% sand). After germination, one seedling was kept in each pot. Five replicated seedlings were used. At the second true leaf stage, each plant was inoculated with a 5.0 mL mixture suspension containing 2000 eggs of SCN. The pots were then arranged in a completely random experimental design in a greenhouse at 28 °C for 16 h light each day and were watered regularly. The soybean plants and soil were taken from the pots after 35 days and soaked in water for at least 30 min. Females were extracted from the roots and collected using an 80-μm-pore sieve [19].
2.3. Statistics
The female index was calculated as follows: FI = (mean number of females on test soybean line per mean number of females on Lee74) × 100. SCN populations were confirmed by HG Types classification schemes based on avirulence (FI < 10) or virulence (FI > 10) response [24]. The reaction levels for female index (FI) were R, resistant = 0–9%, MR, moderately resistant = 10–30%, MS, moderately susceptible = 31–60%, S, susceptible = >60% [27]. All the lines were included for statistical analysis. Data from two tests for HG types 7 and 1.3.4.7 were combined for the analysis of variance of the FIs by the SPSS statistics 26.0 (SPSS, Chicago, IL, USA). Means were separated using Fisher’s LSD based on a significant F test.
3. Results
3.1. Response of Indicator Lines to SCN Populations HG Types 7 and 1.3.4.7
The first population had FI > 10 on PI 548316 was confirmed as HG type 7. The second population had FI > 10 on PI 548402, PI 90763, PI 437654, and PI548316 was classified as HG type 1.3.4.7. According to race test, the first population had FI < 10 on PI 548402, PI 88788, PI 90763, Pickett was classified as race 3. and the second population had FI > 10 on Pickett was classified as race 14 (Table 1).

Table 1.
Response of indicator lines to SCN populations HG Types 7 and 1.3.4.7.
3.2. Resistance Response of Soybean Cultivars to SCN Populations HG Type 7
Among 110 soybean cultivars, several cultivars with various levels of resistance to HG type 7 were shown in Table 1. There were five soybean cultivars displaying resistant (FI = 0–9%) to HG type 7, accounting for 4.55% of the total. Nineteen cultivars were moderately resistant (FI = 10–30%), accounting for 17.27% of the total. The number of HG type 7 with moderately susceptible (63) and susceptible (23) was 86 (Figure 1). Of these cultivars, the FI for the SCN populations ranged from 6.19 to 135.87. Five soybean cultivars from Heilongjiang, including Kangxian 2, Kangxian 5, Kangxian 7, Kangxian 12, and Qingdou 13 (also called Kangxian 13), showed resistance to SCN HG Type 7 (Table 2).
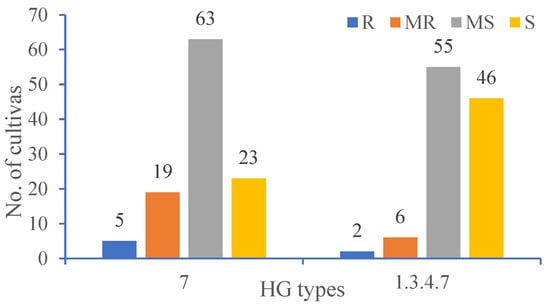
Figure 1.
Frequency of cultivars assessed as R, resistant = 0–9%, MR, moderately resistant = 10–30%, MS, moderately susceptible = 31–60%, S, susceptible = >60% against SCN HG types 7 and 1.3.4.7.

Table 2.
Reaction of soybean cultivars to SCN HG types 7 and 1.3.4.7.
3.3. Resistance Response of Soybean Cultivars to SCN Populations HG Type 1.3.4.7
Two cultivars (Kangxian 12 and Qingdou 13) were resistant to HG type 1.3.4.7 (Race 14), accounting for 1.83% of the total. There were six cultivars with moderate resistance, accounting for 5.50% of the total number. The rest of the cultivars were moderately susceptible and susceptible (Figure 1). Among these cultivars, a minimum FI of 11.41 was evaluated for Kangxian 12 and a maximum FI of 141.93 was evaluated for Hefeng 25.
Among the 110 soybean cultivars, eight cultivars were resistant or moderately resistance to HG type 1.3.4.7. Six soybean cultivars showed moderate resistance to SCN HG type 1.3.4.7, including Bainong 9, Fengdou 3, Kangxian 10, Kangxian 7, Kangxian 6, and Kangxian 5. Bainong 9 is from Jilin, the other cultivars are from Heilongjiang. Kangxian 12 and Qingdou 13 showed resistance not only to SCN HG type 7 but also to SCN HG type 1.3.4.7 (Table 2).
3.4. Agronomic Characters of SCN Resistant Cultivars
Twenty-five soybean cultivars moderately resistant to HG type 7 have yellow seed coats. With regard to seed hilum color, almost 3/4 of the cultivars (72%, 18 out of 25) had brown-hilum-pigmented, the rest having either yellow or black seed hilum color. Six high-oil soybean cultivars, Dongnong 43, Nengfeng 18, Nengfeng 19, Kangxian 6, Pengdou 158 and Qinong 2, had the average contents of fat (oil) in the seeds more than 22%. The content of fat and protein in the seeds is one of the SCN-resistance breeding objectives. These cultivars have favorable agronomic characteristics, making them suitable for use as donor parents in SCN resistance breeding programs [19]. Twenty-two of the cultivars were cataloged as MG 0, whereas MG I had 3 sources. The hundred-seed weight and desirable agronomic traits of these cultivars were listed in Table 3. Agronomic traits were adopted from Qiu et al. and Lai et al. [28,29,30].

Table 3.
Soybean cultivars with SCN resistance having desirable agronomic characteristics.
4. Discussion
This study evaluated the levels of resistance to two HG Types 7 and 1.3.4.7, in 110 commercial soybean cultivars from northeastern China. Kangxian 12 and Qingdou 13 showed resistance not only to SCN HG type 7 but also to SCN HG type 1.3.4.7. Kangxian 12 and Qingdou 13 had been reported to be resistant to HG type 2.5.7 and moderately resistant to both HG type 1.2.3.5.6.7, respectively [18]. Although some commercial cultivars were released as resistant to SCN type 0, the FI for these cultivars across the two HG types in our investigation was greater than 10% and 30%. Pickett was included in our study for the comparison of races between the two SCN populations so as to give soybean growers more information, although race and HG type belong to different classification systems.
In order to effectively manage SCN, it is crucial to understand the virulence phenotypes of SCN and resistant sources [31,32,33]. Soybean growers can select suitable resistant cultivars according to HG types and agronomic performance. In China, although a series of black-seed soybeans have multiple resistance to SCN, it is rarely used in commercial breeding programs due to lack of good agronomic traits [34]. SCN resistance was strongly associated with black seed coat. Soybean breeders need to make numerous backcrosses for improving undesirable traits related to the linkage of genetic background [17]. In this study, some cultivars which have yellow seed coat and good agronomic characteristics will be used in breeding programs as SCN resistance sources.
In previous studies, Kangxian 12 and Qingdou 13 were found to be resistant to HG types 2.5.7 and moderately resistant to HG type 1.2.3.5.6.7, respectively [18]. Here, we found that Kangxian 12 and Qingdou 13 has a broad spectrum resistance to SCN (HG Types 7, 1.2.5.7, HG types 2.5.7, and HG type 1.2.3.5.6.7). Furthermore, in order to create better soybean cultivars with resistance to SCN, the cultivar Kangxian 12 was used as a source of SCN resistance in soybean breeding programs. Some SCN resistant soybean cultivars, such as Nongqingdou 24 and Andou 162, were bred using Kangxian 12 as the male parent. Recently, a new cultivar ‘Heinong 531’ has been bred by means of systematic selection from the hybrids of Pengdou 158 × a male parental line F1 (Hefeng 55 × Kangxian 12) [21].
Kangxian12 were derived from the generations of the cross between Nongda 5129 and Heikang 002-24 which has SCN-resistance from Peking. Kangxian 12 carried resistant types of Forrest (rhg1-a GmSNAP18 and Rhg4 GmSHMT08) [21]. In fact, most of the SCN-resistant soybeans were almost Peking-type in Heilongjiang province. However, in the USA, only a few SCN-resistant varieties come from Peking (PI 548402) and PI 437654, and most of the resistant soybean cultivars have the resistance gene of PI88788 in their pedigree.
Planting single soybean cultivar for many years may lead to the loss of yield due to the adaptation of SCN [35]. Broad-spectrum SCN resistance may be increased by stacking numerous sources of resistance [36]. Some resistant cultivars (Pengdou 158 and Qingdou 13) contain a complex genetic background. In the early soybean genetic improvement, many Chinese black beans and other resistance gene resources were aggregated into SCN resistant cultivars [37]. These include Huipizhiheidou, Wuzhaiheidou, Yingxianheidou, PI 548402, PI 437654, and PI 548316. In addition, PI 90763 and PI 209332 can also be used as sources of H. glycines resistance.
Although some cultivar resources are highly resistant to SCN, the majority of the soybean cultivar is susceptible to SCN. PI 437654 was identified as resistant to all SCN virulence types. However, the isolate TN27 could reproduce on PI 437654 were reported in USA, and the new virulence type (X12) could reproduce on all the indicator lines of both race and HG type tests in China [38,39]. The soybean cultivar from Northeast China breeding program was resistant to HG 0, whereas field populations of SCN exhibit variability in their parasitism of soybean cultivars. Thus, resistant varieties must be matched to the virulence phenotype of SCN [33].
Phenotyping identification is important for complicating the selection of resistant lines or the evaluation of management strategies [40]. In order to make better use of soybean cultivars with SCN resistance, virulence phenotype need to be carefully monitored in a manner similar to what is recommended by the SCN Coalition (www.thescncoalition.com). PI 88788 and PI 548402 with different ways of controlling SCN infection were used as a SCN resistant cultivar for many years. Thus, rotating different derived cultivars can reduce SCN density for sustainable management [41,42,43]. However, the yield of H. glycines resistant cultivars (Heinong 531 and Qingdou 13) is still lower than the local cultivars, soybean cultivars with excellent agronomic traits, and H. glycines resistance are still recommended in SCN infected fields. The source of SCN resistance in cultivated soybean gene pool is limited [44]. It may be a new strategy to identify SCN resistant gene resources from wild soybean to develop new cultivars [45].
5. Conclusions
This research provided important information on the reaction of 110 soybean cultivars to SCN populations HG Types 7 and 1.3.4.7. Of the local cultivars evaluated, five accessions were classified as resistant or moderately resistant to HG Type 7 and also displayed resistance to HG Type 1.3.4.7. Broad-based SCN resistance cultivars, Kangxian 12 and Qingdou 13, which had the resistance to the main or likely to be prevalent virulence phenotypes of H. glycines, would be valuable materials and can be used directly in the SCN resistance breeding program. Kangxian 12 with favorable agronomic characteristics is a valuable genetic reservoir for SCN resistance of soybean improvement. Our results provide guidance for the implementation of the strategy of using resistant cultivars to control SCN. The soybean germplasm collection and identification of SCN resistance are of paramount importance and will undoubtedly contribute to the development of different source against SCN.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life13010248/s1. Table S1. List of soybean cultivars evaluated in previous study.
Author Contributions
Conceptualization, J.C. and L.C., methodology, J.C. and Y.H., validation, Y.Z., formal analysis, J.C., investigation, H.L., D.Z., C.Z. and M.S., resources, J.C., L.Z. and G.C., data curation, J.C. and Y.Z., writing—original draft preparation, J.C., Y.Z. and R.S., writing—review and editing, G.X. and Y.H., supervision, Y.H. and L.C., funding acquisition, J.C. and G.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Supported by the Science and Technology Research Program of Chongqing Municipal Education Commission (Grant Nos. KJQN 202201222 and 202101205), the Youth Innovation Promotion Association of CAS (No. 2020236), the Project of Chongqing Science and Technology Commission (cstc2021jcyj-msxmX0377), and the Modern Agricultural Industry Technology System of China (Grant No. CARS-04-CES-07).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, X.; Li, J.; Zhang, D. History and status of soybean cyst nematode in China. J. Nematol. 1997, 7, 18–25. [Google Scholar]
- Ichinohe, M. On the soybean nematode, Heterodera glycines n. sp., from Japan. Mag. Appl. Zool. 1952, 17, 1–4. [Google Scholar]
- Peng, D.; Jiang, R.; Peng, H.; Liu, S. Soybean cyst nematodes: A destructive threat to soybean production in China. Phytopathol. Res. 2021, 3, 1–16. [Google Scholar] [CrossRef]
- Peng, D.; Peng, H.; Wu, D.; Huang, W.; Ye, W.; Cui, J. First report of soybean cyst nematode (Heterodera glycines) on soybean from Gansu and Ningxia China. Plant Dis. 2016, 100, 229. [Google Scholar] [CrossRef]
- Wang, D.; Duan, Y.; Wang, Y.; Zhu, X.; Chen, L.; Liu, X.; Chen, J. First report of soybean cyst nematode, Heterodera glycines, on soybean from Guangxi, Guizhou, and Jiangxi provinces, China. Plant Dis. 2015, 99, 893. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, Y.; Li, X.; Zhao, L.; Chen, S. First report of the soybean cyst nematode, Heterodera glycines, on soybean in Zhejiang, eastern China. Plant Dis. 2009, 93, 319. [Google Scholar] [CrossRef]
- Ou, S.; Peng, D.; Liu, X.; Li, Y.; Moens, M.J.N. Identification of Heterodera glycines using PCR with sequence characterised amplified region (SCAR) primers. Nematology 2008, 10, 397–403. [Google Scholar]
- Lee, J.D.; Kim, H.J.; Robbins, R.T.; Wrather, J.A.; Bond, J.; Nguyen, H.T.; Shannon, J.G. Reaction of soybean cyst nematode resistant plant introductions to root-knot and reniform nematodes. Plant Breed. Biotechnol. 2015, 3, 346–354. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Zhu, X.; Liu, R.; Xiang, P.; Chen, J.; Liu, X.; Duan, Y.; Chen, L. Management of the soybean cyst nematode Heterodera glycines with combinations of different rhizobacterial strains on soybean. PLoS ONE 2017, 12, e0182654. [Google Scholar] [CrossRef]
- Jiao, Y.; Vuong, T.D.; Liu, Y.; Meinhardt, C.; Liu, Y.; Joshi, T.; Cregan, P.B.; Xu, D.; Shannon, J.G.; Nguyen, H.T. Identification and evaluation of quantitative trait loci underlying resistance to multiple HG types of soybean cyst nematode in soybean PI 437655. Theor. Appl. Genet. 2015, 128, 15–23. [Google Scholar] [CrossRef]
- Vuong, T.D.; Sonah, H.; Meinhardt, C.G.; Deshmukh, R.; Kadam, S.; Nelson, R.L.; Shannon, J.G.; Nguyen, H.T. Genetic architecture of cyst nematode resistance revealed by genome-wide association study in soybean. BMC Genom. 2015, 16, 593. [Google Scholar] [CrossRef] [PubMed]
- Brim, C.A.; Ross, J.P. Regitration of pickett soybeans. Crop Sci. 1966, 6, 305. [Google Scholar] [CrossRef]
- Chen, S.; Porter, P.; Orf, J.; Reese, C.; Stienstra, W.; Young, N.; Walgenbach, D.; Schaus, P.; Arlt, T.; Breitenbach, F.J.P.D. Soybean cyst nematode population development and associated soybean yields of resistant and susceptible cultivars in Minnesota. Plant Dis. 2001, 85, 760–766. [Google Scholar] [CrossRef] [PubMed]
- De Bruin, J.L.; Pedersen, P.J.A.J. Yield improvement and stability for soybean cultivars with resistance to Heterodera glycines Ichinohe. Agron. J. 2008, 100, 1354–1359. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Chen, J.; Yan, X.; Liu, X.; Duan, Y.; Zhu, X.; Chen, L. Incidence and disease index of soybean diseases in the northeast of China in 2015. Soybean Sci. 2016, 35, 643–648. [Google Scholar]
- Chen, J.; Li, X.; Li, Z.; Zhou, C.; Luo, X.; Wang, D.; Duan, Y.; Chen, L. Identification of the virulence type of soybean cyst nematode under continuous cropping in Daqing and Anda. Soybean Sci. 2015, 34, 675–678. [Google Scholar]
- Lian, Y.; Koch, G.; Bo, D.; Wang, J.; Nguyen, H.T.; Li, C.; Lu, W. The spatial distribution and genetic diversity of the soybean cyst nematode, Heterodera glycines, in China: It is time to take measures to control soybean cyst nematode. Front. Plant Sci. 2022, 13, 927773. [Google Scholar] [CrossRef]
- Hua, C.; Li, C.; Hu, Y.; Mao, Y.; You, J.; Wang, M.; Chen, J.; Tian, Z.; Wang, C. Identification of HG types of soybean cyst nematode Heterodera glycines and resistance screening on soybean genotypes in northeast China. J. Nematol. 2018, 50, 41–50. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Y.; Wang, Y.; Fan, H.; Liu, X.; Wang, D.; Zhao, D.; Duan, Y.; Zhu, X.; Chen, L. Characterization of virulence phenotypes of Heterodera glycines in Heilongjiang, Northeast China. Plant Dis. 2021, 105, 2056–2060. [Google Scholar] [CrossRef]
- Yan, X.; Liu, J.; Li, M.; Zhu, X.; Duan, Y. Distribution and virulence phenotypes of soybean cyst nematode (Heterodera glycines) based upon host differentials in Jilin province. Int. J. Agric. Biol. 2021, 25, 735–741. [Google Scholar] [CrossRef]
- Wang, J.; Kong, L.; Zhang, L.; Shi, X.; Yu, B.; Li, J.; Zhang, B.; Gao, M.; Liu, X.; Li, X.; et al. Breeding a soybean cultivar Heinong 531 with Peking-type cyst nematode resistance, enhanced yield and high seed-oil contents. Phytopathology 2022, 112, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Gao, G.; Zhou, C.; Du, Z.; Wu, Y.; Wang, M.; Yang, L.; Li, J. Study on the variation of soybean cyst nematode. Soybean Sci. 2007, 26, 290–292. [Google Scholar]
- Huang, M.; Qin, R.; Li, C.; Wang, M.; Jiang, Y.; Yu, J.; Chang, D.; Tian, Z.; Chen, Q.; Guo, X. Response of soybean genotypes from Northeast China to Heterodera glycines races 4 and 5, and characterisation of rhg1 and Rhg4 genes for soybean resistance. J. Nematol. 2021, 24, 333–345. [Google Scholar] [CrossRef]
- Niblack, T.; Arelli, P.; Noel, G.; Opperman, C.; Orf, J.; Schmitt, D.; Shannon, J.; Tylka, G. A revised classification scheme for genetically diverse populations of Heterodera glycines. J. Nematol. 2002, 34, 279–288. [Google Scholar] [PubMed]
- Arelli, P.R.; Sleper, D.A.; Yue, P.; Wilcox, J.A. Soybean reaction to races 1 and 2 of Heterodera glycines. Crop Sci. 2000, 40, 824–826. [Google Scholar] [CrossRef]
- Arelli, A.; Wilcox, J.; Myers, O., Jr.; Gibson, P.T. Soybean germplasm resistant to races 1 and 2 of Heterodera glycines. Crop Sci. 1997, 37, 1367–1369. [Google Scholar] [CrossRef]
- Schmitt, D.P.; Shannon, G. Differentiating soybean responses to Heterodera glycines races. Crop Sci. 1992, 32, 275–277. [Google Scholar] [CrossRef]
- Lai, Y.; Bi, Y. Heilongjiang Soybean Varieties and Key Parents in Recent Ten Years; Harbin Engineering University Press: Harbin, China, 2020. [Google Scholar]
- Qiu, L.; Wang, S. Chinese Soybean Varieties (1993–2004); China Agricultural Publishing House: Beijing, China, 2007. [Google Scholar]
- Qiu, L.; Wang, S. Chinese Soybean Varieties (2005–2014); China Agricultural Publishing Press: Beijing, China, 2018. [Google Scholar]
- Acharya, K.; Tande, C.; Byamukama, E. Determination of Heterodera glycines virulence phenotypes occurring in South Dakota. Plant Dis. 2016, 100, 2281–2286. [Google Scholar] [CrossRef]
- Chowdhury, I.A.; Yan, G.; Plaisance, A.; Markell, S. Characterization of virulence phenotypes of soybean cyst nematode (Heterodera glycines) populations in North Dakota. Phytopathology 2021, 111, 2100–2109. [Google Scholar] [CrossRef]
- Nissan, N.; Mimee, B.; Cober, E.R.; Golshani, A.; Smith, M.; Samanfar, B. A broad review of soybean research on the ongoing race to overcome soybean cyst nematode. Biology 2022, 11, 211. [Google Scholar] [CrossRef]
- Guo, W.; Chen, J.; Zhang, F.; Li, Z.; Chen, H.; Zhang, C.; Chen, L.; Yuan, S.; Li, R.; Cao, D.; et al. Characterization of Pingliang xiaoheidou (ZDD 11047), a soybean variety with resistance to soybean cyst nematode Heterodera glycines. Plant Mol. Biol. 2020, 103, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Wendimu, G.Y.; Iqbal, M. Cyst nematode (Heterodera glycines) problems in soybean (Glycine max L.) crops and its management. Adv. Agric. 2022, 2022, 7816951. [Google Scholar] [CrossRef]
- Shaibu, A.S.; Li, B.; Zhang, S.; Sun, J. Soybean cyst nematode-resistance: Gene identification and breeding strategies. Crop J. 2020, 8, 892–904. [Google Scholar] [CrossRef]
- Lin, F.; Chhapekar, S.S.; Vieira, C.C.; Da Silva, M.P.; Rojas, A.; Lee, D.; Liu, N.; Pardo, E.M.; Lee, Y.C.; Dong, Z.; et al. Breeding for disease resistance in soybean: A global perspective. Theor. Appl. Genet. 2022, 135, 3773–3872. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Guo, J.; Li, H.; Wu, Y.; Wei, H.; Wang, J.; Li, J.; Lu, W. A new race (X12) of soybean cyst nematode in China. J. Nematol. 2017, 49, 168–176. [Google Scholar] [CrossRef]
- Bekal, S.; Niblack, T.L.; Lambert, K.N. A chorismate mutase from the soybean cyst nematode Heterodera glycines shows polymorphisms that correlate with virulence. Mol. Plant-Microbe Interact. 2003, 16, 439–446. [Google Scholar] [CrossRef]
- Yan, G.; Baidoo, R. Current research status of Heterodera glycines resistance and its implication on soybean breeding. Engineering 2018, 4, 534–541. [Google Scholar] [CrossRef]
- Young, L.D. Influence of soybean cropping sequences on seed yield and female index of the soybean cyst nematode. Plant Dis. 1998, 82, 615–619. [Google Scholar] [CrossRef]
- Rocha, L.F.; Pimentel, M.F.; Bailey, J.; Wyciskalla, T.; Davidson, D.; Fakhoury, A.M.; Bond, J.P. Impact of wheat on soybean cyst nematode population density in double-cropping soybean production. Front. Plant Sci. 2021, 12, 640714. [Google Scholar] [CrossRef]
- Meinhardt, C.; Howland, A.; Ellersieck, M.; Scaboo, A.; Diers, B.; Mitchum, M.G. Resistance gene pyramiding and rotation to combat widespread soybean cyst nematode virulence. Plant Dis. 2021, 105, 3238–3243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Song, B.H. RNA-seq data comparisons of wild soybean genotypes in response to soybean cyst nematode (Heterodera glycines). Genom. Data 2017, 14, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Kofsky, J.; Zhang, H.; Song, B.H. Novel resistance strategies to soybean cyst nematode (SCN) in wild soybean. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).