An In Vitro Study of the Effect of CO2 Laser Power Output on Ablative Properties in Porcine Tongue
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Estimation and Allocation
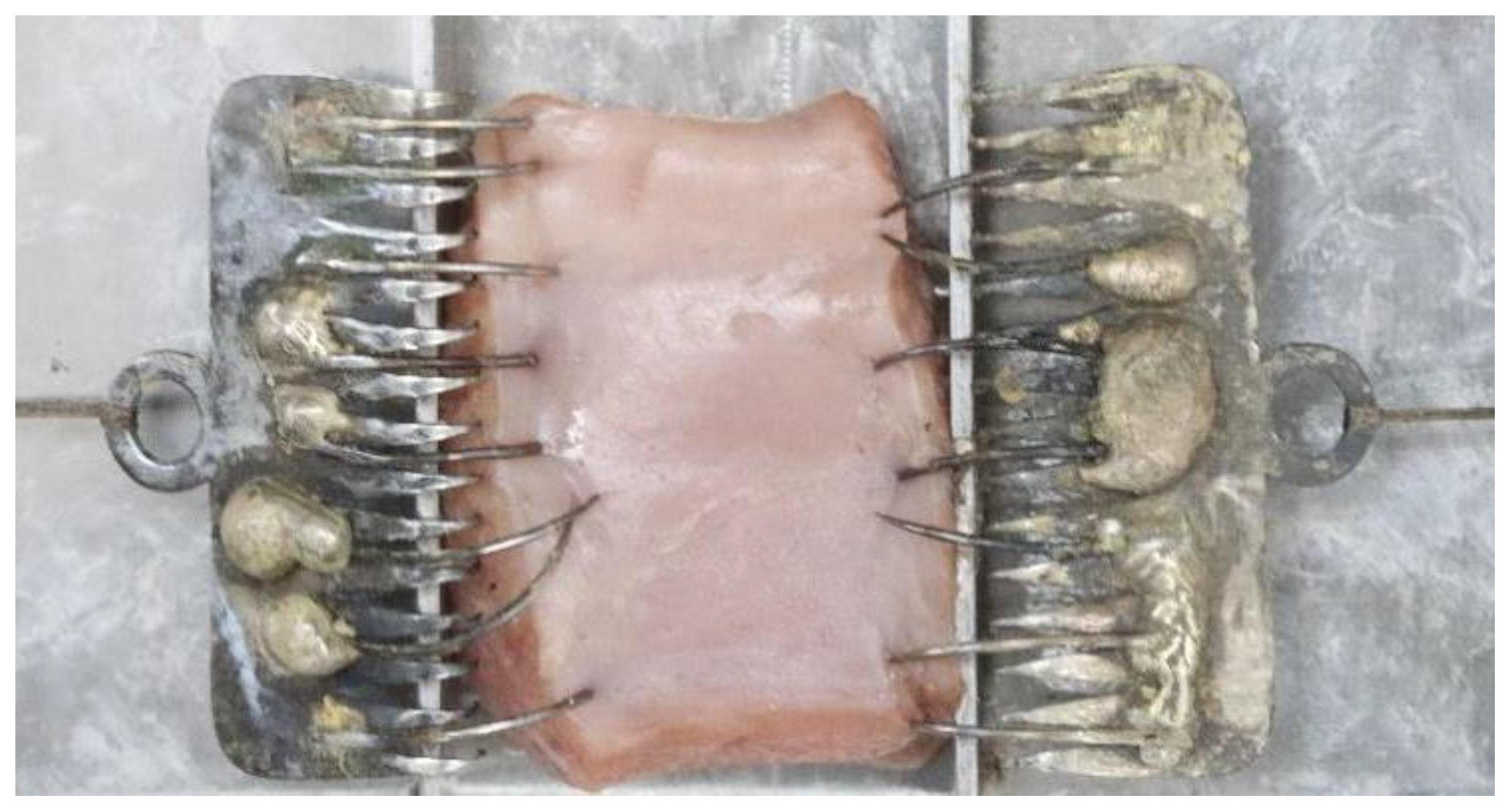
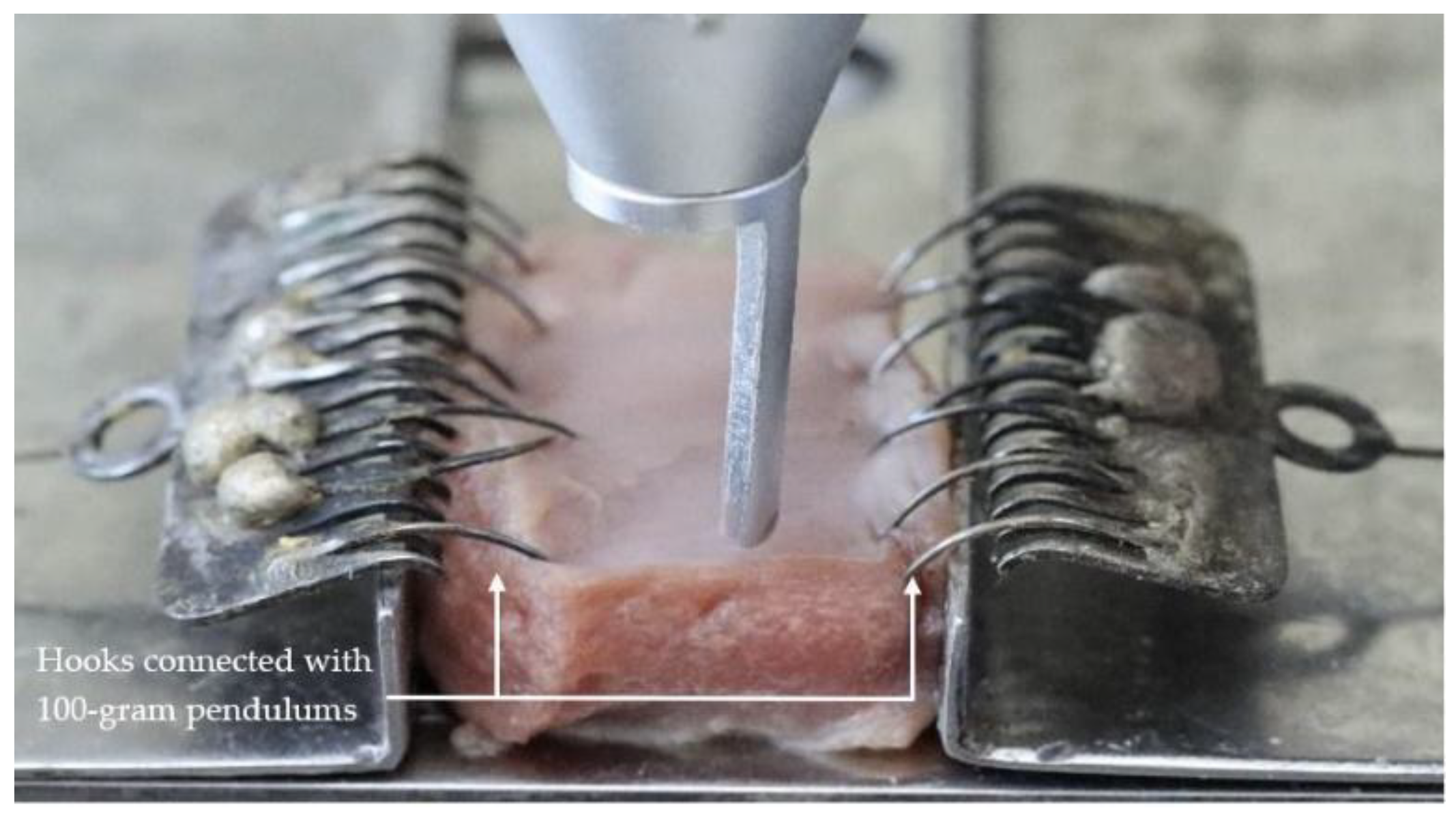
2.2. Sample Preparation
2.3. Laser Parameters
2.4. Method
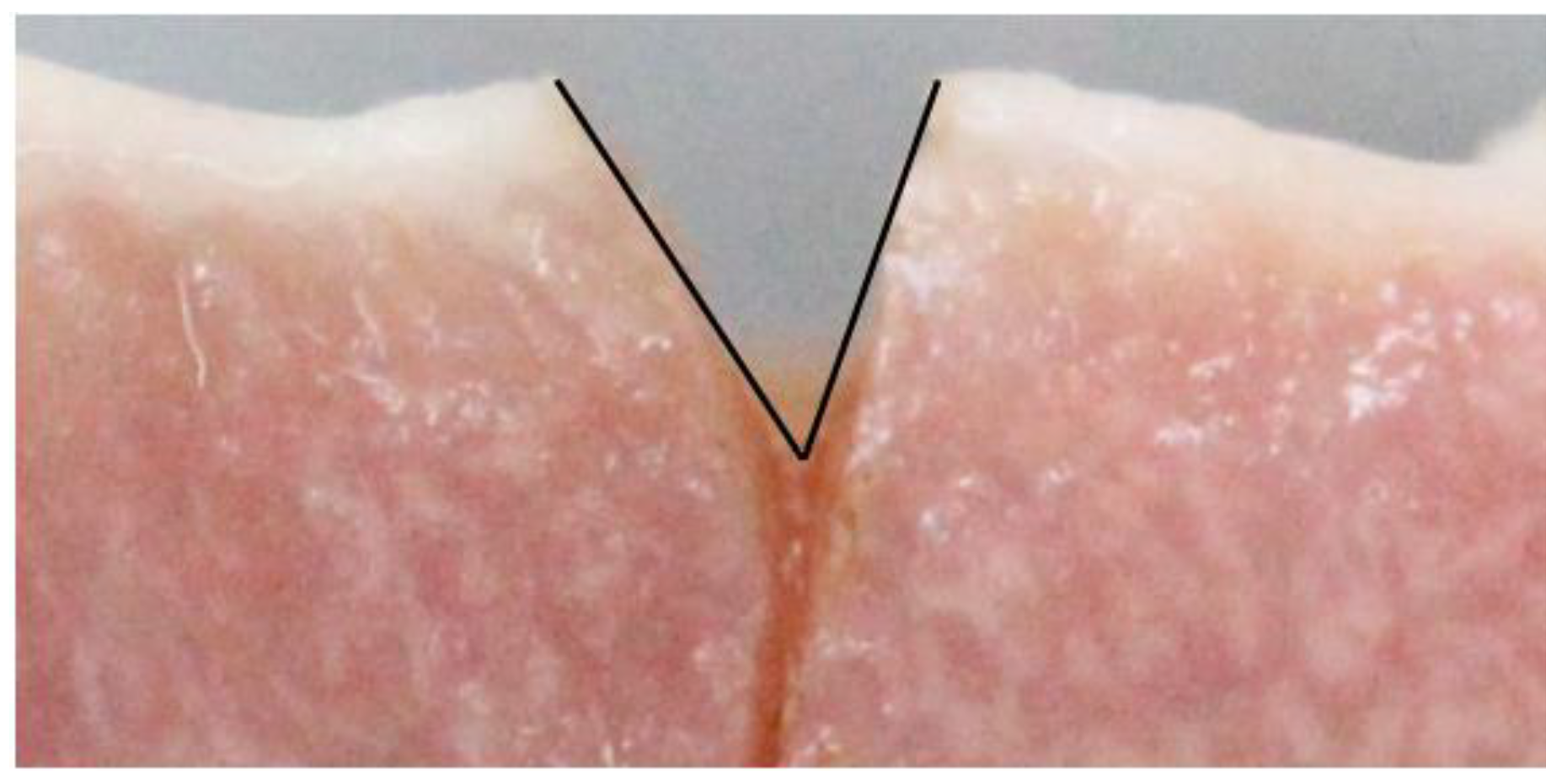
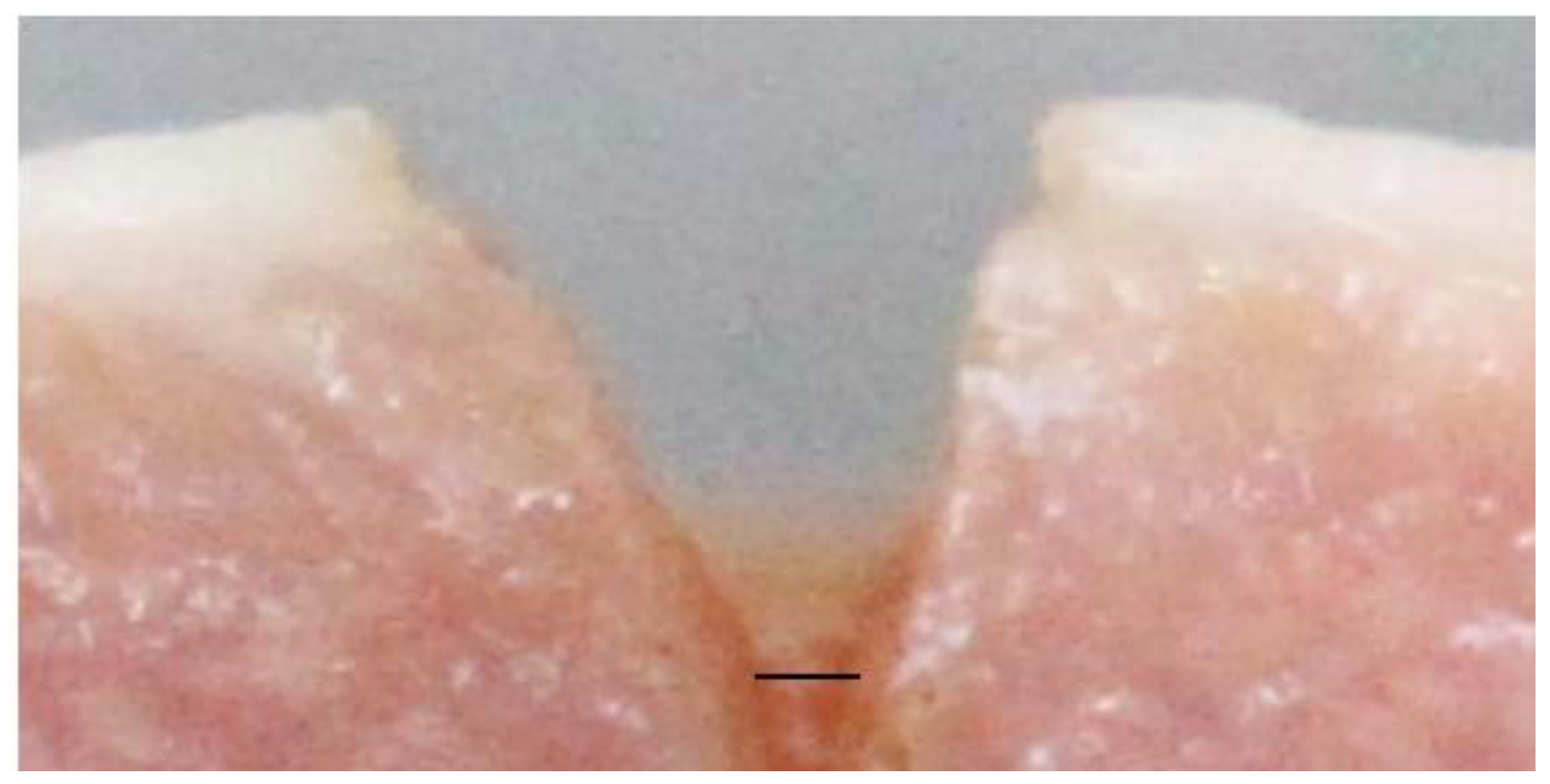
2.5. Outcome Measurement
2.6. Statistical Analyses
3. Results
3.1. Reliability Test
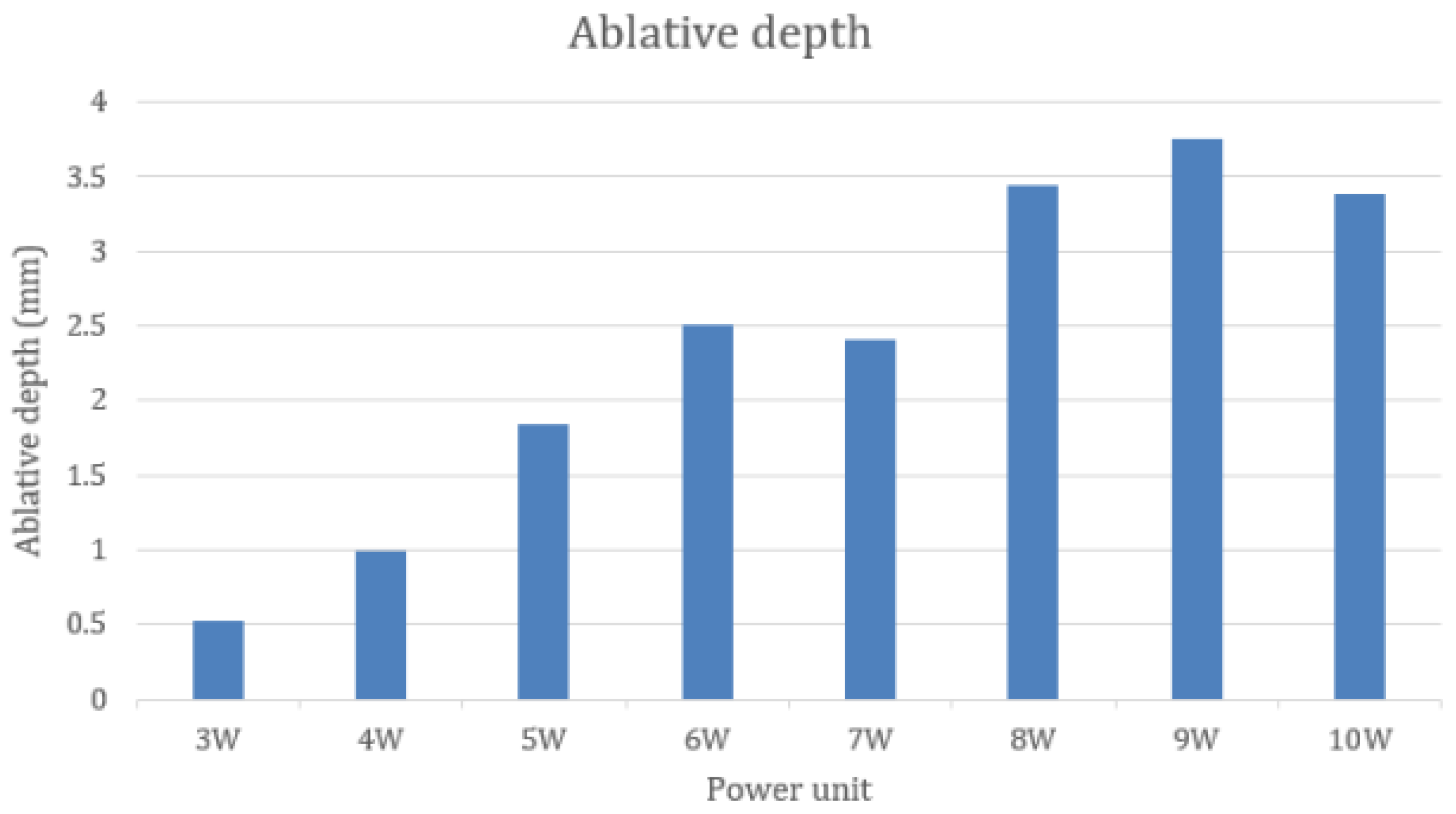
3.2. Comparing Ablative Depth by Different Power Settings of a CO2 Laser
3.3. Relation between the Power Unit of a CO2 Laser and Ablative Depth
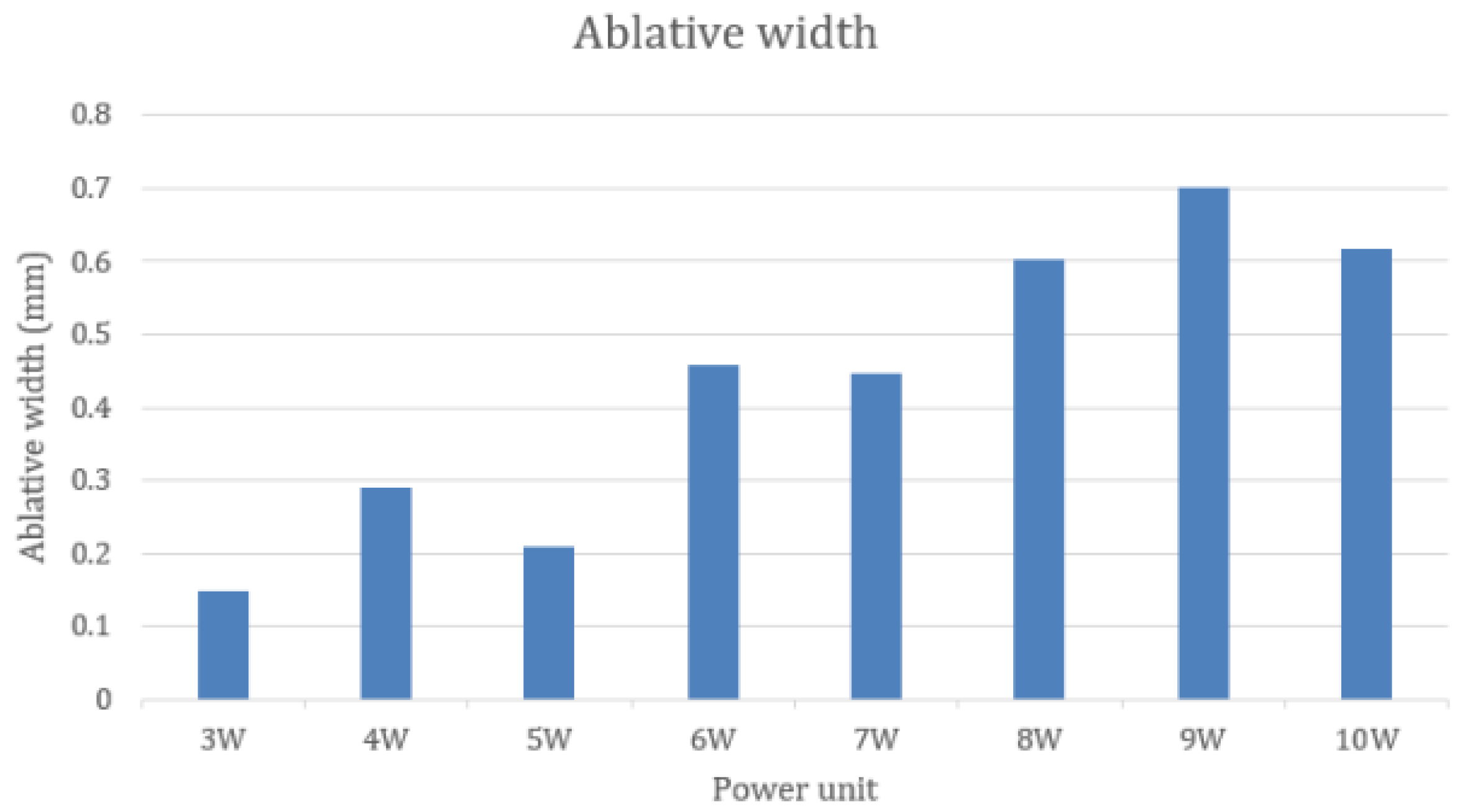
3.4. Comparing Ablative Width by Different Power Settings of a CO2 Laser
3.5. Relation between the Power Unit of a CO2 Laser and the Ablative Width
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coluzzi, D.J. Fundamentals of dental lasers: Science and instruments. Dent. Clin. N. Am. 2004, 48, 751–770. [Google Scholar] [CrossRef] [PubMed]
- Saibene, A.M. Managing Benign and Malignant Oral Lesions with Carbon Dioxide Laser: Indications, Techniques, and Outcomes for Outpatient Surgery. Surg. J. 2019, 5, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Brugnera, A.; Nammour, S. Laser Dentistry: Current Clinical Applications by the World Federation for Laser Dentistry; Universal Publishers, Inc.: California, FL, USA, 2018; pp. 123–140. [Google Scholar]
- van der Hem, P.S.; Egges, M.; van der Wal, J.E.; Roodenburg, J.L.N. CO2 laser evaporation of oral lichen planus. Int. J. Oral Maxillofac. Surg. 2008, 37, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari, H.R.; Ziaei, N.; Nazari, H.; Amiri, S.M.; Sharifi, R. Oral lichen planus treatment by CO2 laser: A systematic review. Asian J. Sci. Res. 2017, 10, 1–9. [Google Scholar]
- Nammour, S.; Zeinoun, T.; Namour, A.; Vanheusden, A.; Vescovi, P. Evaluation of Different Laser-Supported Surgical Protocols for the Treatment of Oral Leukoplakia: A Long-Term Follow-Up. Photomed. Laser Surg. 2017, 35, 629–638. [Google Scholar] [CrossRef]
- Singh, K.; Mir, G.M.; Jeelani, U.; Gupta, S.; Koul, P.; Kalsotra, P. Carbon Dioxide Laser Surgery in Management of Oral Leukoplakia. Int. J. Med. Res. 2015, 3, 3565–3567. [Google Scholar]
- López-Jornet, P.; Camacho-Alonso, F. Comparison of pain and swelling after removal of oral leukoplakia with CO2 laser and cold knife: A randomized clinical trial. Med. Oral Patol. Oral Cir. Bucal. 2013, 18, 38–44. [Google Scholar] [CrossRef]
- Tambuwala, A.; Sangle, A.; Khan, A.; Sayed, A. Excision of Oral Leukoplakia by CO2 Lasers Versus Traditional Scalpel: A Comparative Study. J. Maxillofac. Oral Surg. 2014, 13, 320–327. [Google Scholar] [CrossRef]
- Mir, G.H.M.; Singh, K.P.; Gupta, S.; Manhas, A.; Malik, A.A.; Kalsotra, P. Oral Soft Tissue Benign Lesions-Carbon Dioxide Laser as a Surgical Tool. Int. J. Contemp. Med. 2017, 4, 205–207. [Google Scholar]
- Truschnegg, A.; Acham, S.; Kqiku, L.; Jakse, N.; Beham, A. Minimally Invasive Excision of Epulides with a CO2 Laser: A Retrospective Study of 90 Patients. Photomed. Laser Surg. 2017, 35, 472–478. [Google Scholar] [CrossRef]
- Toledano-Serrabona, J.; López-Ramírez, M.; Sánchez-Torres, A.; España-Tost, A.; Gay-Escoda, C. Recurrence rate of oral squamous cell papilloma after excision with surgical scalpel or laser therapy: A retrospective cohort study. Med. Oral Patol. Oral Cir. Bucal. 2019, 24, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Nammour, S.; Gerges, E.; Bou Tayeh, R.; Zeinoun, T. Oral crest lengthening for increasing removable denture retention by means of CO2 laser. Sci. World J. 2014, 2014, 738643. [Google Scholar] [CrossRef][Green Version]
- Lazăr, L.; Manu, D.R.; Dako, T.; Mârțu, M.-A.; Suciu, M.; Ormenișan, A.; Păcurar, M.; Lazăr, A.-P. Effects of Laser Application on Alveolar Bone Mesenchymal Stem Cells and Osteoblasts: An In Vitro Study. Diagnostics 2022, 12, 2358. [Google Scholar] [CrossRef]
- Martu, M.-A.; Surlin, P.; Lazar, L.; Maftei, G.A.; Luchian, I.; Gheorghe, D.-N.; Rezus, E.; Toma, V.; Foia, L.-G. Evaluation of Oxidative Stress before and after Using Laser and Photoactivation Therapy as Adjuvant of Non-Surgical Periodontal Treatment in Patients with Rheumatoid Arthritis. Antioxidants 2021, 10, 226. [Google Scholar] [CrossRef] [PubMed]
- Kastek, M.; Piatkowski, T.; Polakowski, H.; Zajac, A. Investigation of thermal effects caused by interaction of laser radiation with soft tissues. Proceed. SPIE—Int. Soc. Opt. Eng. 2012, 8354, 276–285. [Google Scholar] [CrossRef]
- Wang, H.; Delacroix, S.; Osswald, O.; Anderson, M.; Heil, T.; Lepre, E.; Lopez-Salas, N.; Kaner, R.B.; Smarsly, B.; Strauss, V. Laser-carbonization: Peering into the formation of micro-thermally produced (N-doped) carbons. Carbon 2021, 176, 500–510. [Google Scholar] [CrossRef]
- Wilder-Smith, P.; Dang, J.; Kurosaki, T. Investigating the range of surgical effects on soft tissue produced by a carbon dioxide laser. J. Am. Dent. Assoc. 1997, 128, 583–588. [Google Scholar] [CrossRef]
- Jaleel, B.A.A.; Mahmood, A.S. Evaluation the Effects of CO2 Laser on Soft and Hard Tissues (in vitro study). Iraqi J. Laser 2014, 13, 13–22. [Google Scholar]
- Hanby, D.F.; Gremillion, G.; Zieske, A.W.; Loehn, B.; Whitworth, R.; Wolf, T.; Kakade, A.C.; Walvekar, R.R. Harmonic scalpel versus flexible CO2 laser for tongue resection: A histopathological analysis of thermal damage in human cadavers. World J. Surg. Oncol. 2011, 121, 1–6. [Google Scholar]
- Roush, J.; Bustillo, K.; Low, E. Measurement Error Between a Goniometer and the NIH ImageJ Program for Measuring Quadriceps Angle. Int. J. Allied Health Sci. Pract. 2008, 6, 7. [Google Scholar] [CrossRef]
- Jeffcoate, W.J.; Musgrove, A.J.; Lincoln, N.B. Using image J to document healing in ulcers of the foot in diabetes. Int. Wound J. 2017, 1137–1139. [Google Scholar] [CrossRef] [PubMed]
- Sattayut, S.; Nakkyo, P.; Phusrinuan, P.; Sangiamsak, T.; Phiolueang, R. CO2 laser oral soft tissue welding: An in vitro study. Laser Ther. 2013, 22, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Sattayut, S.; Bradley, P. A comparative study of the central vaporization with peripheral coagulation of Nd:YAG laser. Int. Congr. Ser. 2003, 1248, 371–376. [Google Scholar] [CrossRef]
- Goharkhay, K.; Moritz, A.; Wilder-Smith, P.; Schoop, U.; Kluger, W.; Jakolitsch, S.; Sperr, W. Effects on oral soft tissue produced by a diode laser in vitro. Lasers Surg. Med. 1999, 25, 401–406. [Google Scholar] [CrossRef]
- Romeo, U.; Palaia, G.; Del Vecchio, A.; Tenore, G.; Gambarini, G.; Gutknecht, N.; De Luca, M. Effects of KTP laser on oral soft tissues. An in vitro study. Lasers Med. Sci. 2010, 25, 539–543. [Google Scholar] [CrossRef]
- Kinikoglu, B.; Hemar, J.; Hasirci, V.; Breton, P.; Damour, O. Feasibility of a porcine oral mucosa equivalent: A preclinical study. Artif. Cells Blood Substit. Immobil. Biotechnol. 2012, 40, 271–274. [Google Scholar] [CrossRef]
- Sattayut, S.; Hortong, K.; Kitichaiwan, C. The Ablation Properties of CO2 Laser Irradiating to Absorption Media: An In Vitro Study. Int. J. Dent. 2012, 2012, 230967. [Google Scholar] [CrossRef]
- Beer, F.; Körpert, W.; Passow, H.; Steidler, A.; Meinl, A.; Buchmair, A.G.; Moritz, A. Reduction of collateral thermal impact of diode laser irradiation on soft tissue due to modified application parameters. Lasers Med. Sci. 2012, 27, 917–921. [Google Scholar] [CrossRef]
- Matthews, M.; Trapp, J.; Guss, G.; Rubenchik, A. Direct measurements of laser absorptivity during metal melt pool formation associated with powder bed fusion additive manufacturing processes. J. Laser Appl. 2018, 30, 032302. [Google Scholar] [CrossRef]
- Holmstrup, P.; Vedtofte, P.; Reibel, J.; Stoltze, K. Long-term treatment outcome of oral premalignant lesions. Oral Oncol. 2006, 42, 461–474. [Google Scholar] [CrossRef]
- Romeo, U.; Mohsen, M.; Palaia, G.; Bellisario, A.; Del Vecchio, A.; Tenore, G. CO2 laser ablation of oral leukoplakia: With or without extension of margins? Clin. Ter. 2020, 171, 209–215. [Google Scholar]
- Levine, R.; Vitruk, P. Use of a 10,600-nm CO2 Laser Mandibular Vestibular Extension in a Patient With a Chromosomal Abnormality. Compend. Contin. Educ. Dent. 2016, 37, 527–533. [Google Scholar]
- Ozturan, S.; Ay, E.; Sagir, S. Case series of laser-assisted treatment of excessive gingival display: An alternative treatment. Photomed. Laser Surg. 2014, 32, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Kongsong, W.; Sessirisombat, S. Treatment outcomes of carbon dioxide laser for trigeminal neuralgia. Laser Dent. Sci. 2020, 4, 61–66. [Google Scholar] [CrossRef]
- Song, J.E.; Um, Y.J.; Kim, C.S.; Choi, S.H.; Cho, K.S.; Kim, C.K.; Chai, J.K.; Jung, U.W. Thickness of posterior palatal masticatory mucosa: The use of computerized tomography. J. Periodontol. 2008, 79, 406–412. [Google Scholar] [CrossRef] [PubMed]


| The Depth of the Incision | ||||||||
|---|---|---|---|---|---|---|---|---|
| 3 W | 4 W | 5 W | 6 W | 7 W | 8 W | 9 W | 10 W | |
| Median (mm) | 0.527 | 0.996 | 1.842 | 2.507 | 2.403 | 3.447 | 3.750 | 3.388 |
| 25th percen tile | 0.474 | 0.842 | 1.326 | 2.063 | 3.362 | 2.589 | 3.362 | 3.151 |
| 75th percen tile | 0.817 | 1.205 | 2.199 | 2.705 | 4.118 | 4.870 | 4.118 | 4.453 |
| The Pairwise Comparisons of the Ablative Depth | |||||||
|---|---|---|---|---|---|---|---|
| 4 W | 5 W | 6 W | 7 W | 8 W | 9 W | 10 W | |
| 3 W | 1 | 0.283 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 |
| 4 W | 1 | 0.098 | 0.021 | <0.001 | <0.001 | <0.001 | |
| 5 W | 1 | 1 | 0.017 | 0.002 | 0.009 | ||
| 6 W | 1 | 1 | 0.269 | 0.89 | |||
| 7 W | 1 | 0.916 | 1 | ||||
| 8 W | 1 | 1 | |||||
| 9 W | 1 | ||||||
| The Mean and 95% Confidence Interval | |
|---|---|
| 3 W | 0.147 (0.110–0.184) |
| 4 W | 0.290 (0.198–0.382) |
| 5 W | 0.208 (0.163–0.254) |
| 6 W | 0.458 (0.366–0.550) |
| 7 W | 0.446 (0.322–0.570) |
| 8 W | 0.602 (0.469–0.735) |
| 9 W | 0.700 (0.541–0.860) |
| 10 W | 0.618 (0.452–0.785) |
| The Tukey Post-Hoc Test of Ablative Width | |||||||
|---|---|---|---|---|---|---|---|
| 4 W | 5 W | 6 W | 7 W | 8 W | 9 W | 10 W | |
| 3 W | 0.559 | 0.992 | 0.002 | 0.003 | <0.001 | <0.001 | <0.001 |
| 4 W | 0.960 | 0.345 | 0.444 | 0.002 | <0.001 | 0.001 | |
| 5 W | 0.028 | 0.044 | <0.001 | <0.001 | <0.001 | ||
| 6 W | 1 | 0.548 | 0.037 | 0.408 | |||
| 7 W | 0.441 | 0.023 | 0.313 | ||||
| 8 W | 0.898 | 1 | |||||
| 9 W | 0.959 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mungmee, A.; Sattayut, S. An In Vitro Study of the Effect of CO2 Laser Power Output on Ablative Properties in Porcine Tongue. Life 2023, 13, 162. https://doi.org/10.3390/life13010162
Mungmee A, Sattayut S. An In Vitro Study of the Effect of CO2 Laser Power Output on Ablative Properties in Porcine Tongue. Life. 2023; 13(1):162. https://doi.org/10.3390/life13010162
Chicago/Turabian StyleMungmee, Amontep, and Sajee Sattayut. 2023. "An In Vitro Study of the Effect of CO2 Laser Power Output on Ablative Properties in Porcine Tongue" Life 13, no. 1: 162. https://doi.org/10.3390/life13010162
APA StyleMungmee, A., & Sattayut, S. (2023). An In Vitro Study of the Effect of CO2 Laser Power Output on Ablative Properties in Porcine Tongue. Life, 13(1), 162. https://doi.org/10.3390/life13010162








