Evaluation of Cerebral Volume Changes in Patients with Tremor Treated by MRgFUS Thalamotomy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Evaluation
2.3. MRI Evaluation and Volumetric/Statistical Analysis
2.4. Statistical Analysis
3. Results
3.1. Patients Population
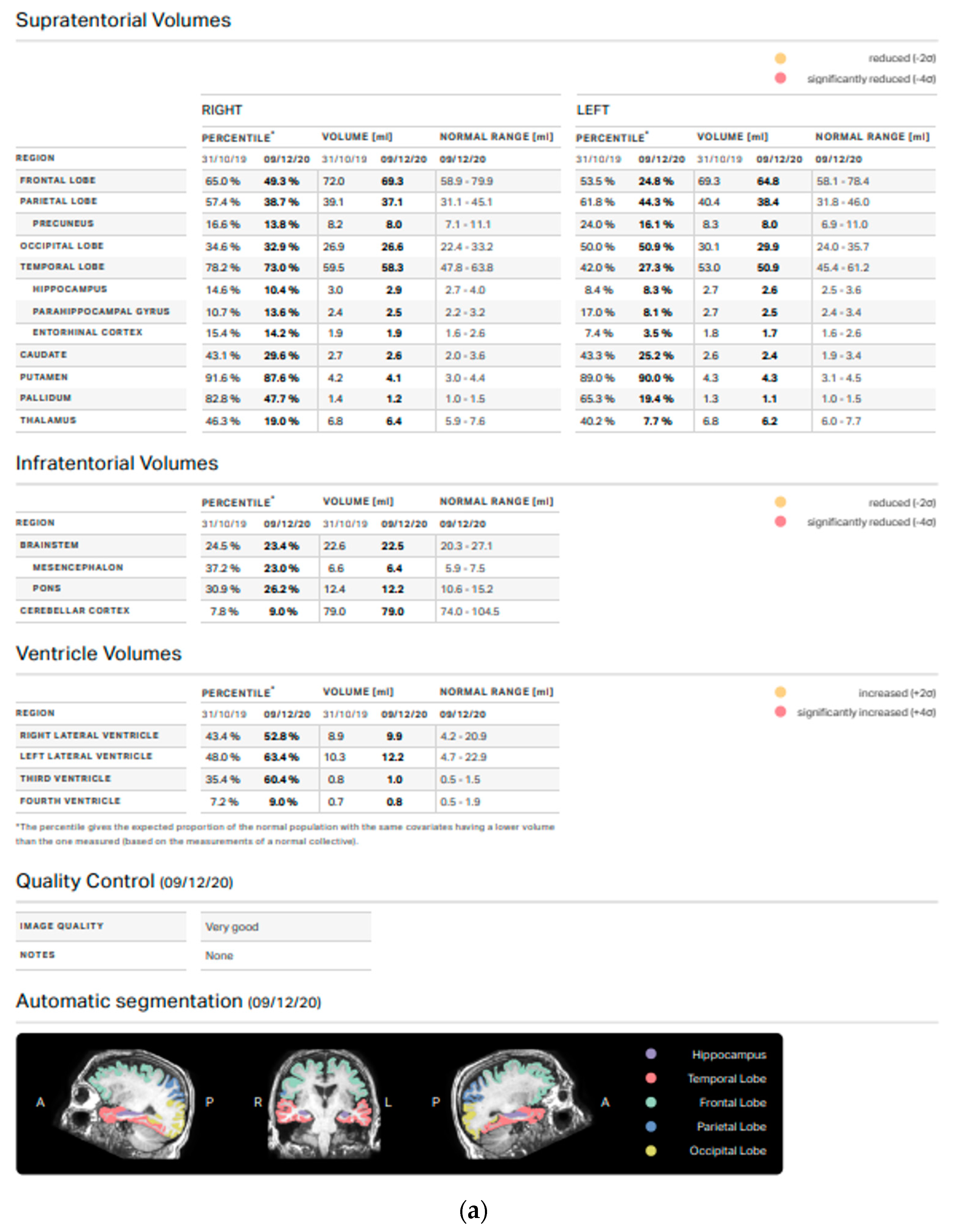
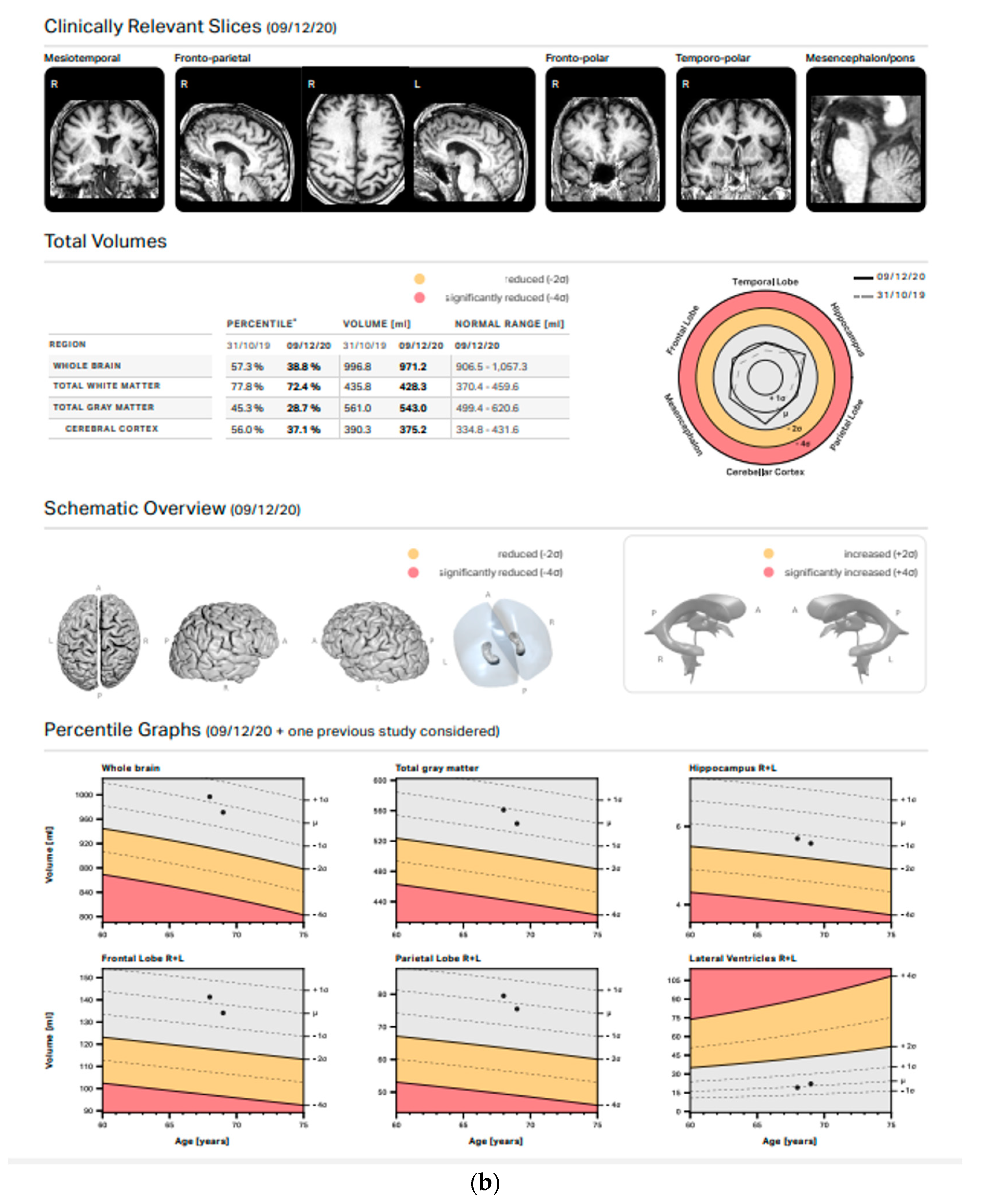
3.2. Volumetric Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weiss, H.D. Clinical Pearls in Tremor and Other Hyperkinetic Movement Disorders. Semin. Neurol. 2016, 36, 335–341. [Google Scholar] [CrossRef]
- Louis, E.D.; Faust, P.L. Essential tremor pathology: Neurodegeneration and reorganization of neuronal connections. Nat. Rev. Neurol. 2020, 16, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M. Parkinson’s disease tremor: Pathophysiology. Parkinsonism Relat. Disord. 2012, 18 (Suppl. S1), S85–S86. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, T. Pathophysiologic Basis of Movement Disorders. Prog. Neurol. Surg. 2018, 33, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Bruno, F.; Catalucci, A.; Arrigoni, F.; Sucapane, P.; Cerone, D.; Cerrone, P.; Ricci, A.; Marini, C.; Masciocchi, C. An experience-based review of HIFU in functional interventional neuroradiology: Transcranial MRgFUS thalamotomy for treatment of tremor. Radiol. Med. 2020, 125, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.W.; Park, C.K.; Lipsman, N.; Schwartz, M.L.; Ghanouni, P.; Henderson, J.M.; Gwinn, R.; Witt, J.; Tierney, T.S.; Cosgrove, G.R.; et al. A prospective trial of magnetic resonance-guided focused ultrasound thalamotomy for essential tremor: Results at the 2-year follow-up. Ann. Neurol. 2018, 83, 107–114. [Google Scholar] [CrossRef]
- Jameel, A.; Bain, P.; Nandi, D.; Jones, B.; Gedroyc, W. Device profile of exAblate Neuro 4000, the leading system for brain magnetic resonance guided focused ultrasound technology: An overview of its safety and efficacy in the treatment of medically refractory essential tremor. Expert Rev. Med. Devices 2021, 18, 429–437. [Google Scholar] [CrossRef]
- Agren, R.; Awad, A.; Blomstedt, P.; Fytagoridis, A. Voxel-Based Morphometry of Cerebellar Lobules in Essential Tremor. Front. Aging Neurosci. 2021, 13, 667854. [Google Scholar] [CrossRef]
- Lin, C.H.; Chen, C.M.; Lu, M.K.; Tsai, C.H.; Chiou, J.C.; Liao, J.R.; Duann, J.R. VBM Reveals Brain Volume Differences between Parkinson’s Disease and Essential Tremor Patients. Front. Hum. Neurosci. 2013, 7, 247. [Google Scholar] [CrossRef]
- Mavroudis, I.; Petrides, F.; Karantali, E.; Chatzikonstantinou, S.; McKenna, J.; Ciobica, A.; Iordache, A.C.; Dobrin, R.; Trus, C.; Kazis, D. A Voxel-Wise Meta-Analysis on the Cerebellum in Essential Tremor. Medicina 2021, 57, 264. [Google Scholar] [CrossRef]
- Bruno, F.; Catalucci, A.; Arrigoni, F.; Gagliardi, A.; Campanozzi, E.; Corridore, A.; Tommasino, E.; Pagliei, V.; Pertici, L.; Palumbo, P.; et al. Comprehensive Evaluation of Factors Affecting Tremor Relapse after MRgFUS Thalamotomy: A Case-Control Study. Brain Sci. 2021, 11, 1183. [Google Scholar] [CrossRef] [PubMed]
- Bruno, F.; Catalucci, A.; Varrassi, M.; Arrigoni, F.; Sucapane, P.; Cerone, D.; Pistoia, F.; Torlone, S.; Tommasino, E.; De Santis, L.; et al. Comparative evaluation of tractography-based direct targeting and atlas-based indirect targeting of the ventral intermediate (Vim) nucleus in MRgFUS thalamotomy. Sci. Rep. 2021, 11, 13538. [Google Scholar] [CrossRef] [PubMed]
- Zur, G.; Lesman-Segev, O.H.; Schlesinger, I.; Goldsher, D.; Sinai, A.; Zaaroor, M.; Assaf, Y.; Eran, A.; Kahn, I. Tremor Relief and Structural Integrity after MRI-guided Focused US Thalamotomy in Tremor Disorders. Radiology 2020, 294, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Seasons, G.M.; Mazerolle, E.L.; Sankar, T.; Martino, D.; Kiss, Z.H.T.; Pichardo, S.; Pike, G.B. Predicting high-intensity focused ultrasound thalamotomy lesions using 2D magnetic resonance thermometry and 3D Gaussian modeling. Med. Phys. 2019, 46, 5722–5732. [Google Scholar] [CrossRef] [PubMed]
- Stanziano, M.; Golfre Andreasi, N.; Messina, G.; Rinaldo, S.; Palermo, S.; Verri, M.; Demichelis, G.; Medina, J.P.; Ghielmetti, F.; Bonvegna, S.; et al. Resting State Functional Connectivity Signatures of MRgFUS Vim Thalamotomy in Parkinson’s Disease: A Preliminary Study. Front. Neurol. 2021, 12, 786734. [Google Scholar] [CrossRef] [PubMed]
- Tommasino, E.; Bruno, F.; Catalucci, A.; Varrassi, M.; Sucapane, P.; Cerone, D.; Pistoia, F.; Di Cesare, E.; Barile, A.; Ricci, A.; et al. Prognostic value of brain tissues’ volumes in patients with essential tremor treated with MRgFUS thalamotomy. J. Clin. Neurosci. 2021, 92, 33–38. [Google Scholar] [CrossRef]
- Prasad, S.; Shah, A.; Bhalsing, K.S.; Ingalhalikar, M.; Saini, J.; Pal, P.K. Clinical correlates of abnormal subcortical volumes in Essential Tremor. J. Neural Transm. (Vienna) 2019, 126, 569–576. [Google Scholar] [CrossRef]
- Cameron, E.; Dyke, J.P.; Hernandez, N.; Louis, E.D.; Dydak, U. Cerebral gray matter volume losses in essential tremor: A case-control study using high resolution tissue probability maps. Parkinsonism Relat. Disord. 2018, 51, 85–90. [Google Scholar] [CrossRef]
- Benito-Leon, J.; Serrano, J.I.; Louis, E.D.; Holobar, A.; Romero, J.P.; Povalej-Brzan, P.; Kranjec, J.; Bermejo-Pareja, F.; Del Castillo, M.D.; Posada, I.J.; et al. Essential tremor severity and anatomical changes in brain areas controlling movement sequencing. Ann. Clin. Transl. Neurol. 2019, 6, 83–97. [Google Scholar] [CrossRef]
- Choi, S.M.; Kim, B.C.; Chang, J.; Choi, K.H.; Nam, T.S.; Kim, J.T.; Lee, S.H.; Park, M.S.; Yoon, W.; de Leon, M.J. Comparison of the Brain Volume in Essential Tremor and Parkinson’s Disease Tremor Using an Automated Segmentation Method. Eur. Neurol. 2015, 73, 303–309. [Google Scholar] [CrossRef]
- Bostan, A.C.; Dum, R.P.; Strick, P.L. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn. Sci. 2013, 17, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Cerasa, A.; Messina, D.; Pugliese, P.; Morelli, M.; Lanza, P.; Salsone, M.; Novellino, F.; Nicoletti, G.; Arabia, G.; Quattrone, A. Increased prefrontal volume in PD with levodopa-induced dyskinesias: A voxel-based morphometry study. Mov. Disord. 2011, 26, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Benito-Leon, J.; Alvarez-Linera, J.; Hernandez-Tamames, J.A.; Alonso-Navarro, H.; Jimenez-Jimenez, F.J.; Louis, E.D. Brain structural changes in essential tremor: Voxel-based morphometry at 3-Tesla. J. Neurol. Sci. 2009, 287, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Kern, D.S.; Uy, D.; Rhoades, R.; Ojemann, S.; Abosch, A.; Thompson, J.A. Discrete changes in brain volume after deep brain stimulation in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2020, 91, 928–937. [Google Scholar] [CrossRef]
- Younce, J.R.; Campbell, M.C.; Perlmutter, J.S.; Norris, S.A. Thalamic and ventricular volumes predict motor response to deep brain stimulation for Parkinson’s disease. Park. Relat. Disord. 2019, 61, 64–69. [Google Scholar] [CrossRef]
- Prodoehl, J.; Planetta, P.J.; Kurani, A.S.; Comella, C.L.; Corcos, D.M.; Vaillancourt, D.E. Differences in brain activation between tremor- and nontremor-dominant Parkinson disease. JAMA Neurol. 2013, 70, 100–106. [Google Scholar] [CrossRef]
- Jellinger, K.A. Post mortem studies in Parkinson’s disease--is it possible to detect brain areas for specific symptoms? J. Neural Transm. Suppl. 1999, 56, 1–29. [Google Scholar] [CrossRef]
- Jung, N.Y.; Park, C.K.; Chang, W.S.; Jung, H.H.; Chang, J.W. Effects on cognition and quality of life with unilateral magnetic resonance-guided focused ultrasound thalamotomy for essential tremor. Neurosurg. Focus 2018, 44, E8. [Google Scholar] [CrossRef]
- Gallay, M.N.; Moser, D.; Jeanmonod, D. Safety and accuracy of incisionless transcranial MR-guided focused ultrasound functional neurosurgery: Single-center experience with 253 targets in 180 treatments. J. Neurosurg. 2018, 130, 1234–1243. [Google Scholar] [CrossRef]
- Lehman, V.T.; Lee, K.H.; Klassen, B.T.; Blezek, D.J.; Goyal, A.; Shah, B.R.; Gorny, K.R.; Huston, J.; Kaufmann, T.J. MRI and tractography techniques to localize the ventral intermediate nucleus and dentatorubrothalamic tract for deep brain stimulation and MR-guided focused ultrasound: A narrative review and update. Neurosurg. Focus 2020, 49, E8. [Google Scholar] [CrossRef]
- Miller, T.R.; Zhuo, J.; Eisenberg, H.M.; Fishman, P.S.; Melhem, E.R.; Gullapalli, R.; Gandhi, D. Targeting of the dentato-rubro-thalamic tract for MR-guided focused ultrasound treatment of essential tremor. Neuroradiol. J. 2019, 32, 401–407. [Google Scholar] [CrossRef] [PubMed]
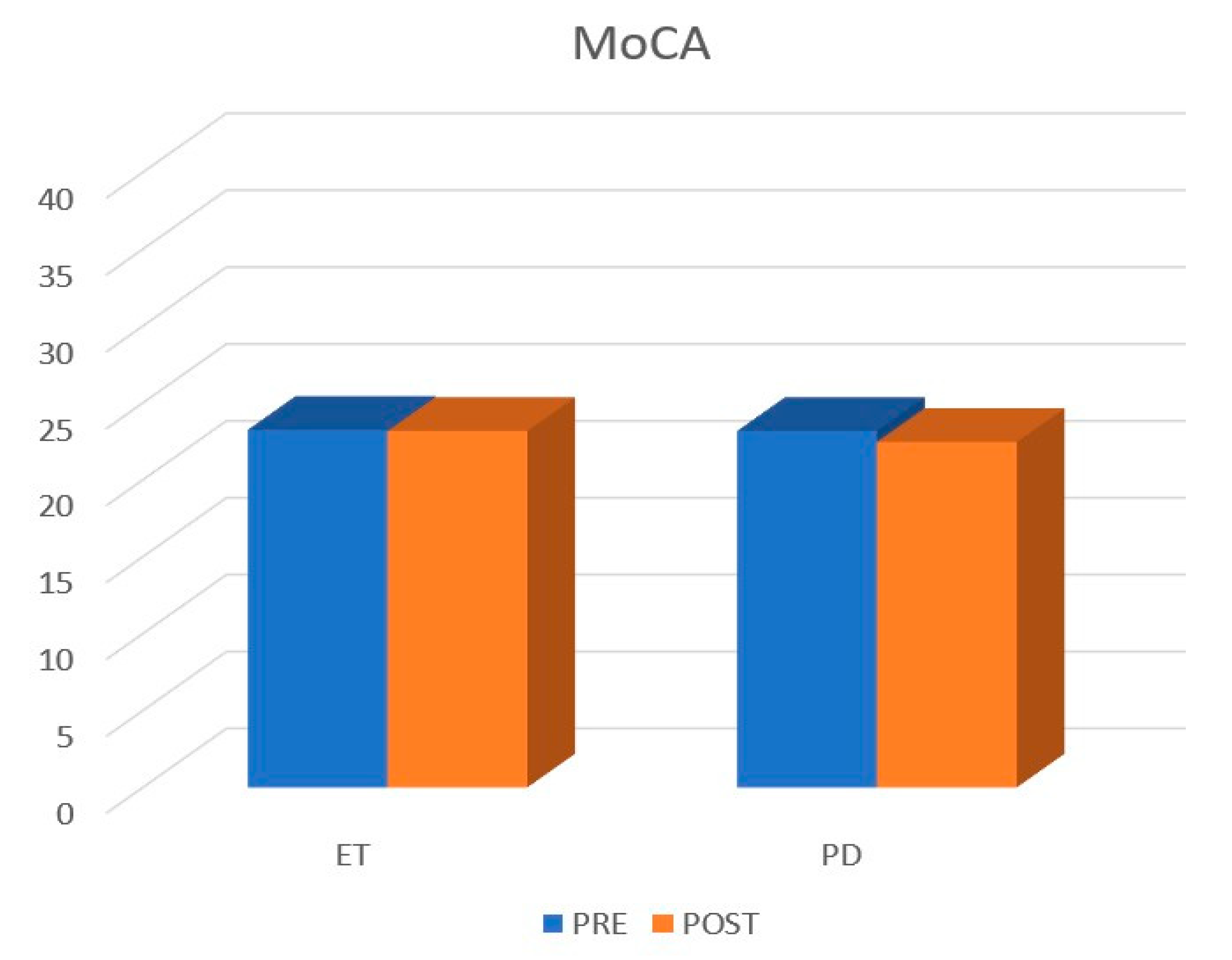
| PD | ET | p-Value | |
|---|---|---|---|
| Age | 68 ± 9.89 | 68 ± 9.79 | 0.12 |
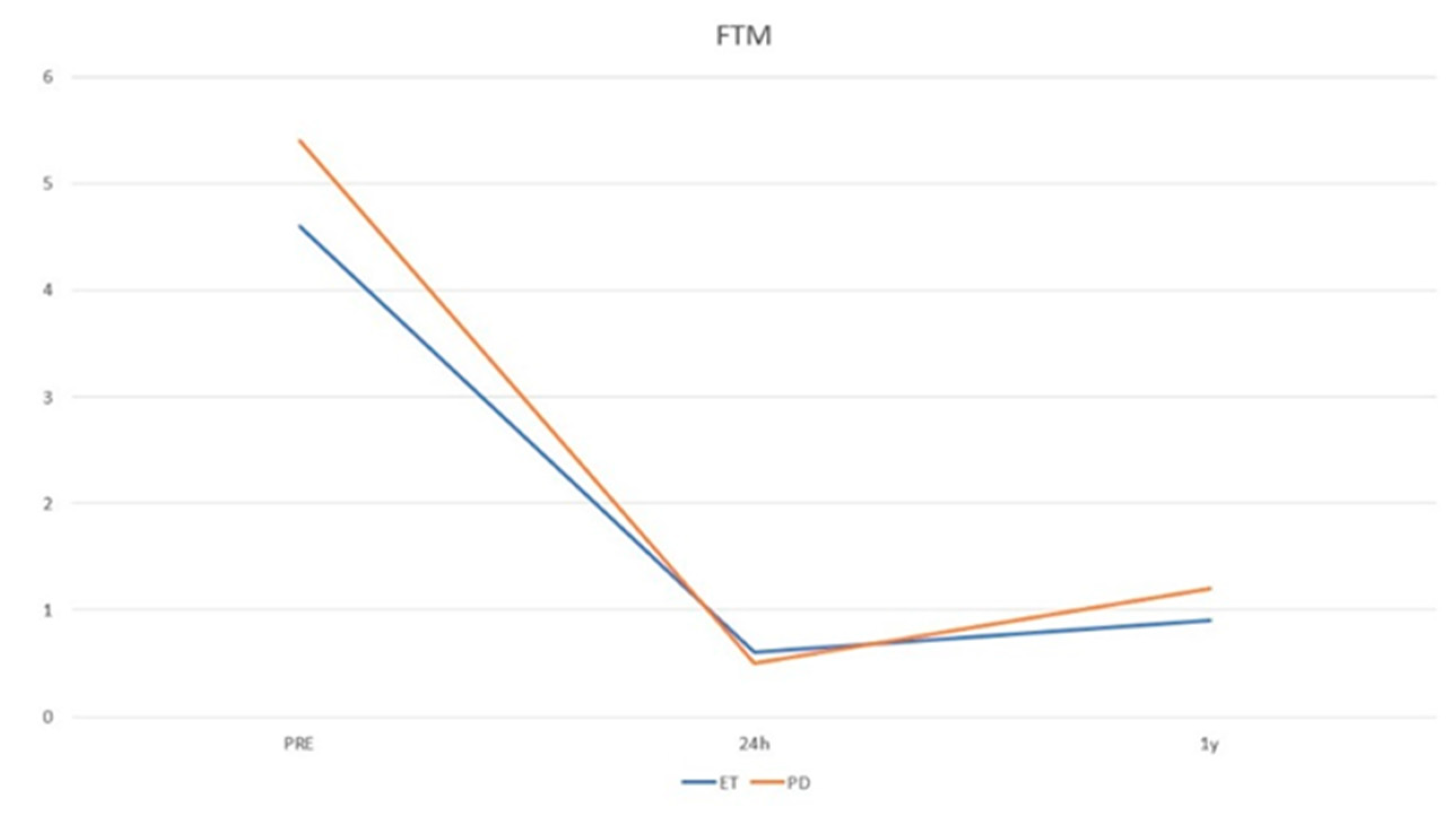
| Pre-FTM | 5.43 ± 2.39 | 4.60 ± 2.09 | 0.23 |
| Pre-MoCa | 23.18 ± 4.63 | 23.25 ± 5.09 | 0.34 |
| Cerebral Area | Pre-Treatment Volume (mL) | Post-Treatment Volume (mL) | p Value |
|---|---|---|---|
| Total white matter | 473.11 ± 53.47 | 471.96 ± 55.75 | 0.72 |
| Total grey matter | 654.50 ± 57.29 | 650.38 ± 62.31 | 0.27 |
| Right thalamus | 7.56 ± 0.66 | 7.50 ± 0.76 | 0.24 |
| Left thalamus | 7.64 ± 0.70 | 7.38 ± 0.87 | 0.001 |
| Right Temporal lobe | 65.68 ± 6.50 | 65.58 ± 7.51 | 0.84 |
| Left Temporal lobe | 61.32 ± 5.82 | 60.85 ± 6.52 | 0.37 |
| Right Putamen | 4.18 ± 4.86 | 4.14 ± 0.46 | 0.23 |
| Left Putamen | 4.32 ± 5.36 | 4.22 ± 0.43 | 0.001 |
| Right Parietal lobe | 75.37 ± 5.36 | 46.24 ± 4.53 | 0.33 |
| Left Parietal lobe | 46.73 ± 4.54 | 45.56 ± 46.70 | 0.33 |
| Right Pallidus | 1.35 ± 0.18 | 1.30 ± 0.17 | 0.06 |
| Left Pallidus | 1.36 ± 0.17 | 0.28 ± 0.17 | 0.004 |
| Right Caudate | 3.58 ± 0.55 | 3.63 ± 0.56 | 0.24 |
| Left Caudate | 3.36 ± 0.65 | 3.38 ± 0.68 | 0.53 |
| Right Occipital lobe | 32.625 ± 3.52 | 32.30 ± 4.31 | 0.47 |
| Left Occipital lobe | 34.12 ± 3.59 | 34.09 ± 4.65 | 0.95 |
| Right Frontal lobe | 32.62 ± 8.21 | 32.30 ± 9.02 | 0.47 |
| Left Frontal lobe | 34.12 ± 8.25 | 34.09 ± 9.11 | 0.95 |
| Cerebral cortex | 451.69 ± 42.36 | 451.93 ± 47.36 | 0.93 |
| Cerebellar Cortex | 91.11 ± 12.69 | 84.99 ± 14.69 | 0.02 |
| Cerebral Area | Pre-Treatment Volume (mL) | Post-Treatment Volume (mL) | p Value |
|---|---|---|---|
| Total white matter | 500.36 ± 54.21 | 493.08 ± 55.21 | 0.07 |
| Total grey matter | 655.56 ± 69.43 | 651.23 ± 67.69 | 0.25 |
| Right thalamus | 7.67 ± 0.77 | 7.66 ± 0.71 | 0.83 |
| Left thalamus | 7.68 ± 0.80 | 7.58 ± 0.67 | 0.23 |
| Right Temporal lobe | 67.48 ± 8.60 | 67.00 ± 8.16 | 0.23 |
| Left Temporal lobe | 63.18 ± 8.43 | 62.59 ± 7.69 | 0.18 |
| Right Putamen | 4.03 ± 0.91 | 4.03 ± 0.68 | 1.00 |
| Left Putamen | 4.22 ± 0.70 | 4.18 ± 0.57 | 0.42 |
| Right Parietal lobe | 45.81 ± 4.84 | 45.63 ± 4.85 | 0.76 |
| Left Parietal lobe | 45.46 ± 5.54 | 45.91 ± 4.92 | 0.51 |
| Right Pallidus | 1.31 ± 0.27 | 1.29 ± 0.22 | 0.63 |
| Left Pallidus | 1.36 ± 0.27 | 1.35 ± 0.22 | 0.68 |
| Right Caudate | 3.17 ± 0.52 | 3.21 ± 0.61 | 0.63 |
| Left Caudate | 2.92 ± 0.55 | 2.95 ± 0.49 | 0.64 |
| Right Occipital lobe | 33.26 ± 3.88 | 33.41 ± 3.84 | 0.68 |
| Left Occipital lobe | 34.89 ± 3.16 | 34.61 ± 2.98 | 0.56 |
| Right Frontal lobe | 84.24 ± 11.88 | 84.70 ± 12.56 | 0.69 |
| Left Frontal lobe | 82.13 ± 12.54 | 82.75 ± 10.91 | 0.67 |
| Cerebral cortex | 456.48 ± 53.76 | 456.60 ± 52.65 | 0.97 |
| Cerebellar Cortex | 88.20 ± 13.83 | 86.61 ±16.35 | 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruno, F.; Tommasino, E.; Catalucci, A.; Pastorelli, C.; Borea, F.; Caldarelli, G.; Bellini, M.; Badini, P.; Mancini, S.; Santobuono, C.; et al. Evaluation of Cerebral Volume Changes in Patients with Tremor Treated by MRgFUS Thalamotomy. Life 2023, 13, 16. https://doi.org/10.3390/life13010016
Bruno F, Tommasino E, Catalucci A, Pastorelli C, Borea F, Caldarelli G, Bellini M, Badini P, Mancini S, Santobuono C, et al. Evaluation of Cerebral Volume Changes in Patients with Tremor Treated by MRgFUS Thalamotomy. Life. 2023; 13(1):16. https://doi.org/10.3390/life13010016
Chicago/Turabian StyleBruno, Federico, Emanuele Tommasino, Alessia Catalucci, Cristina Pastorelli, Francesco Borea, Giulia Caldarelli, Mattia Bellini, Pierfrancesco Badini, Sara Mancini, Chiara Santobuono, and et al. 2023. "Evaluation of Cerebral Volume Changes in Patients with Tremor Treated by MRgFUS Thalamotomy" Life 13, no. 1: 16. https://doi.org/10.3390/life13010016
APA StyleBruno, F., Tommasino, E., Catalucci, A., Pastorelli, C., Borea, F., Caldarelli, G., Bellini, M., Badini, P., Mancini, S., Santobuono, C., Martino, S., Pagliei, V., Manco, G., Cerone, D., Pistoia, F., Palumbo, P., Arrigoni, F., Di Cesare, E., Marini, C., ... Masciocchi, C. (2023). Evaluation of Cerebral Volume Changes in Patients with Tremor Treated by MRgFUS Thalamotomy. Life, 13(1), 16. https://doi.org/10.3390/life13010016