Fluid Management, Intra-Abdominal Hypertension and the Abdominal Compartment Syndrome: A Narrative Review
Abstract
1. Background
- To reduce the pressure in the compartment by improving compliance (e.g., muscle relaxation) and, or opening different compartments (e.g., through escharotomy or decompressive surgery).
- Individualized fluid management strategies and supportive therapy.
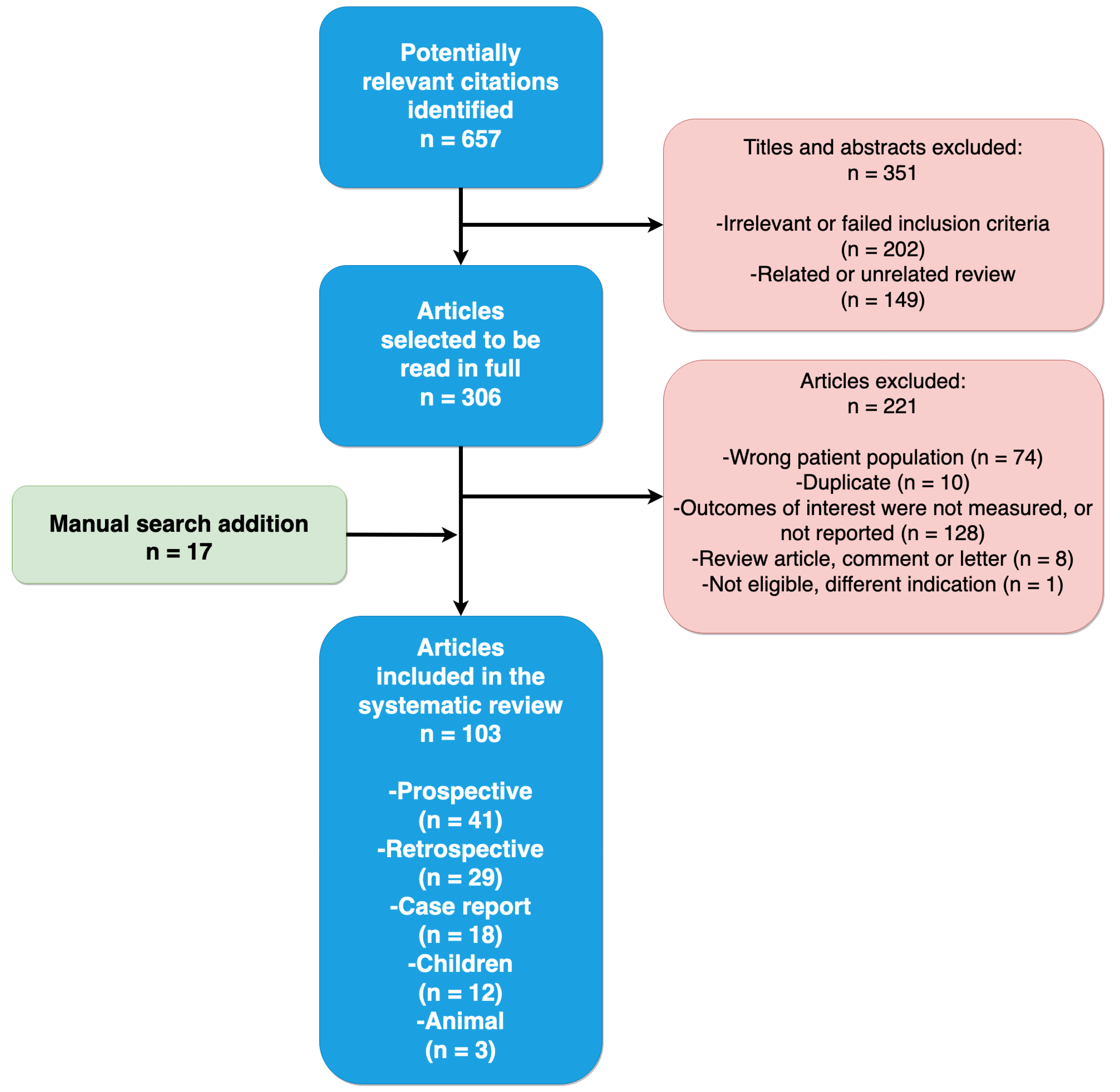
2. Methods
Search Strategy
3. Results
3.1. Data on Associations between Fluid Administration and IAH
3.1.1. Study selection and characteristics
3.1.2. Severe burn patients
Prevalence
Resuscitation Fluids & Risk Factors
Management
Outcome
3.1.3. Severe acute pancreatitis
Prevalence
Resuscitation Fluid and Risk Factors
Management
Outcome
3.1.4. Trauma patients
Prevalence
Resuscitation Fluid and Risk Factors
Management
Outcome
3.1.5. Medical patients
Incidence
Resuscitation Fluid and Management
Outcome
3.1.6. Surgical patients
Incidence
Resuscitation Fluid and Risk Factors
Outcome
3.1.7. Mixed ICU patients
Incidence
Independent Predictors for IAH
Outcome
| Author | Year | Type of Study | Patients | Resuscitation Fluids | IAP (mmHg) | Results |
|---|---|---|---|---|---|---|
| Severe burn patients | ||||||
| Ivy et al. [19] | 2000 | Prospective | n = 10 (7 IAH; 2 ACS) | IAH: volume of fluid 9 to 35 L 579 ACS: volume of fluid 33 to 48 L | IAP: 9 to 44 | 2 DL; 2 patients died |
| O’Mara et al. [23] | 2005 | Observational | Crystalloid (n = 15) vs. Plasma (n = 16) | 561 mL/kg crystalloid 360 mL/kg plasma | Crystalloid: 32.5 Plasma: 16.4 | Crystalloid group:
|
| Oda et al. [21] | 2006 | Observational | HLS (n = 14) LR (n = 22) (≥40% TBSA) | Needed to maintain UO: HLS 3.1 ± 0.9 mL/24 h/kg/% TBSA vs. LR 5.2 ± 1.2 mL24 h/kg/% TBSA | HLS 14% vs. LR 50% developed IAH | HLS resuscitation can reduce risk of secondary ACS with lower fluid load than LR solution |
| Oda et al. [22] | 2006 | Observational | n = 48 | ACS patients received 398.7 ± 105.5 mL/kg fluid the first 24 h after injury | IAP (49 ± 12 cm H2O) ACS: n = 8 | >300 mL/kg/24 h fluid resuscitation → ACS |
| Ennis et al. [18] | 2008 | Prospective | n = 56 BRG group n = 62 control group | >250 mL/kg volume in the first 24 h | Not reported | ACS and mortality significantly lower in BRG group (p = 0.03) |
| Ruiz-Castilla et al. [24] | 2014 | Observational | n = 25 (>20% TBSA) | 10473 mL in pts with IAH vs. 4100 mL in no IAH (p = 0.03) | 13 vs. 10 | IAH pts:
|
| Wise et al. [26] | 2016 | Observational | n = 56 | ACS 13.6 ± 16 L vs. No ACS 7.6 ± 4.1 L | IAH: n = 44 ACS: n = 16 | Non-survivors:
|
| Mbiine et al. [20] | 2017 | Observational | n = 64 (adults and children) | IAH in fluid overloaded patients: 16 vs. 13 IAH in patients not fluid overloaded: 10 vs. 9 | Prevalence IAH: 57.8% 3.3 times increased risk of mortality with IAH Mortality with IAH: 82.6% | More IAH among the fluid overloaded patients, but difference not significant, probably due to small sample size |
| Talizin et al. [25] | 2018 | Prospective | n = 46 (38 IAH; 8 no IAH) | 48 h fluid balance: With IAH: 5370 (3857–8828) mL Without IAH: 3894 (2411–5946) mL (p = 0.091) | Not applicable | IAH was associated with↑ mortality rate: 24 IAH + vs. 1 IAH – p = 0.016 |
| Severe acute pancreatitis | ||||||
| Mao et al. [43] | 2009 | RCT | n = 76 | Amount of crystalloid and colloid on admission day (mL):
| Incidence of ACS 72.2% in group I vs. 32.5% in group II | Total amount of fluid sequestration, rate of mechanical ventilation, incidence of ACS and mortality were significantly higher in group I |
| Du et al. [41] | 2011 | RCT | HES = 20 RL = 21 | Total infusion volumes not significantly different between 2 groups | HES: 11.25 ± 2.35 RL: 17.08 ± 4.98 | HES group (p < 0.05): IAP lower; more urine production, earlier negative fluid balance and fewer patients received mechanical ventilation |
| Ke et al. [42] | 2012 | Observational | n = 58 | 24 h fluid balance: IAH: 503 (373–1431) mL No IAH 74 (−31–409) mL | Median max IAP 13.1 mmHg 36 patients developed IAH 7 patients developed ACS | Risk factors for IAH include 24 h positive fluid balance (first day), number of fluid collections, and serum calcium level |
| Zhao et al. [40] | 2013 | RCT | n = 120 | NS: 61.79 ± 7.61 mL/kg/day SH (NS + HES): 46.93 ± 12.38 mL/kg/day SHG (SH + glutamine) 44.75 ± 8.53) mL/kg/day (p < 0.05) | IAP in NS significant higher | Compared to the NS group: patients in the SH and SHG groups:
|
| Trauma | ||||||
| Raeburn et al. [51] | 2001 | Observational | n = 77 | 28 patients with ACS vs. 49 patients no ACS | Mortality ACS 43% vs. no ACS 22% (p = 0.002) |
|
| Balogh et al. [47] | 2002 | Prospective | n = 128 total n = 11 ACS | 26 ± 2 U RCC 38 ± 3 L crystalloid | Mortality ACS: 54% | |
| Balogh et al. [48] | 2003 | Observational | n = 188 | Amount of crystalloid (L) received in:
| ACS: Primary 11 patients vs. Secondary 15 patients Mortality ACS (prim 64% vs. sec 53% vs. no ACS 17% |
|
| Cotton et al. [74] | 2009 | Prospective | Pre-TEP: n = 141 TEP: n = 125 | Blood products intraoperative:
| Higher 30-day survival in TEP 56.8% vs. 37.6% pre-TEP (p = 0.001) |
|
| Neal et al. [50] | 2012 | Multi-centre, Prospective | n = 452 |
| Overall mortality 22.6% | Patients with a ratio > 1.5:1 Crystalloid: RCC have 70% higher risk of MOF and 2-fold higher risk of ARDS and ACS |
| Mahmood et al. [49] | 2014 | Observational | n = 117 DL = 102 No DL = 15 | Crystalloid (L):
| 16.7% developed IAP > 20 mmHg in DL Mortality: 6% in DL vs. 20% in no DL p = 0.05 | Blood transfusion and IV fluids significant correlation with IAP >20 mmHg and more metabolic acidosis |
| Vatankhah et al. [52] | 2018 | Observational | n = 100 28 ACS vs. 72 no ACS | Crystalloid:
| 21% mortality in ACS | Mean volume of fluids significantly higher in pts. with ACS |
| Medical | ||||||
| Daugherty et al. [86] | 2007 | Observational | n = 40 | Positive fluid balance > 5 L/24 h | n = 34 IAP > 12 mmHg n = 13 IAP > 20 mmHg n = 10 ACS | 25% of patients with 5 L or > positive fluid balance in 24 h developed ACS |
| Cordemans et al. [87] | 2012 | Observational | n = 123 | Cumulative fluid balance:
| 20% IAH | Not achieving CLFM & being non-responder: strong independent predictors of mortality |
| Dorigatti et al. [85] | 2019 | Observational | n = 25 | Accumulated fluid balance (mL): n = 13 (death): 15165.4 ± 12719.2 vs. n = 12 (survival): 6194.5 ± 6517.1 | IAP 14.1 ± 4.2 vs. 9.4 ± 2.0 | Higher admission and consecutive SOFA score of > 7 associated with higher ACS incidence and higher mortality rate. |
| Surgical | ||||||
| Biancofiore et al. [92] | 2003 | Observational | n = 34 IAH n = 74 no IAH | IAH:
| Not Reported | High IAP pressure:
|
| Šerpytis et al. [97] | 2008 | Observational | n = 77 | Not reported | POD 1: 45.5% IAH POD 2: 41.7% IAH POD 3: 35.6% IAH | Positive correlation between 24-h fluid balance and IAP |
| Makar et al. [95] | 2009 | Prospective | n = 14 eEVR n = 16 eOR | Units RCC: (p ≤ 0.001)
| 1 ACS in eEVR, 1 ACS eOR | Correlation between IAP and the following:
|
| Dalfino et al. [93] | 2013 | Observational | n = 22 IAH n = 47 no IAH | Positive fluid balance: independent risk factor for IAH | Not Reported | Mortality IAH 53% vs. 27% (p = 0.02) |
| Muturi et al. [96] | 2017 | Observational | n = 113 | IV fluid over 24 h (mL): IAH: 3946.6 vs. No IAH: 2931.1 (p = 0.003) | n = 76 IAH n = 37 no IAH n = 5 ACS | Of those who had IAH; age, amount of iv fluids over 24 h, fluid balance & ventilator mode were significant determinants of risk of progression to ACS |
| Kotlińska-Hasiec et al. [94] | 2017 | Observational | Liberal: n = 32 vs. Restrictive: n = 31) | Liberal = 2822 ± 606 mL Restrictive = 823 ± 223 mL (p < 0.001) | Significant higher IAP in pts receiving liberal crystalloid therapy | Correlation between IAP and ECW |
| Medical-surgical | ||||||
| Biffl et al. [104] | 2001 | Prospective | 14 ACS: 8 trauma 6 medical | Averages administered: 16.7 ± 3.0 L crystalloid 13.3 ± 2.9 RBC | 10 patients underwent DL |
|
| Malbrain et al. [110] | 2004 | Observational | n = 97 | Patients with IAH:
| IAH 50.5% ACS 8.2% |
|
| Malbrain et al. [109] | 2005 | Observational | n = 265 | Not reported | IAH: 32.1% on admission Mortality 27.5% | Fluid resuscitation was independent predictor for IAH (OR, 1.88; 95% CI, 1.04– 3.42; p = 0.04) |
| Dalfino et al. [107] | 2008 | Observational | n = 123 | Cumulative fluid balance in ml
| Primary IAH: 27.1% Secondary IAH: 67.5% ACS: 5.4% | Acute renal failure: 19.7% in IAH vs. 8.1% in no IAH Age, cumulative fluid balance and shock are all independent predictive factors of IAH |
| Vidal et al. [113] | 2008 | Prospective | n = 83 | Intense fluid resuscitation, was significantly greater in patients with IAH and in non-survivors | 53 patients with IAH 30 patients with no IAH | IAH associated with organ failure and mortality |
| Reintam Blaser et al. [116] | 2011 | Observational | n = 563 | >5 L fluid resuscitation/24 h:
| No IAH: 381 patients IAH: 182 patients 33% mortality in ACS patients |
|
| Kim et al. [114] | 2012 | Observational | n = 100 52 medical, 37 surgical, 11 trauma | No correlation with 24-h fluid balance | 42% IAH, 4% ACS |
|
| Iyer et al. [111] | 2014 | Observational | n = 403 | IAH: 4.24 (2.54–5.56) L No IAH: 2.75 (1.75–4.05) L (p < 0.001) | 39% IAH 2% ACS | IV fluid > 2.3 L is an independent predictor of IAH |
| Malbrain et al. [115] | 2014 | Systematic review | n = 1669 | Not reported | Overall mean IAP:
|
|
| Dąbrowski et al. [5] | 2015 | Observational | n = 120 48 surgical 72 medical | Cut-off points for development of IAH:
| Not Reported | IAP strongly correlates with ECW |
| Murphy et al. [108] | 2018 | Observational | n = 285 | No IAH: 1135 (145–2685) mL IAH: 2019 (716–4.000) mL (p < 0.001) | 45% IAH, 3% ACS Mortality: 30% IAH vs. 11% no IAH | 24-h fluid balance > 3 L is an independent predictor for IAH |
| Reintam Blaser et al. [112] | 2019 | Observational | n = 491 | 48.9% IAH (46.3% primary vs. 53.7% secondary). IAH vs. no IAH: 5 L fluid resuscitation before ICU (p = 0.036) | 6.3% ACS | Positive fluid balance and BMI and PEEP ≥ 7 cmH2O associated with development of IAH |
| Author | Year | Population | Patients | Resuscitation Fluids | IAP (mmHg) | Intervention | Results |
|---|---|---|---|---|---|---|---|
| Boehm et al. [27] | 2019 | Burn | 38 ACS+ vs. control | Average FB/day: ACS vs. control 13.3 L ± 7.7 L vs. control 7.9 L ± 7.9 L (NS) | Not reported | Not reported | ↑ mortality rate of 84% in ACS+ vs. 32% in control (p = 0.00008) |
| Hershberger et al. [28] | 2007 | Burn | 25 ACS+ | Mean fluid infused 2102 mL/h before DL | Mean IAP 57 ± 4.2 | DL | 22 patients (88%) died |
| Hobsen et al. [29] | 2002 | Burn | n = 1014 10 ACS | 3.1 mL/kg/% TBSA for the first 12 h | Mean 40 ± 10 | DL | 40% of ACS patients survived |
| Markell et al. [30] | 2009 | Burn | n = 1825 ACS: 32 | 6.02 mL/kg/% TBSA | >30 | DL | 90% mortality for ACS |
| McBeth et al. [31] | 2014 | Burn | 110 | 48-h FB: 25.6 (± 11.1) L exceeding predicted Parkland formula estimates by 86% (± 32) | 12.1 (± 4.2) | 3 patients DL | 39 patients died |
| Park et al. [32] | 2012 | Burn | 159 | Pre-protocol 4.6 ± 2.3 mL/kg/% TBS. Post-protocol: 4.2 ± 1.7 mL/kg/% TBS, mean ± SD; p not significant | Pre-protocol:
| DL, n (%) Pre-protocol: 6 Post-protocol: 0 (p < 0.05) | Mortality, n (%)
|
| Britt et al. [38] | 2005 | Burn, trauma | 10 ACS | Mean volume in the first 24 h: 33 L (12.4–69) | Mean 44.6 | DL |
|
| Reed et al. [39] | 2006 | Trauma, burn, solid organ injury | 12 | 12 L of fluids or >500 mL/h for 4 consecutive hours | Average before and after catheter insertion 44.8 and 58.7 | 2 patients DL, 8 patients intra-abdominal catheters | 7 patients survived |
| Gracias et al. [54] | 2002 | Trauma | 5 ACS vs. 15 control | ACS: 37 L crystalloid vs. Control: 16.1 L crystalloid | >25 | Decompression | 60% in ACS vs. 7% in control |
| Balogh et al. [53] | 2003 | Trauma | 71 N vs. 85 SN | SN vs. LR infusion:
| SN vs. LR:
| Not reported | Mortality SN vs. LR: 27% vs. 11% (p < 0.05) |
| He et al. [55] | 2019 | Trauma | 455 pts (44 IAH; 5 ACS) | Volume of IV fluids over 24 h: 3.965 ± 739 mL | Mean IAP 24.4 ± 8.5 | DL |
|
| Hwabejire et al. [56] | 2016 | Trauma | n = 1976 of which 122 ACS | Total fluid/kg:
| Not reported | 98.4% DL | ACS+: 37.7% vs. ACS-: 14.6% (p < 0.001) Rise in ACS risk after total volume + 1302 mL/kg |
| Joseph et al. [57] | 2014 | Trauma | 799 |
| 18 patients ACS | DL in 18.9% |
|
| Macedo et al. [58] | 2016 | Trauma | 10 |
| Not reported | DL | 60% overall mortality |
| Shaheen et al. [62] | 2016 | Trauma | 28 | >10 U of RCC in 24 h | 60.7% developed ACS | Not reported | - 30-day mortality was 32.1% |
| Madigan et al. [59] | 2008 | Trauma | ACS (n = 48) vs. control (n = 48) | Net fluid for DC until 48 h post-admission was 18.2 L vs. 5.1 L (p < 0.0001) | Not reported | DL | Mortality 60% ACS vs. 2% controls (p < 0.0001) |
| Maxwell et al. [60] | 1999 | Trauma | 46 | Mean 19 ± 5 L crystalloid 29 ± 10 U RCC | Mean: 33± 3 | DL | 67% mortality |
| Rodas et al. [61] | 2005 | Trauma | 5 | Crystalloid: 15 ± 1.7 L Blood: 11 ± 0.4 U | NR | DL | No mortality |
| Strang et al. [75] | 2015 | Trauma | 567 509 no IAH 58 IAH | No IAH: 4.2 L Crystalloid vs. IAH: 6 L crystalloid; no IAH: 1.5 L colloids vs. IAH: 2.5 L colloids; no IAH: 2 U RCC vs. IAH: 17 U | 30 patients ACS | NR | IAH: 25.9% vs. 12.2% no IAH; p = 0.012). |
| Zaydfudim et al. [69] | 2010 | Trauma | 39 pre-TEP vs. 36 TEP | Pre-TEP: 12 U RCC vs. TEP: 12.5 U RCC Pre-TEP: 4 U FFP, vs. TEP: 8 U FFP; p < 0.01 Pre-TEP: 1 U platelets vs. TEP: 2 U platelets; p < 0.01 Pre-TEP: 6 L of crystalloids vs. TEP: 4 L crystalloids; p < 0.01 | 20% ACS in pre-TEP vs. 0% ACS in TEP | NR | pre-TEP cohort: 31% 30-day survival TEP cohort: 53% 30-day survival |
| Cothren et al. [106] | 2007 | Surgical & Medical patients | 54 patients | Total fluid resuscitation before DL:
| Medical: 33.5 ± 1.1 vs.
| DL | MOF:
|
| Cordemans et al. [78] | 2012 | ALI | 57 PAL vs. 57 control | Cumulative FB after 1 week 8.027 ± 5.254 mL/day vs. −1.451 ± 7.761 (p < 0.001) | IAP at baseline: PAL: 10 ± 4.2 Control: 8 ± 3.7 (p = 0.013) | PAL treatment |
|
| Pupelis et al. [44] | 2012 | Pancreatitis | 130 patients 75 CVVH 55 control | Not reported | CVVH: 19.6 ± 7.1 Control: 16.3 ± 5.5 p = 0.05 | DL n = 36 | 11.7% CVVH and 13.8% no CVVH NS |
| Struck et al. [79] | 2012 | TEN | 29 patients 5 ACS | + FB 4.6 ± 1.2 L | 33 ± 7 | DL | Mortality: ACS+ 100% vs. ACS- 0% |
| Aik-Yong et al. [105] | 2014 | Surgical & medical patients | 17 patients: 14 primary ACS 3 secondary ACS | >3.5 L in 24 h | DL | Overall mortality 47.1% | |
| McNelis et al. [99] | 2002 | Surgery | 22 ACS vs. 22 control | 24-h FB: ACS: 15.9 ± 10.3 L vs. Control: 7 ± 3.5 L (p < 0.05) | Not reported | Not reported | Mortality: 66.7% in ACS vs. none in control |
| Rubenstein et al. [89] | 2015 | rAAA open repair. 44 pts (60%) EVAR: 29 pts (40%) | 73 | Intraoperative fluid higher in EVAR patients ACS+ vs. ACS-
| ACS% 34% in open21% in EVARp not significant | DL | Overall mortality 42%:
|
| Leclerc et al. [98] | 2017 | rAAA | 47 | ACS+: 5.250 (4.625; 9.375) L ACS-: 4.125 (2.925; 5.500) L (p = 0.053) | 8 patients developed ACS | 30-day mortality in ACS+ higher (p = 0.108) | |
| Miranda et al. [88] | 2018 | rAAA | 25 |
| 12% (n = 3) developed ACS |
|
| Author | Year | Population | Resuscitation Fluids/Fluid Balance | IAP (mmHg) | Intervention | Results |
|---|---|---|---|---|---|---|
| Fietsam et al. [101] | 1989 | Surgery | >25 L of fluid | NR | DL | NR |
| Burrows et al. [63] | 1995 | Surgery | 21 L of crystalloid; 4 U RCC | NR | DL | Alive |
| Burrows et al. [63] | 1995 | Trauma | Pre-op: 7.3 mL/kg/h vs. Postop: 14.2 mL/kg/h | 39 | DL | NR |
| Burrows et al. [63] | 1995 | Trauma | Pre-op: 9.2 mL/kg/h vs. Postop: 5.5 mL/kg/h | 40 | DL | Died |
| Burrows et al. [63] | 1995 | Trauma | Pre-op: 14.7 mL/kg/h vs. Postop: 3.2 mL/kg/h | NR | DL | Alive |
| Ivy et al. [33] | 1999 | Burn | 32 L | 49 | DL | Died |
| Ivy et al. [33] | 1999 | Burn | 24 L | 50 | Escharotomy | Died |
| Ivy et al. [33] | 1999 | Burn | 32 L | 36 | None | Died |
| Kopelman et al. [65] | 2000 | Trauma | + FB: 25 L | 34 | DL | Died |
| Kopelman et al. [65] | 2000 | Trauma | 26 L of crystalloid | 25 | DL | Died |
| Kopelman et al. [65] | 2000 | Trauma | + FB: 29.5 L | 22 | DL | Died |
| Kopelman et al. [65] | 2000 | Trauma | + FB: 10 L | 26 | DL | Alive |
| Kopelman et al. [65] | 2000 | Trauma | + FB: 5 L | 46 | DL | Alive |
| Macalino et al. [77] | 2002 | Sepsis | 14 L crystalloids | 27 | NMB | Died |
| Kula et al. [72] | 2004 | Sepsis | 10 L + FB first 96 h. 4:1 (crystalloid: colloid) | >25 | DL CVVH | Died |
| Kula et al. [72] | 2004 | Sepsis | 12.5 L + FB first 96 h (crystalloids) | 29 | CVVH | Died |
| Shiiya et al. [103] | 2005 | Surgery | 34.1 L crystalloids vs. 13.7 L blood products | NR | DL | Alive |
| Parra et al. [34] | 2006 | Burn/Trauma | 25.55 L of crystalloid 12 U RCC | 34 | DL | Alive |
| De Wolf et al. [100] | 2008 | Surgery | Massive fluid resuscitation | 24 in 1st patient 27 in 2nd patient | DL | Alive |
| Tsuang et al. [76] | 2007 | Sepsis | 17 L fluid during first 20 h | 54 | DL | Alive |
| Chamisa et al. [64] | 2008 | Trauma | Not reported | >35 | DL | Died |
| Kula et al. [73] | 2008 | Trauma | 7.5 L + FB first 48 h. 4:1 (crystalloid: colloid) | 26 | CVVH | NR |
| Kula et al. [73] | 2008 | Trauma | 17 L + FB first 96 h. 3:1 (crystalloid: colloid) | 28 | CVVH | NR |
| Augustin et al. [90] | 2010 | Surgery | 16 L + FB | 19 | DL | Died |
| Augustin et al. [90] | 2010 | Surgery | 23 L + FB | 35 | None | Died |
| Rabbi et al. [102] | 2012 | Surgery | Not reported | 50 | DL | Alive |
| Park et al. [46] | 2014 | SAP | Not reported | 31 | PCD | Alive |
| Bressan et al. [91] | 2016 | Surgery | 4 L crystalloids 2 RCC during first 24 h | 21 | DL | Alive |
| Michel et al. [66] | 2016 | Trauma | 10.5 L (crystalloids, colloids & blood products) | NR | DL | Alive |
| Lee et al. [45] | 2019 | SAP | 6 L | 28 | DL | Alive |
| Author | Year | Type of Study | Population | Resuscitation Fluids | IAP (mmHg) | Intervention | Results |
|---|---|---|---|---|---|---|---|
| Divarci et al. [81] | 2016 | Prospective | Sepsis | NR | 14 patients with IAH (13–15) 6 patients ACS (17–24) | Decompressive measures DL | 1 Dead |
| Ranjit et al. [84] | 2018 | Prospective | Sepsis | ST group (n = 30): 17.8 (10.8–25.2) L TI group (n = 38): 10.02 (5.7–18.2) L (p = 0.009) | NR | Percutaneous drainage of ACS, n (%) ST group: 9 (30) TI group: 3 (7.9) (p = 0.01) | Mortality: ST: 8 (26%) TI: 1 (2.6%) p = 0.008 |
| DeCou et al. [70] | 2000 | Case report | Trauma | Crystalloids and 16 U RCC and 4 U FFP | NR | Silo decompression | Alive |
| DeCou et al. [70] | 2000 | Case report | Trauma | Replacement of 2 x blood volume | NR | Silo decompression | Alive |
| DeCou et al. [70] | 2000 | Case report | Sepsis | NR | 26 | Silo decompression | Alive |
| Perks et al. [68] | 2005 | Case report | Trauma | NR | NR | Surgical decompression | Alive |
| Jensen et al. [37] | 2006 | Case report | Burn | 5990 mL crystalloids | >22 | DL | Dead |
| Jensen et al. [37] | 2006 | Case report | Burn | 8580 mL crystalloids + 990 mL blood products + 805 mL albumin | NR | Abdominal wall escharotomy and NMB and peritoneal dialysis catheter | Alive |
| Jensen et al. [37] | 2006 | Case report | Burn | 10300 mL crystalloids | 44 | Surgical decompression | Dead |
| Jensen et al. [37] | 2006 | Case report | Trauma | 1950 mL crystalloids | 26 | Silo decompression | Alive |
| Morell et al. [67] | 2007 | Case report | Trauma | 10000 mL crystalloids and 10 U RCC | NR | Laparotomy | Alive |
| Lam et al. [83] | 2008 | Case report | Sepsis | 272 mL/kg | 35 | Paracentesis | Died |
| Lam et al. [83] | 2008 | Case report | Sepsis | 220 mL/kg | NR | DL | Died |
| Lam et al. [83] | 2008 | Case report | Reanimated after drowning | 334 mL/kg | NR | DL | Died |
| Lam et al. [83] | 2008 | Case report | Sepsis | 500 mL/kg | 120 | None | Died |
| Lam et al. [83] | 2008 | Case report | Sepsis | NR | NR | Peritoneal catheter | Alive |
| Dauplaise et al. [80] | 2010 | Case report | Sepsis | 70 mL/kg in first h and 330 mL/kg in first 24 h | 43 | DL | Alive |
| Gala et al. [82] | 2012 | Case report | Sepsis | NR | NR | Paracentesis | Alive |
| Streit et al. [35] | 2013 | Case report | Burn | NR | 27 | Decompression | Alive |
| Sun et al. [36] | 2015 | Case report | Burn | 5600 mL LR during first 24 h | 22 | NMB, diuresis; percutaneous drain | Alive |
| Kobayashi et al. [71] | 2016 | Case report | Trauma | 560 mL RCC. 960 mL FFP. 400 mL platelets and fluids | NR | Laparotomy | Alive |
3.2. Animal data
| Author | Year | Population | Intervention | Results |
|---|---|---|---|---|
| Schachtrupp et al. [119] | 2005 | 12 Pigs:
| Fluid intake: Intervention group vs. control (p < 0.01) 10570 ± 1928 mL vs. 3918 ± 1042 mL | Acidosis, liver, bowel, kidney and lung damage higher in intervention group (p < 0.01) |
| Moore-Olufemi et al. [117] | 2005 | 44 Rats Experiment 1: 20 mL/kg saline Experiment 2: 80 mL/kg saline In each experiment 4 groups
| A mesenteric venous hypertension/gut edema model was created to evaluate whether gut edema caused by acute mesenteric venous hypertension and/or crystalloid resuscitation is associated with impaired intestinal transit, mucosal barrier dysfunction, and/or injury | Delayed intestinal transit, increased permeability, and decreased epithelial resistance are associated with gut edema |
| Chang et al. [118] | 2016 | 48 rats:
| Induced portal hypertension, hemorrhage to a MAP of 40 mmHg for 2 h (except for sham group) Collected blood reinfused and treatment with:
| Melatonin use associated with less inflammatory and oxidative injury, less intestinal permeability and injury, lower incidence of secondary IAH |
4. Discussion
4.1. Type of Patients
4.2. Type of Resuscitation Fluids
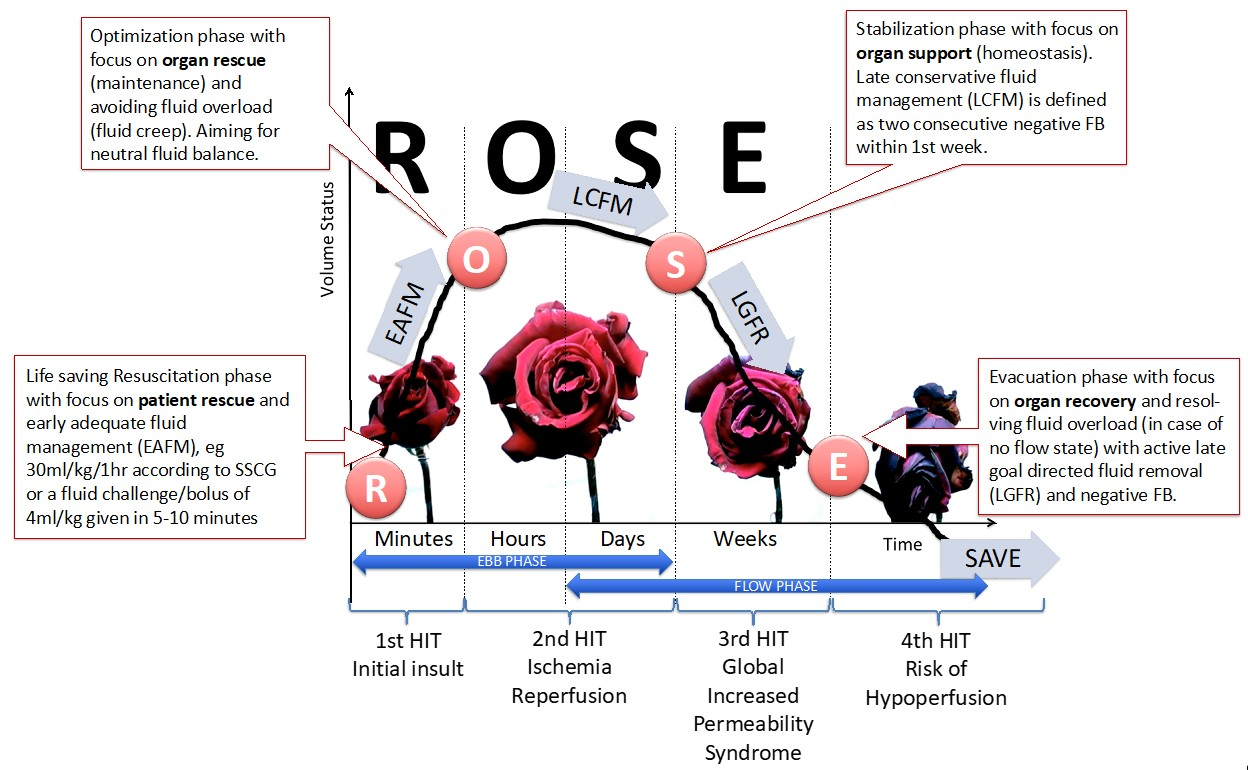
4.3. Fluid Resuscitation Strategies
4.4. Interventions with Potential Beneficial Effects That Need Further Investigation
4.5. Limitations
- There is a relationship between fluid resuscitation, fluid accumulation, and secondary IAH. This signal, from the limited number of RCTs, needs further confirmation.
- Crystalloids are associated with a more positive fluid balance and a greater likelihood of developing IAH compared to colloids or hypertonic solutions.
- Fluid resuscitation in IAH may preserve cardiac output, however, it does not prevent organ damage.
- Delivery of blood products in a 3:2 ratio of RCC: FFP (red blood cells: fresh frozen plasma) and 5:1 for RCC: platelets, may reduce MOF and infectious complications, and increase ventilator-free days [63].
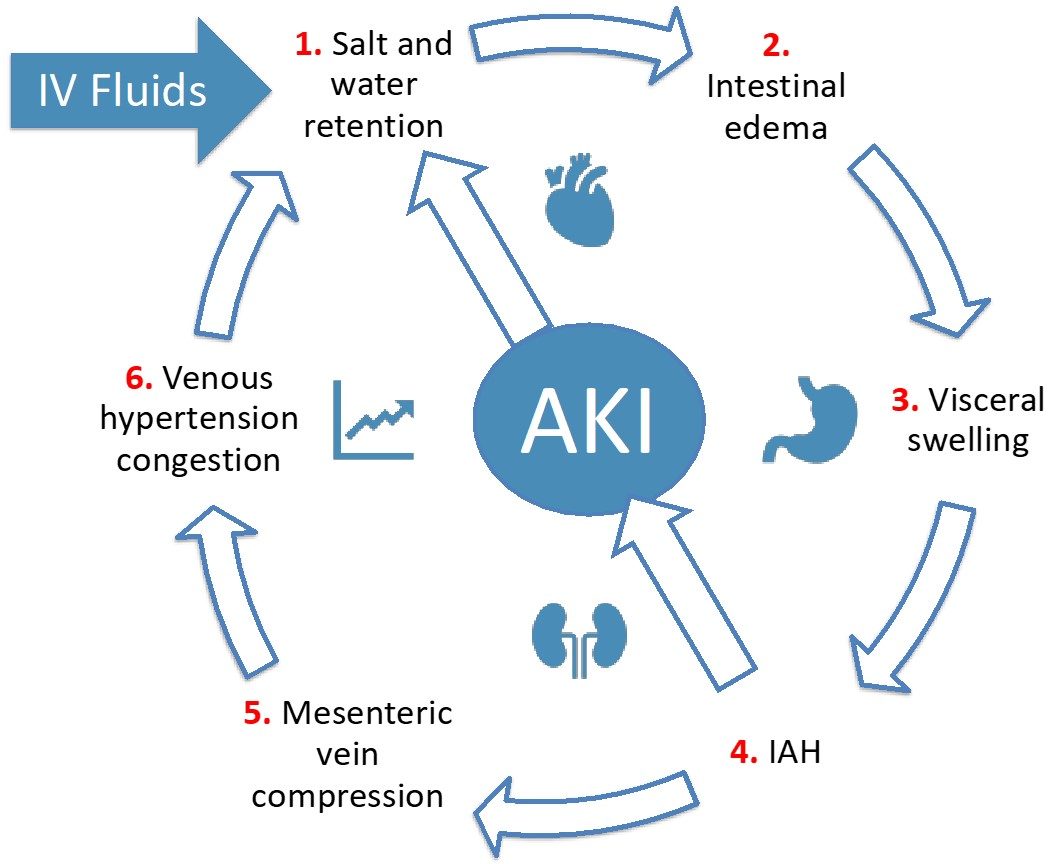
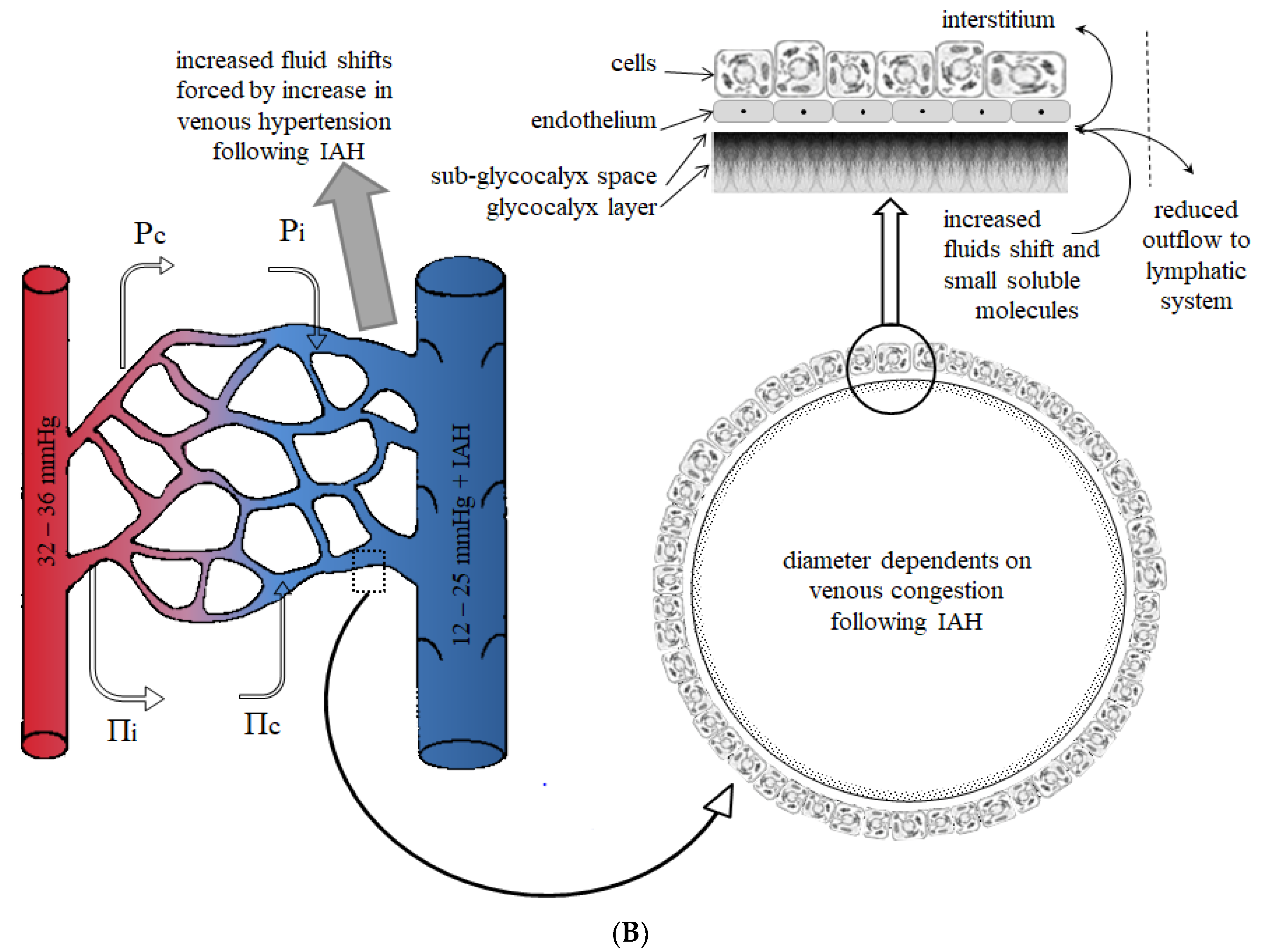
- Fluid resuscitation leads to IAH and venous congestion (or venous hypertension), contributing to gut edema and diminished gut contractility.
- The relationship between fluid resuscitation, fluid accumulation, and secondary IAH holds in the setting of sepsis (capillary leak), severe burn injury, emergency surgery, and trauma with the presence of the deadly triad (coagulopathy, acidosis, hypothermia).
- Fluid removal with diuretics or CVVH may restore cumulative fluid balance and may reduce IAP. The time to initiate RRT in this setting remains unclear.
- Bladder pressure measurements should be performed after infusion of more than 25 mL during the acute resuscitation phase, and one should check for peak inspiratory pressures greater than 40 cm H2O.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Ethics Approval and Consent to Participate
Abbreviations
| ACS | abdominal compartment syndrome |
| ADH | anti-diuretic hormone |
| ALI | acute lung injury |
| APP | abdominal perfusion pressure |
| ARDS | acute respiratory distress syndrome |
| BMI | body mass index |
| BMT | bone marrow transplantation |
| BRG | burn resuscitation guidelines |
| CLFM | conservative late fluid management |
| CLI | capillary leak index |
| CO | cardiac output |
| CPB | cardiopulmonary bypass |
| CR | case report |
| CVVH | continuous veno-venous hemofiltration |
| CVP | central venous pressure |
| d | day |
| DL | damage control laparotomy |
| ECMO | extra-corporeal membrane oxygenation |
| ECW | extracellular body water |
| EGL | endothelial glycocalyx layer |
| eOR | emergency open repair |
| EVAR | endovascular aortic repair |
| EVLWI | extravascular lung water index |
| eEVR | emergency endovascular repair |
| FOAM | free open access medical education |
| FB | fluid balance |
| FFP | fresh frozen plasma |
| HES | hydroxyethyl starch |
| HLS | hypertonic lactated saline |
| IAP | intra-abdominal pressure |
| IAH | intra-abdominal hypertension |
| ICP | intra-cranial pressure |
| ICU | intensive care unit |
| ITP | intra-thoracic pressure |
| IV | intra-venous |
| L | liters |
| LR | ringer’s lactate solution |
| LOS | length of stay |
| M | medical |
| MAP | mean arterial pressure |
| MOF | multiple organ failure |
| MV | mechanical ventilation |
| NMB | neuromuscular blocker |
| NGT | nasogastric tube |
| NR | not reported |
| NS | 0.9% saline |
| OF | organ failure |
| PAL | positive end-expiratory pressure, albumin, and Lasix® (furosemide) |
| PCD | percutaneous catheter drainage |
| PCS | poly-compartment syndrome |
| PEEP | positive end-expiratory pressure |
| POCUS | point-of-care ultrasound |
| pts | patients |
| rAAAs | ruptured abdominal aortic aneurysms |
| RCC | red cell concentrate |
| RF | risk factor |
| S | surgical |
| SAP | severe acute pancreatitis |
| SAPS II | Simplified Acute Physiology Score II |
| SH group | combination of 0.9% saline and hydroxyethyl starch (HES) |
| SHG group | combination of 0.9% saline, hydroxyethyl starch and glutamine |
| SHINE | shock induced endotheliopathy |
| SN | supranormal resuscitation group |
| SOFA | Sequential Organ Failure Assessment Score |
| ST group | standard therapy |
| TBSA | total body surface area |
| TEP | trauma exsanguination protocol |
| TI group | targeted intervention |
| U | units |
| UO | urine output |
| WSACS | The Abdominal Compartment Society |
References
- De Keulenaer, B.L.; Regli, A.; Dabrowski, W.; Kaloiani, V.; Bodnar, Z.; Cea, J.I.; Litvin, A.A.; Davis, W.A.; Palermo, A.M.; De Waele, J.J.; et al. Does femoral venous pressure measurement correlate well with intrabladder pressure measurement? A multicenter observational trial. Intensive Care Med. 2011, 37, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Kotlinska-Hasiec, E.; Dabrowski, W.; Rzecki, Z.; Rybojad, B.; Pilat, J.; De Keulenaer, B.; Lng Malbrain, M. Association between intra-abdominal pressure and jugular bulb saturation in critically ill pa-tients. Minerva Anestesiol. 2014, 80, 785–795. [Google Scholar] [PubMed]
- Druml, W. [Intestinal cross-talk: The gut as motor of multiple organ failure]. Med. Klin. Intensivmed. Notfmed. 2018, 113, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Holte, K.; Sharrock, N.E.; Kehlet, H. Pathophysiology and clinical implications of perioperative fluid excess. Br. J. Anaesth. 2002, 89, 622–632. [Google Scholar] [CrossRef]
- Dabrowski, W.; Kotlinska-Hasiec, E.; Jaroszynski, A.; Zadora, P.; Pilat, J.; Rzecki, Z.; Zaluska, W.; Schneditz, D. Intra-abdominal pressure correlates with extracellular water content. PLoS ONE 2015, 10, e0122193. [Google Scholar] [CrossRef]
- Reintam, A.; Parm, P.; Kitus, R.; Kern, H.; Starkopf, J. Primary and secondary intra-abdominal hypertension--different impact on ICU outcome. Intensive Care Med. 2008, 34, 1624–1631. [Google Scholar] [CrossRef]
- Holodinsky, J.K.; Roberts, D.J.; Ball, C.G.; Blaser, A.R.; Starkopf, J.; Zygun, D.A.; Stelfox, H.T.; Malbrain, M.L.; Jaeschke, R.C.; Kirkpatrick, A.W. Risk factors for intra-abdominal hypertension and abdominal compartment syndrome among adult intensive care unit patients: A systematic review and meta-analysis. Crit. Care 2013, 17, R249. [Google Scholar] [CrossRef]
- Scalea, T.M.; Bochicchio, G.V.; Habashi, N.; McCunn, M.; Shih, D.; McQuillan, K.; Aarabi, B. Increased intra-abdominal, intrathoracic, and intracranial pressure after severe brain injury: Multiple compartment syndrome. J. Trauma 2007, 62, 647–656; discussion 656. [Google Scholar] [CrossRef]
- De Waele, J.J.; Malbrain, M.L.; Kirkpatrick, A.W. The abdominal compartment syndrome: Evolving concepts and future directions. Crit Care 2015, 19, 211. [Google Scholar] [CrossRef]
- Malbrain, M.; De Laet, I.; De Waele, J. The polycompartment syndrome: What’s all the fuss about? Yearbook of Intensive Care and Emergency Medicine, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 465–484. [Google Scholar]
- Bodnar, Z. Polycompartment syndrome—intra-abdominal pressure measurement. Anaesthesiol. Intensive Ther. 2019, 51, 316–322. [Google Scholar] [CrossRef]
- Malbrain, M.L.; Roberts, D.J.; Sugrue, M.; De Keulenaer, B.L.; Ivatury, R.; Pelosi, P.; Verbrugge, F.; Wise, R.; Mullens, W. The polycompartment syndrome: A concise state-of-the-art review. Anaesthesiol. Intensive Ther. 2014, 46, 433–450. [Google Scholar] [CrossRef]
- Malbrain, M.L.; Wilmer, A. The polycompartment syndrome: Towards an understanding of the interactions between different compartments! Intensive Care Med. 2007, 33, 1869–1872. [Google Scholar] [CrossRef]
- Malbrain, M. Respiratory effects of increased intra-abdominal pressure. Réanimation 2007, 16, 49–60. [Google Scholar] [CrossRef]
- Armanious, M.; Bacon, L.N.; Harris, J.; George, S.; Goulbourne, K.K.; Danner, O.; Matthews, L.R.; Wilson, K.L. Decompressive laparotomy for reduction of incessant increased intracranial pressure in the absence of abdominal compartment syndrome: A case report. Int. J. Case Rep. Images 2013, 4, 419–422. [Google Scholar] [CrossRef]
- Mackay, E.J.; Nunn, A.M.; Cannon, J.W.; Martin, N.D. Secondary extremity compartment syndrome after traumatic cardiac arrest. Trauma 2016, 2016, 4. [Google Scholar] [CrossRef]
- Malbrain, M.; Van Regenmortel, N.; Saugel, B.; De Tavernier, B.; Van Gaal, P.J.; Joannes-Boyau, O.; Teboul, J.L.; Rice, T.W.; Mythen, M.; Monnet, X. Principles of fluid management and stewardship in septic shock: It is time to consider the four D’s and the four phases of fluid therapy. Ann. Intensive Care 2018, 8, 66. [Google Scholar] [CrossRef]
- Ennis, J.L.; Chung, K.K.; Renz, E.M.; Barillo, D.J.; Albrecht, M.C.; Jones, J.A.; Blackbourne, L.H.; Cancio, L.C.; Eastridge, B.J.; Flaherty, S.F.; et al. Joint Theater Trauma System implementation of burn resuscitation guidelines improves outcomes in severely burned military casualties. J. Trauma 2008, 64, S146–S151. discussion S142–S151. [Google Scholar] [CrossRef]
- Ivy, M.E.; Atweh, N.A.; Palmer, J.; Possenti, P.P.; Pineau, M.; D’Aiuto, M. Intra-abdominal hypertension and abdominal compartment syndrome in burn patients. J. Trauma 2000, 49, 387–391. [Google Scholar] [CrossRef]
- Mbiine, R.; Alenyo, R.; Kobusingye, O.; Kuteesa, J.; Nakanwagi, C.; Lekuya, H.M.; Kituuka, O.; Galukande, M. Intra-abdominal hypertension in severe burns: Prevalence, incidence and mortality in a sub-Saharan African hospital. Int. J. Burns Trauma 2017, 7, 80–87. [Google Scholar]
- Oda, J.; Ueyama, M.; Yamashita, K.; Inoue, T.; Noborio, M.; Ode, Y.; Aoki, Y.; Sugimoto, H. Hypertonic lactated saline resuscitation reduces the risk of abdominal compartment syndrome in severely burned patients. J. Trauma 2006, 60, 64–71. [Google Scholar] [CrossRef]
- Oda, J.; Yamashita, K.; Inoue, T.; Harunari, N.; Ode, Y.; Mega, K.; Aoki, Y.; Noborio, M.; Ueyama, M. Resuscitation fluid volume and abdominal compartment syndrome in patients with major burns. Burns 2006, 32, 151–154. [Google Scholar] [CrossRef] [PubMed]
- O’Mara, M.S.; Slater, H.; Goldfarb, I.W.; Caushaj, P.F. A prospective, randomized evaluation of intra-abdominal pressures with crystalloid and colloid resuscitation in burn patients. J. Trauma 2005, 58, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Castilla, M.; Barret, J.P.; Sanz, D.; Aguilera, J.; Serracanta, J.; Garcia, V.; Collado, J.M. Analysis of intra-abdominal hypertension in severe burned patients: The Vall d’Hebron experience. Burns 2014, 40, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Talizin, T.B.; Tsuda, M.S.; Tanita, M.T.; Kauss, I.A.M.; Festti, J.; Carrilho, C.; Grion, C.M.C.; Cardoso, L.T.Q. Acute kidney injury and intra-abdominal hypertension in burn patients in intensive care. Rev. Bras. Ter. Intensiva. 2018, 30, 15–20. [Google Scholar] [CrossRef]
- Wise, R.; Jacobs, J.; Pilate, S.; Jacobs, A.; Peeters, Y.; Vandervelden, S.; Van Regenmortel, N.; De Laet, I.; Schoonheydt, K.; Dits, H.; et al. Incidence and prognosis of intra-abdominal hypertension and abdominal compartment syndrome in severely burned patients: Pilot study and review of the literature. Anaesthesiol. Intensive Ther. 2016, 48, 95–109. [Google Scholar] [CrossRef]
- Boehm, D.; Schroder, C.; Arras, D.; Siemers, F.; Siafliakis, A.; Lehnhardt, M.; Dadras, M.; Hartmann, B.; Kuepper, S.; Czaja, K.U.; et al. Fluid Management as a Risk Factor for Intra-abdominal Compartment Syndrome in Burn Patients: A Total Body Surface Area-Independent Multicenter Trial Part I. J. Burn Care Res. 2019, 40, 500–506. [Google Scholar] [CrossRef]
- Hershberger, R.C.; Hunt, J.L.; Arnoldo, B.D.; Purdue, G.F. Abdominal compartment syndrome in the severely burned patient. J. Burn Care Res. 2007, 28, 708–714. [Google Scholar] [CrossRef]
- Hobson, K.G.; Young, K.M.; Ciraulo, A.; Palmieri, T.L.; Greenhalgh, D.G. Release of abdominal compartment syndrome improves survival in patients with burn injury. J. Trauma 2002, 53, 1129–1133; discussion 1133–1124. [Google Scholar] [CrossRef]
- Markell, K.W.; Renz, E.M.; White, C.E.; Albrecht, M.E.; Blackbourne, L.H.; Park, M.S.; Barillo, D.A.; Chung, K.K.; Kozar, R.A.; Minei, J.P.; et al. Abdominal complications after severe burns. J. Am. Coll. Surg. 2009, 208, 940–947; discussion 947–949. [Google Scholar] [CrossRef]
- McBeth, P.B.; Sass, K.; Nickerson, D.; Ball, C.G.; Kirkpatrick, A.W. A necessary evil? Intra-abdominal hypertension complicating burn patient resuscitation. J. Trauma Manag. Outcomes 2014, 8, 12. [Google Scholar] [CrossRef]
- Park, S.H.; Hemmila, M.R.; Wahl, W.L. Early albumin use improves mortality in difficult to resuscitate burn patients. J. Trauma Acute Care Surg. 2012, 73, 1294–1297. [Google Scholar] [CrossRef]
- Ivy, M.E.; Possenti, P.P.; Kepros, J.; Atweh, N.A.; D’Aiuto, M.; Palmer, J.; Pineau, M.; Burns, G.A.; Caushaj, P.F. Abdominal compartment syndrome in patients with burns. J. Burn. Care Rehabil. 1999, 20, 351–353. [Google Scholar] [CrossRef]
- Parra, M.W.; Al-Khayat, H.; Smith, H.G.; Cheatham, M.L. Paracentesis for resuscitation-induced abdominal compartment syndrome: An alternative to decompressive laparotomy in the burn patient. J. Trauma 2006, 60, 1119–1121. [Google Scholar] [CrossRef]
- Streit, S.; Hebra, A. Abdominal compartment syndrome in a three year old child following a severe burn injury. J. Pediatric Surg. Case Rep. 2013, 1, 177–179. [Google Scholar] [CrossRef]
- Sun, K.; Hancock, B.J.; Logsetty, S. Ischemic bowel as a late sequela of abdominal compartment syndrome secondary to severe burn injury. Plast. Surg. 2015, 23, 218–220. [Google Scholar] [CrossRef]
- Jensen, A.R.; Hughes, W.B.; Grewal, H. Secondary abdominal compartment syndrome in children with burns and trauma: A potentially lethal complication. J. Burn Care Res. 2006, 27, 242–246. [Google Scholar] [CrossRef]
- Britt, R.C.; Gannon, T.; Collins, J.N.; Cole, F.J.; Weireter, L.J.; Britt, L.D. Secondary abdominal compartment syndrome: Risk factors and outcomes. Am. Surg. 2005, 71, 982–985. [Google Scholar] [CrossRef]
- Reed, S.F.; Britt, R.C.; Collins, J.; Weireter, L.; Cole, F.; Britt, L.D. Aggressive surveillance and early catheter-directed therapy in the management of intra-abdominal hypertension. J. Trauma 2006, 61, 1359–1363; discussion 1363–1355. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, J.G.; Wu, H.S.; Tao, J.; Qin, Q.; Deng, S.C.; Liu, Y.; Liu, L.; Wang, B.; Tian, K.; et al. Effects of different resuscitation fluid on severe acute pancreatitis. World J. Gastroenterol. 2013, 19, 2044–2052. [Google Scholar] [CrossRef]
- Du, X.J.; Hu, W.M.; Xia, Q.; Huang, Z.W.; Chen, G.Y.; Jin, X.D.; Xue, P.; Lu, H.M.; Ke, N.W.; Zhang, Z.D.; et al. Hydroxyethyl starch resuscitation reduces the risk of intra-abdominal hypertension in severe acute pancreatitis. Pancreas 2011, 40, 1220–1225. [Google Scholar] [CrossRef]
- Ke, L.; Ni, H.B.; Sun, J.K.; Tong, Z.H.; Li, W.Q.; Li, N.; Li, J.S. Risk factors and outcome of intra-abdominal hypertension in patients with severe acute pancreatitis. World J. Surg. 2012, 36, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Mao, E.Q.; Tang, Y.Q.; Fei, J.; Qin, S.; Wu, J.; Li, L.; Min, D.; Zhang, S.D. Fluid therapy for severe acute pancreatitis in acute response stage. Chin. Med. J. 2009, 122, 169–173. [Google Scholar] [PubMed]
- Pupelis, G.; Plaudis, H.; Zeiza, K.; Drozdova, N.; Mukans, M.; Kazaka, I. Early continuous veno-venous haemofiltration in the management of severe acute pancreatitis complicated with intra-abdominal hypertension: Retrospective review of 10 years’ experience. Ann. Intensive Care 2012, 2 (Suppl. S1), S21. [Google Scholar] [CrossRef]
- Lee, A.H.H.; Lee, W.S.; Anderson, D. Severe pancreatitis complicated by abdominal compartment syndrome managed with decompressive laparotomy: A case report. BMC Surg. 2019, 19, 113. [Google Scholar] [CrossRef]
- Park, S.; Lee, S.; Lee, H.D.; Kim, M.; Kim, K.; Jeong, Y.; Park, S.M. Abdominal compartment syndrome in severe acute pancreatitis treated with percutaneous catheter drainage. Clin. Endosc. 2014, 47, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Balogh, Z.; McKinley, B.A.; Cocanour, C.S.; Kozar, R.A.; Holcomb, J.B.; Ware, D.N.; Moore, F.A. Secondary abdominal compartment syndrome is an elusive early complication of traumatic shock resuscitation. Am. J. Surg. 2002, 184, 538–543; discussion 543–534. [Google Scholar] [CrossRef]
- Balogh, Z.; McKinley, B.A.; Holcomb, J.B.; Miller, C.C.; Cocanour, C.S.; Kozar, R.A.; Valdivia, A.; Ware, D.N.; Moore, F.A. Both primary and secondary abdominal compartment syndrome can be predicted early and are harbingers of multiple organ failure. J. Trauma 2003, 54, 848–859; discussion 859–861. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, I.; Mahmood, S.; Parchani, A.; Kumar, S.; El-Menyar, A.; Zarour, A.; Al-Thani, H.; Latifi, R. Intra-abdominal hypertension in the current era of modern trauma resuscitation. ANZ J. Surg. 2014, 84, 166–171. [Google Scholar] [CrossRef]
- Neal, M.D.; Hoffman, M.K.; Cuschieri, J.; Minei, J.P.; Maier, R.V.; Harbrecht, B.G.; Billiar, T.R.; Peitzman, A.B.; Moore, E.E.; Cohen, M.J.; et al. Crystalloid to packed red blood cell transfusion ratio in the massively transfused patient: When a little goes a long way. J. Trauma Acute Care Surg. 2012, 72, 892–898. [Google Scholar] [CrossRef]
- Raeburn, C.D.; Moore, E.E.; Biffl, W.L.; Johnson, J.L.; Meldrum, D.R.; Offner, P.J.; Franciose, R.J.; Burch, J.M. The abdominal compartment syndrome is a morbid complication of postinjury damage control surgery. Am. J. Surg. 2001, 182, 542–546. [Google Scholar] [CrossRef]
- Vatankhah, S.; Sheikhi, R.A.; Heidari, M.; Moradimajd, P. The relationship between fluid resuscitation and intra-abdominal hypertension in patients with blunt abdominal trauma. Int. J. Crit. Illn. Inj. Sci. 2018, 8, 149–153. [Google Scholar] [CrossRef]
- Balogh, Z.; McKinley, B.A.; Cocanour, C.S.; Kozar, R.A.; Valdivia, A.; Sailors, R.M.; Moore, F.A. Supranormal trauma resuscitation causes more cases of abdominal compartment syndrome. Arch. Surg. 2003, 138, 637–642; discussion 642–633. [Google Scholar] [CrossRef]
- Gracias, V.H.; Braslow, B.; Johnson, J.; Pryor, J.; Gupta, R.; Reilly, P.; Schwab, C.W. Abdominal compartment syndrome in the open abdomen. Arch. Surg. 2002, 137, 1298–1300. [Google Scholar] [CrossRef]
- He, L.; Yi, C.; Hou, Z.; Hak, D.J. Intraabdominal hypertension/abdominal compartment syndrome after pelvic fractures: How they occur and what can be done? Injury 2019, 50, 919–925. [Google Scholar] [CrossRef]
- Hwabejire, J.O.; Nembhard, C.E.; Oyetunji, T.A.; Seyoum, T.; Siram, S.M.; Cornwell, E.E., 3rd; Greene, W.R. Abdominal compartment syndrome in traumatic hemorrhagic shock: Is there a fluid resuscitation inflection point associated with increased risk? Am. J. Surg. 2016, 211, 733–738. [Google Scholar] [CrossRef]
- Joseph, B.; Zangbar, B.; Pandit, V.; Vercruysse, G.; Aziz, H.; Kulvatunyou, N.; Wynne, J.; O’Keeffe, T.; Tang, A.; Friese, R.S.; et al. The conjoint effect of reduced crystalloid administration and decreased damage-control laparotomy use in the development of abdominal compartment syndrome. J. Trauma Acute Care Surg. 2014, 76, 457–461. [Google Scholar] [CrossRef]
- Macedo, F.I.; Sciarretta, J.D.; Otero, C.A.; Ruiz, G.; Ebler, D.J.; Pizano, L.R.; Namias, N. Secondary abdominal compartment syndrome after complicated traumatic lower extremity vascular injuries. Eur. J. Trauma Emerg. Surg. 2016, 42, 207–211. [Google Scholar] [CrossRef]
- Madigan, M.C.; Kemp, C.D.; Johnson, J.C.; Cotton, B.A. Secondary abdominal compartment syndrome after severe extremity injury: Are early, aggressive fluid resuscitation strategies to blame? J. Trauma 2008, 64, 280–285. [Google Scholar] [CrossRef]
- Maxwell, R.A.; Fabian, T.C.; Croce, M.A.; Davis, K.A. Secondary abdominal compartment syndrome: An underappreciated manifestation of severe hemorrhagic shock. J. Trauma 1999, 47, 995–999. [Google Scholar] [CrossRef]
- Rodas, E.B.; Malhotra, A.K.; Chhitwal, R.; Aboutanos, M.B.; Duane, T.M.; Ivatury, R.R. Hyperacute abdominal compartment syndrome: An unrecognized complication of massive intraoperative resuscitation for extra-abdominal injuries. Am. Surg. 2005, 71, 977–981. [Google Scholar] [CrossRef]
- Shaheen, A.W.; Crandall, M.L.; Nicolson, N.G.; Smith-Singares, E.; Merlotti, G.J.; Jalundhwala, Y.; Issa, N.M. Abdominal compartment syndrome in trauma patients: New insights for predicting outcomes. J. Emerg. Trauma Shock 2016, 9, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Burrows, R.; Edington, J.; Robbs, J.V. A wolf in wolf’s clothing--the abdominal compartment syndrome. S. Afr. Med. J. 1995, 85, 46–48. [Google Scholar] [PubMed]
- Chamisa, I. Secondary Abdominal Compartment Syndrome in a Patientwith Isolated Extraperitoneal Injuries. Eur. J. Trauma Emerg. Surg. 2008, 34, 313–314. [Google Scholar] [CrossRef] [PubMed]
- Kopelman, T.; Harris, C.; Miller, R.; Arrillaga, A. Abdominal compartment syndrome in patients with isolated extraperitoneal injuries. J. Trauma 2000, 49, 744–747; discussion 747–749. [Google Scholar] [CrossRef]
- Michel, P.; Wahnert, D.; Freistuhler, M.; Laukoetter, M.G.; Rehberg, S.; Raschke, M.J.; Garcia, P. Acute transfusion-related abdominal injury in trauma patients: A case report. J. Med. Case Rep. 2016, 10, 294. [Google Scholar] [CrossRef][Green Version]
- Morrell, B.J.; Vinden, C.; Singh, R.N.; Kornecki, A.; Fraser, D.D. Secondary abdominal compartment syndrome in a case of pediatric trauma shock resuscitation. Pediatr. Crit. Care Med. 2007, 8, 67–70. [Google Scholar] [CrossRef]
- Perks, D.H.; Grewal, H. Abdominal compartment syndrome in the pediatric patient with blunt trauma. J. Trauma Nurs. 2005, 12, 50–54. [Google Scholar] [CrossRef]
- Zaydfudim, V.; Dutton, W.D.; Feurer, I.D.; Au, B.K.; Pinson, C.W.; Cotton, B.A. Exsanguination protocol improves survival after major hepatic trauma. Injury 2010, 41, 30–34. [Google Scholar] [CrossRef]
- DeCou, J.M.; Abrams, R.S.; Miller, R.S.; Gauderer, M.W. Abdominal compartment syndrome in children: Experience with three cases. J. Pediatric Surg. 2000, 35, 840–842. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kubota, M.; Arai, Y.; Ohyama, T.; Yokota, N.; Miura, K.; Ishikawa, H.; Soma, D.; Takizawa, K.; Sakata, J.; et al. Staged laparotomies based on the damage control principle to treat hemodynamically unstable grade IV blunt hepatic injury in an eight-year-old girl. Surg. Case Rep. 2016, 2, 134. [Google Scholar] [CrossRef][Green Version]
- Kula, R.; Szturz, P.; Sklienka, P.; Neiser, J.; Jahoda, J. A role for negative fluid balance in septic patients with abdominal compartment syndrome? Intensive Care Med. 2004, 30, 2138–2139. [Google Scholar] [CrossRef]
- Kula, R.; Szturz, P.; Sklienka, P.; Neiser, J. Negative fluid balance in patients with abdominal compartment syndrome--case reports. Acta Chir. Belg. 2008, 108, 346–349. [Google Scholar] [CrossRef]
- Cotton, B.A.; Au, B.K.; Nunez, T.C.; Gunter, O.L.; Robertson, A.M.; Young, P.P. Predefined massive trans-fusion protocols are associated with a reduction in organ failure and postinjury complications. J. Trauma 2009, 66, 41–48; discussion 48–49. [Google Scholar]
- Strang, S.; Van Imhoff, D.; Van Lieshout, E.; D’Amours, S.; Van Waes, O. Identifying patients at risk for high-grade intra-abdominal hypertension following trauma laparotomy. Injury 2015, 46, 843–848. [Google Scholar] [CrossRef]
- Tsuang, W.; Pohlman, M.; Hall, J. A 25-year-old woman with acute pancreatitis and hypotension refractory to aggressive fluid resuscitation. Diagnosis: Abdominal compartment syndrome. Chest 2007, 132, 1702–1705. [Google Scholar] [CrossRef]
- Macalino, J.U.; Goldman, R.K.; Mayberry, J.C. Medical management of abdominal compartment syndrome: Case report and a caution. Asian J. Surg. 2002, 25, 244–246. [Google Scholar] [CrossRef]
- Cordemans, C.; De Laet, I.; Van Regenmortel, N.; Schoonheydt, K.; Dits, H.; Huber, W.; Malbrain, M.L. Fluid management in critically ill patients: The role of extravascular lung water, abdominal hypertension, capillary leak, and fluid balance. Ann. Intensive Care 2012, 2 (Suppl. S1), S1. [Google Scholar] [CrossRef]
- Struck, M.F.; Illert, T.; Schmidt, T.; Reichelt, B.; Steen, M. Secondary abdominal compartment syndrome in patients with toxic epidermal necrolysis. Burns 2012, 38, 562–567. [Google Scholar] [CrossRef]
- Dauplaise, D.J.; Barnett, S.J.; Frischer, J.S.; Wong, H.R. Decompressive abdominal laparotomy for abdominal compartment syndrome in an unengrafted bone marrow recipient with septic shock. Crit. Care Res. Pract. 2010, 2010, 102910. [Google Scholar] [CrossRef]
- Divarci, E.; Karapinar, B.; Yalaz, M.; Ergun, O.; Celik, A. Incidence and prognosis of intraabdominal hypertension and abdominal compartment syndrome in children. J. Pediatric Surg. 2016, 51, 503–507. [Google Scholar] [CrossRef]
- Gala, H.C.; Avasthi, B.S.; Lokeshwar, M.R. Dengue shock syndrome with two atypical complications. Indian J. Pediatr 2012, 79, 386–388. [Google Scholar] [CrossRef]
- Lam, M.C.; Yang, P.T.; Skippen, P.W.; Kissoon, N.; Skarsgard, E.D. Abdominal compartment syndrome complicating paediatric extracorporeal life support: Diagnostic and therapeutic challenges. Anaesth Intensive Care 2008, 36, 726–731. [Google Scholar] [CrossRef]
- Ranjit, S.; Ramanathan, G.; Ramakrishnan, B.; Kissoon, N. Targeted Interventions in Critically Ill Children with Severe Dengue. Indian J. Crit. Care Med. 2018, 22, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Dorigatti, A.; Pereira, B.; Melek, M.; Dos Santos, J.; Teramoto, F.; Fraga, G. Clinical warning signs for intra-abdominal hypertension in septic shock patients. Anaesthesiol. Intensive Ther. 2019, 51, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, E.L.; Hongyan, L.; Taichman, D.; Hansen-Flaschen, J.; Fuchs, B.D. Abdominal compartment syndrome is common in medical intensive care unit patients receiving large-volume resuscitation. J. Intensive Care Med. 2007, 22, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Cordemans, C.; De Laet, I.; Van Regenmortel, N.; Schoonheydt, K.; Dits, H.; Martin, G.; Huber, W.; Malbrain, M.L. Aiming for a negative fluid balance in patients with acute lung injury and increased intra-abdominal pressure: A pilot study looking at the effects of PAL-treatment. Ann. Intensive Care 2012, 2 (Suppl. S1), S15. [Google Scholar] [CrossRef]
- Miranda, E.; Manzur, M.; Han, S.; Ham, S.W.; Weaver, F.A.; Rowe, V.L. Postoperative Development of Abdominal Compartment Syndrome among Patients Undergoing Endovascular Aortic Repair for Ruptured Abdominal Aortic Aneurysms. Ann. Vasc. Surg. 2018, 49, 289–294. [Google Scholar] [CrossRef]
- Rubenstein, C.; Bietz, G.; Davenport, D.L.; Winkler, M.; Endean, E.D. Abdominal compartment syndrome associated with endovascular and open repair of ruptured abdominal aortic aneurysms. J. Vasc. Surg. 2015, 61, 648–654. [Google Scholar] [CrossRef]
- Augustin, P.; Lasocki, S.; Dufour, G.; Rode, J.; Karsenti, A.; Al-Attar, N.; Bazeli, R.; Montravers, P. Abdominal compartment syndrome due to extracorporeal membrane oxygenation in adults. Ann. Thorac. Surg. 2010, 90, e40–e41. [Google Scholar] [CrossRef]
- Bressan, A.K.; Kirkpatrick, A.W.; Ball, C.G. Abdominal intra-compartment syndrome—A non-hydraulic model of abdominal compartment syndrome due to post-hepatectomy hemorrhage in a man with a localized frozen abdomen due to extensive adhesions: A case report. J. Med. Case Rep. 2016, 10, 251. [Google Scholar] [CrossRef][Green Version]
- Biancofiore, G.; Bindi, M.L.; Romanelli, A.M.; Boldrini, A.; Consani, G.; Bisa, M.; Filipponi, F.; Vagelli, A.; Mosca, F. Intra-abdominal pressure monitoring in liver transplant recipients: A prospective study. Intensive Care Med. 2003, 29, 30–36. [Google Scholar] [CrossRef]
- Dalfino, L.; Sicolo, A.; Paparella, D.; Mongelli, M.; Rubino, G.; Brienza, N. Intra-abdominal hypertension in cardiac surgery. Interact. Cardiovasc. Thorac. Surg. 2013, 17, 644–651. [Google Scholar] [CrossRef]
- Kotlinska-Hasiec, E.; Rutyna, R.R.; Rzecki, Z.; Czarko-Wicha, K.; Gagala, J.; Pawlik, P.; Zaluska, A.; Jaroszynski, A.; Zaluska, W.; Dabrowski, W. The effect of crystalloid infusion on body water content and intra-abdominal pressure in patients undergoing orthopedic surgery under spinal anesthesia. Adv. Clin. Exp. Med. 2017, 26, 1189–1196. [Google Scholar] [CrossRef]
- Makar, R.R.; Badger, S.A.; O’Donnell, M.E.; Loan, W.; Lau, L.L.; Soong, C.V. The effects of abdominal compartment hypertension after open and endovascular repair of a ruptured abdominal aortic aneurysm. J. Vasc. Surg. 2009, 49, 866–872. [Google Scholar] [CrossRef][Green Version]
- Muturi, A.; Ndaguatha, P.; Ojuka, D.; Kibet, A. Prevalence and predictors of intra-abdominal hypertension and compartment syndrome in surgical patients in critical care units at Kenyatta National Hospital. BMC Emerg. Med. 2017, 17, 10. [Google Scholar] [CrossRef]
- Serpytis, M.; Ivaskevicius, J. The influence of fluid balance on intra-abdominal pressure after major abdominal surgery. Medicina 2008, 44, 421–427. [Google Scholar] [CrossRef]
- Leclerc, B.; Salomon Du Mont, L.; Besch, G.; Rinckenbach, S. How to identify patients at risk of abdominal compartment syndrome after surgical repair of ruptured abdominal aortic aneurysms in the operating room: A pilot study. Vascular 2017, 25, 472–478. [Google Scholar] [CrossRef]
- McNelis, J.; Marini, C.P.; Jurkiewicz, A.; Fields, S.; Caplin, D.; Stein, D.; Ritter, G.; Nathan, I.; Simms, H.H. Predictive factors associated with the development of abdominal compartment syndrome in the surgical intensive care unit. Arch. Surg. 2002, 137, 133–136. [Google Scholar] [CrossRef]
- De Wolf, A.; Poelaert, J.; Herck, I.; De Waele, J.J. Surgical decompression for abdominal compartment syndrome after emergency cardiac surgery. Ann. Thorac. Surg. 2008, 85, 2133–2135. [Google Scholar] [CrossRef]
- Fietsam, R., Jr.; Villalba, M.; Glover, J.L.; Clark, K. Intra-abdominal compartment syndrome as a complication of ruptured abdominal aortic aneurysm repair. Am. Surg. 1989, 55, 396–402. [Google Scholar]
- Rabbi, J.F.; Valaulikar, G.; Appling, N.A.; Bee, T.K.; Ostrow, B.F.; Weiman, D.S. Secondary abdominal compartment syndrome causing failure to wean from cardiopulmonary bypass. Ann. Thorac. Surg. 2012, 93, e99–e100. [Google Scholar] [CrossRef] [PubMed]
- Shiiya, N.; Matsuzaki, K.; Miyatake, T.; Yoshimoto, K.; Yasuda, K. Abdominal compartment syndrome causing respiratory failure during surgery for a ruptured descending thoracic aneurysm: Report of a case. Surg. Today 2005, 35, 320–322. [Google Scholar] [CrossRef] [PubMed]
- Biffl, W.L.; Moore, E.E.; Burch, J.M.; Offner, P.J.; Franciose, R.J.; Johnson, J.L. Secondary abdominal compartment syndrome is a highly lethal event. Am. J. Surg. 2001, 182, 645–648. [Google Scholar] [CrossRef]
- Aik-Yong, C.; Ye-Xin, K.; Yi, N.S.; Hway, W.T. Abdominal compartment syndrome: Incidence and prognostic factors influencing survival in Singapore. Indian J. Crit. Care. Med. 2014, 18, 648–652. [Google Scholar] [CrossRef]
- Cothren, C.C.; Moore, E.E.; Johnson, J.L.; Moore, J.B. Outcomes in surgical versus medical patients with the secondary abdominal compartment syndrome. Am. J. Surg. 2007, 194, 804–807; discussion 807–808. [Google Scholar] [CrossRef]
- Dalfino, L.; Tullo, L.; Donadio, I.; Malcangi, V.; Brienza, N. Intra-abdominal hypertension and acute renal failure in critically ill patients. Intensive Care Med. 2008, 34, 707–713. [Google Scholar] [CrossRef]
- Murphy, P.B.; Parry, N.G.; Sela, N.; Leslie, K.; Vogt, K.; Ball, I. Intra-Abdominal Hypertension Is More Common Than Previously Thought: A Prospective Study in a Mixed Medical-Surgical ICU. Crit. Care Med. 2018, 46, 958–964. [Google Scholar] [CrossRef]
- Malbrain, M.L.N.G.; Chiumello, D.; Pelosi, P.; Bihari, D.; Innes, R.; Ranieri, V.M.; Del Turco, M.; Wilmer, A.; Brienza, N.; Malcangi, V.; et al. Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: A multiple-center epidemiological study. Crit. Care Med. 2005, 33, 315–322. [Google Scholar] [CrossRef]
- Malbrain, M.L.; Chiumello, D.; Pelosi, P.; Wilmer, A.; Brienza, N.; Malcangi, V.; Bihari, D.; Innes, R.; Cohen, J.; Singer, P.; et al. Prevalence of intra-abdominal hypertension in critically ill patients: A multicentre epidemiological study. Intensive Care Med. 2004, 30, 822–829. [Google Scholar] [CrossRef]
- Iyer, D.; Rastogi, P.; Aneman, A.; D’Amours, S. Early screening to identify patients at risk of developing intra-abdominal hypertension and abdominal compartment syndrome. Acta Anaesthesiol. Scand. 2014, 58, 1267–1275. [Google Scholar] [CrossRef]
- Reintam Blaser, A.; Regli, A.; De Keulenaer, B.; Kimball, E.J.; Starkopf, L.; Davis, W.A.; Greiffenstein, P.; Starkopf, J.; Incidence, R.F.; Outcomes of Intra-Abdominal Study, I. Incidence, Risk Factors, and Outcomes of Intra-Abdominal Hypertension in Critically Ill Patients-A Prospective Multicenter Study (IROI Study). Crit. Care Med. 2019, 47, 535–542. [Google Scholar] [CrossRef]
- Vidal, M.G.; Ruiz Weisser, J.; Gonzalez, F.; Toro, M.A.; Loudet, C.; Balasini, C.; Canales, H.; Reina, R.; Es-tenssoro, E. Incidence and clinical effects of intra-abdominal hypertension in critically ill pa-tients. Crit. Care Med. 2008, 36, 1823–1831. [Google Scholar] [CrossRef]
- Kim, I.B.; Prowle, J.; Baldwin, I.; Bellomo, R. Incidence, risk factors and outcome associations of in-tra-abdominal hypertension in critically ill patients. Anaesth Intensive Care 2012, 40, 79–89. [Google Scholar] [CrossRef]
- Malbrain, M.L.; Chiumello, D.; Cesana, B.M.; Reintam Blaser, A.; Starkopf, J.; Sugrue, M.; Pelosi, P.; Sev-ergnini, P.; Hernandez, G.; Brienza, N.; et al. A systematic review and individual patient data me-ta-analysis on intra-abdominal hypertension in critically ill patients: The wake-up project. World initiative on Abdominal Hypertension Epidemiology, a Unifying Project (WAKE-Up!). Minerva Anestesiol. 2014, 80, 293–306. [Google Scholar]
- Reintam Blaser, A.; Parm, P.; Kitus, R.; Starkopf, J. Risk factors for intra-abdominal hypertension in mechanically ventilated patients. Acta Anaesthesiol. Scand. 2011, 55, 607–614. [Google Scholar] [CrossRef]
- Moore-Olufemi, S.D.; Xue, H.; Attuwaybi, B.O.; Fischer, U.; Harari, Y.; Oliver, D.H.; Weisbrodt, N.; Allen, S.J.; Moore, F.A.; Stewart, R.; et al. Resuscitation-induced gut edema and intestinal dysfunction. J. Trauma 2005, 58, 264–270. [Google Scholar] [CrossRef]
- Chang, M.; Tang, H.; Liu, D.; Li, Y.; Zhang, L. Comparison of Melatonin, Hypertonic Saline, and Hydroxyethyl Starch for Resuscitation of Secondary Intra-Abdominal Hypertension in an Animal Model. PLoS ONE 2016, 11, e0161688. [Google Scholar] [CrossRef][Green Version]
- Schachtrupp, A.; Lawong, G.; Afify, M.; Graf, J.; Toens, C.; Schumpelick, V. Fluid resuscitation preserves cardiac output but cannot prevent organ damage in a porcine model during 24 h of intraabdominal hypertension. Shock 2005, 24, 153–158. [Google Scholar] [CrossRef]
- Regan, A.; Hotwagner, D.T. Burn Fluid Management; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Rezende-Neto, J.B.; Moore, E.E.; Melo de Andrade, M.V.; Teixeira, M.M.; Lisboa, F.A.; Arantes, R.M.; de Souza, D.G.; da Cunha-Melo, J.R. Systemic inflammatory response secondary to abdominal compartment syndrome: Stage for multiple organ failure. J. Trauma 2002, 53, 1121–1128. [Google Scholar] [CrossRef]
- Silversides, J.A.; Perner, A.; Malbrain, M. Liberal versus restrictive fluid therapy in critically ill patients. Intensive Care Med. 2019, 45, 1440–1442. [Google Scholar] [CrossRef]
- Van der Mullen, J.; Wise, R.; Vermeulen, G.; Moonen, P.J.; Malbrain, M. Assessment of hypovolaemia in the critically ill. Anaesthesiol. Intensive Ther. 2018, 50, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Harrell, B.R.; Miller, S. Abdominal Compartment Syndrome as a Complication of Fluid Resuscitation. Nurs. Clin. North Am. 2017, 52, 331–338. [Google Scholar] [CrossRef]
- De Waele, J.J.; Ejike, J.C.; Leppaniemi, A.; De Keulenaer, B.L.; De Laet, I.; Kirkpatrick, A.W.; Roberts, D.J.; Kimball, E.; Ivatury, R.; Malbrain, M.L. Intra-abdominal hypertension and abdominal compartment syndrome in pancreatitis, paediatrics, and trauma. Anaesthesiol. Intensive Ther. 2015, 47, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Peeters, Y.; Bernards, J.; Mekeirele, M.; Hoffmann, B.; De Raes, M.; Malbrain, M.L. Hemodynamic monitoring: To calibrate or not to calibrate? Part 1--Calibrated techniques. Anaesthesiol. Intensive Ther. 2015, 47, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Cosnett, J.E. The origins of intravenous fluid therapy. Lancet 1989, 1, 768–771. [Google Scholar] [CrossRef]
- Myburgh, J.A.; Mythen, M.G. Resuscitation fluids. N. Engl. J. Med. 2013, 369, 1243–1251. [Google Scholar] [CrossRef]
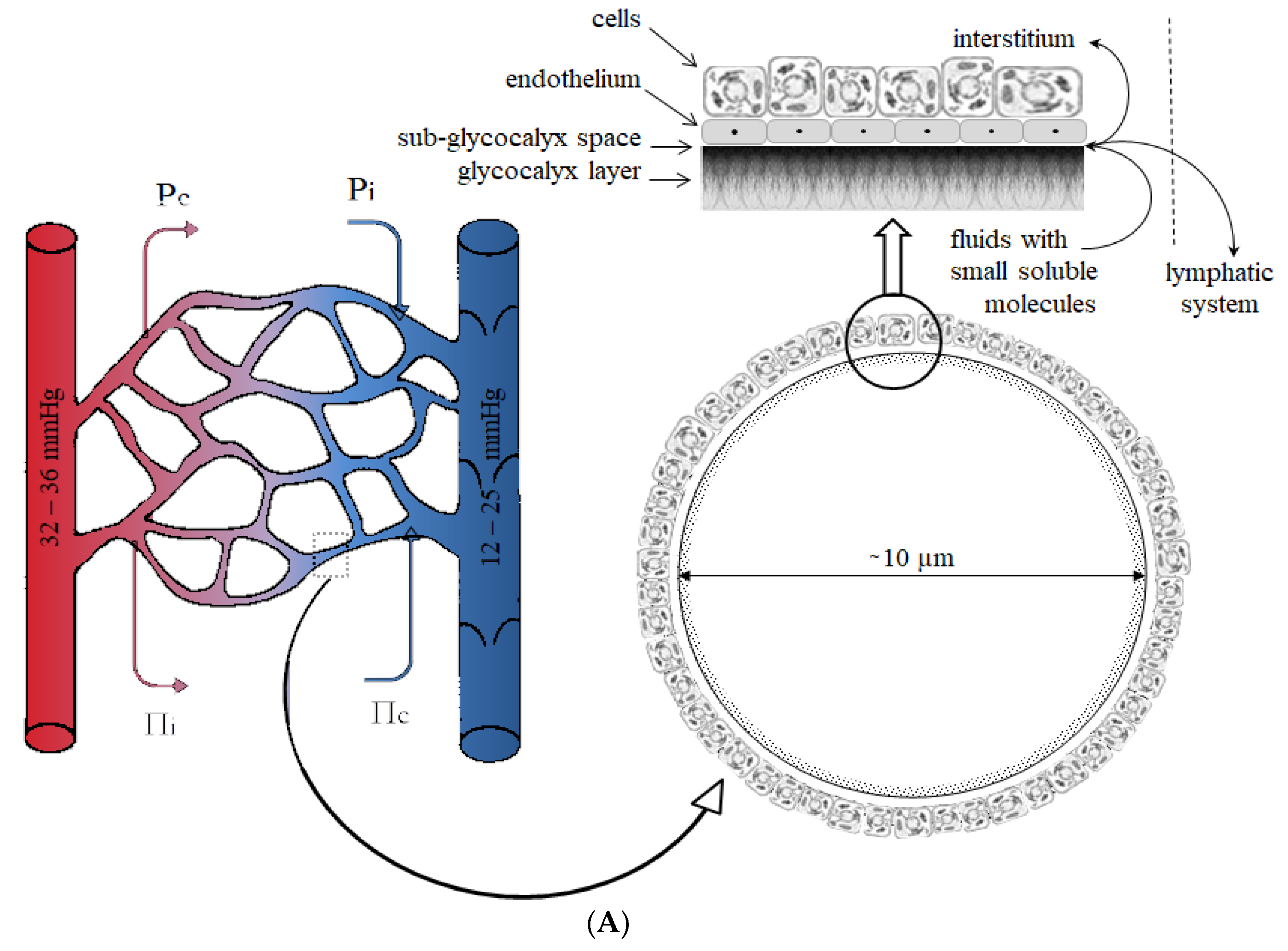
- Reitsma, S.; Slaaf, D.W.; Vink, H.; van Zandvoort, M.A.; oude Egbrink, M.G. The endothelial glycocalyx: Composition, functions, and visualization. Pflug. Arch. 2007, 454, 345–359. [Google Scholar] [CrossRef]
- Henry, C.B.; Duling, B.R. TNF-alpha increases entry of macromolecules into luminal endothelial cell glycocalyx. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H2815–H2823. [Google Scholar] [CrossRef]
- Woodcock, T.E.; Woodcock, T.M. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: An improved paradigm for prescribing intravenous fluid therapy. Br. J. Anaesth 2012, 108, 384–394. [Google Scholar] [CrossRef]
- Erstad, B.L. The Revised Starling Equation: The Debate of Albumin Versus Crystalloids Continues. Ann. Pharmacother. 2020, 54, 921–927. [Google Scholar] [CrossRef]
- Levick, J.R.; Michel, C.C. Microvascular fluid exchange and the revised Starling principle. Cardiovasc. Res. 2010, 87, 198–210. [Google Scholar] [CrossRef]
- Dubniks, M.; Persson, J.; Grande, P.O. Effect of blood pressure on plasma volume loss in the rat under increased permeability. Intensive Care Med. 2007, 33, 2192–2198. [Google Scholar] [CrossRef]
- Jacobs, R.; Lochy, S.; Malbrain, M. Phenylephrine-induced recruitable preload from the venous side. J. Clin. Monit. Comput. 2019, 33, 373–376. [Google Scholar] [CrossRef]
- Schaefer, T.; Nunez Lopez, O. Burn Resuscitation And Management. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430795/ (accessed on 26 May 2022).
- Baxter, C.R.; Shires, T. Physiological response to crystalloid resuscitation of severe burns. Ann. New York Acad. Sci. 1968, 150, 874–894. [Google Scholar] [CrossRef]
- Saffle, J.R. Fluid Creep and Over-resuscitation. Crit. Care Clin. 2016, 32, 587–598. [Google Scholar] [CrossRef]
- Saffle, J.I. The phenomenon of “fluid creep” in acute burn resuscitation. J. Burn Care Res. 2007, 28, 382–395. [Google Scholar] [CrossRef]
- Pruitt, B.A., Jr. Protection from excessive resuscitation: “pushing the pendulum back”. J. Trauma 2000, 49, 567–568. [Google Scholar] [CrossRef]
- Working Group IAPAPAAPG. IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology 2013, 13, e1–e15. [Google Scholar] [CrossRef]
- Huber, W.; Malbrain, M.L. Goal-directed fluid resuscitation in acute pancreatitis: Shedding light on the penumbra by dynamic markers of preload? Intensive Care Med. 2013, 39, 784–786. [Google Scholar] [CrossRef][Green Version]
- Trikudanathan, G.; Navaneethan, U.; Vege, S.S. Current controversies in fluid resuscitation in acute pancreatitis: A systematic review. Pancreas 2012, 41, 827–834. [Google Scholar] [CrossRef]
- Mao, E.Q.; Fei, J.; Peng, Y.B.; Huang, J.; Tang, Y.Q.; Zhang, S.D. Rapid hemodilution is associated with increased sepsis and mortality among patients with severe acute pancreatitis. Chin. Med. J. 2010, 123, 1639–1644. [Google Scholar] [PubMed]
- Johansson, P.I.; Stensballe, J.; Ostrowski, S.R. Shock induced endotheliopathy (SHINE) in acute critical illness—A unifying pathophysiologic mechanism. Crit. Care 2017, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Van Regenmortel, N.; Verbrugghe, W.; Roelant, E.; Van den Wyngaert, T.; Jorens, P.G. Maintenance fluid therapy and fluid creep impose more significant fluid, sodium, and chloride burdens than resuscitation fluids in critically ill patients: A retrospective study in a tertiary mixed ICU population. Intensive Care Med. 2018, 44, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Myles, P.S.; Bellomo, R.; Corcoran, T.; Forbes, A.; Peyton, P.; Story, D.; Christophi, C.; Leslie, K.; McGuinness, S.; Parke, R.; et al. Restrictive versus Liberal Fluid Therapy for Major Abdominal Surgery. N. Engl. J. Med. 2018, 378, 2263–2274. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.L.; Van Regenmortel, N.; Owczuk, R. It is time to consider the four D’s of fluid management. Anaesthesiol. Intensive Ther. 2015, 47, s1–s5. [Google Scholar] [CrossRef]
- Meyhoff, T.S.; Hjortrup, P.B.; Moller, M.H.; Wetterslev, J.; Lange, T.; Kjaer, M.N.; Jonsson, A.B.; Hjortso, C.J.S.; Cronhjort, M.; Laake, J.H.; et al. Conservative vs liberal fluid therapy in septic shock (CLASSIC) trial-Protocol and statistical analysis plan. Acta Anaesthesiol. Scand. 2019, 63, 1262–1271. [Google Scholar] [CrossRef]
- Pereira, B.; Dorigatti, A.; Melek, M.; Dos Santos, J.; Ferreira, M.; Calderan, T.; Carmona, C.; Fraga, G. Septic shock patients admitted to the intensive care unit with higher SOFA score tend to have higher incidence of abdominal compartment syndrome—A preliminary analysis. Anaesthesiol. Intensive Ther. 2019, 51, 370–372. [Google Scholar] [CrossRef]
- Perel, P.; Roberts, I.; Ker, K. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst. Rev. 2013, 2, 1465–1858. [Google Scholar] [CrossRef]
- Hahn, R.G. Adverse effects of crystalloid and colloid fluids. Anaesthesiol. Intensive Ther. 2017, 49, 303–308. [Google Scholar] [CrossRef]
- Iqbal, U.; Anwar, H.; Scribani, M. Ringer’s lactate versus normal saline in acute pancreatitis: A systematic review and meta-analysis. J. Dig. Dis. 2018, 19, 335–341. [Google Scholar] [CrossRef]
- Wu, B.U.; Hwang, J.Q.; Gardner, T.H.; Repas, K.; Delee, R.; Yu, S.; Smith, B.; Banks, P.A.; Conwell, D.L. Lactated Ringer’s solution reduces systemic inflammation compared with saline in patients with acute pancreatitis. Clin. Gastroenterol. Hepatol. 2011, 9, 710–717.e711. [Google Scholar] [CrossRef]
- Semler, M.W.; Self, W.H.; Wanderer, J.P.; Ehrenfeld, J.M.; Wang, L.; Byrne, D.W.; Stollings, J.L.; Kumar, A.B.; Hughes, C.G.; Hernandez, A.; et al. Balanced Crystalloids versus Saline in Critically Ill Adults. N. Engl. J. Med. 2018, 378, 829–839. [Google Scholar] [CrossRef]
- Buxbaum, J.L.; Quezada, M.; Da, B.; Jani, N.; Lane, C.; Mwengela, D.; Kelly, T.; Jhun, P.; Dhanireddy, K.; Laine, L. Early Aggressive Hydration Hastens Clinical Improvement in Mild Acute Pancreatitis. Am. J. Gastroenterol. 2017, 112, 797–803. [Google Scholar] [CrossRef]
- Finfer, S.; Bellomo, R.; Boyce, N.; French, J.; Myburgh, J.; Norton, R.; Investigators, S.S. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N. Engl. J. Med. 2004, 350, 2247–2256. [Google Scholar] [CrossRef]
- Maitland, K.; Kiguli, S.; Opoka, R.O.; Engoru, C.; Olupot-Olupot, P.; Akech, S.O.; Nyeko, R.; Mtove, G.; Reyburn, H.; Lang, T.; et al. Mortality after fluid bolus in African children with severe infection. N. Engl. J. Med. 2011, 364, 2483–2495. [Google Scholar] [CrossRef]
- Caironi, P.; Tognoni, G.; Masson, S.; Fumagalli, R.; Pesenti, A.; Romero, M.; Fanizza, C.; Caspani, L.; Faenza, S.; Grasselli, G.; et al. Albumin replacement in patients with severe sepsis or septic shock. N. Engl. J. Med. 2014, 370, 1412–1421. [Google Scholar] [CrossRef]
- Eljaiek, R.; Heylbroeck, C.; Dubois, M.J. Albumin administration for fluid resuscitation in burn patients: A systematic review and meta-analysis. Burns 2017, 43, 17–24. [Google Scholar] [CrossRef]
- Martensson, J.; Bihari, S.; Bannard-Smith, J.; Glassford, N.J.; Lloyd-Donald, P.; Cioccari, L.; Luethi, N.; Tanaka, A.; Crisman, M.; Rey de Castro, N.; et al. Small volume resuscitation with 20% albumin in intensive care: Physiological effects: The SWIPE randomised clinical trial. Intensive Care Med. 2018, 44, 1797–1806. [Google Scholar] [CrossRef]
- Finfer, S. Reappraising the role of albumin for resuscitation. Curr. Opin. Crit. Care 2013, 19, 315–320. [Google Scholar] [CrossRef]
- Wang, M.D.; Ji, Y.; Xu, J.; Jiang, D.H.; Luo, L.; Huang, S.W. Early goal-directed fluid therapy with fresh frozen plasma reduces severe acute pancreatitis mortality in the intensive care unit. Chin. Med. J. 2013, 126, 1987–1988. [Google Scholar]
- Horton, J.W.; Dunn, C.W.; Burnweit, C.A.; Walker, P.B. Hypertonic saline-dextran resuscitation of acute canine bile-induced pancreatitis. Am. J. Surg. 1989, 158, 48–56. [Google Scholar] [CrossRef]
- Machado, M.C.; Coelho, A.M.; Pontieri, V.; Sampietre, S.N.; Molan, N.A.; Soriano, F.; Matheus, A.S.; Patzina, R.A.; Cunha, J.E.; Velasco, I.T. Local and systemic effects of hypertonic solution (NaCl 7.5%) in experimental acute pancreatitis. Pancreas 2006, 32, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.B.; Ke, L.; Sun, J.K.; Tong, Z.H.; Ding, W.W.; Li, W.Q.; Li, N.; Li, J.S. Beneficial effect of hypertonic saline resuscitation in a porcine model of severe acute pancreatitis. Pancreas 2012, 41, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.J.; Winter, D.C.; Sookhai, S.; Ryan, L.; Kirwan, W.O.; Redmond, H.P. Hypertonic saline attenuates end-organ damage in an experimental model of acute pancreatitis. Br. J. Surg. 2000, 87, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.N.; Cancio, L.C.; Gibran, N.S.; American Burn, A. American Burn Association practice guidelines burn shock resuscitation. J. Burn Care Res. 2008, 29, 257–266. [Google Scholar] [CrossRef]
- Saugel, B.; Malbrain, M.L.; Perel, A. Hemodynamic monitoring in the era of evidence-based medicine. Crit. Care 2016, 20, 401. [Google Scholar] [CrossRef]
- Vandervelden, S.; Malbrain, M.L. Initial resuscitation from severe sepsis: One size does not fit all. Anaesthesiol. Intensive Ther. 2015, 47, s44–s55. [Google Scholar] [CrossRef]
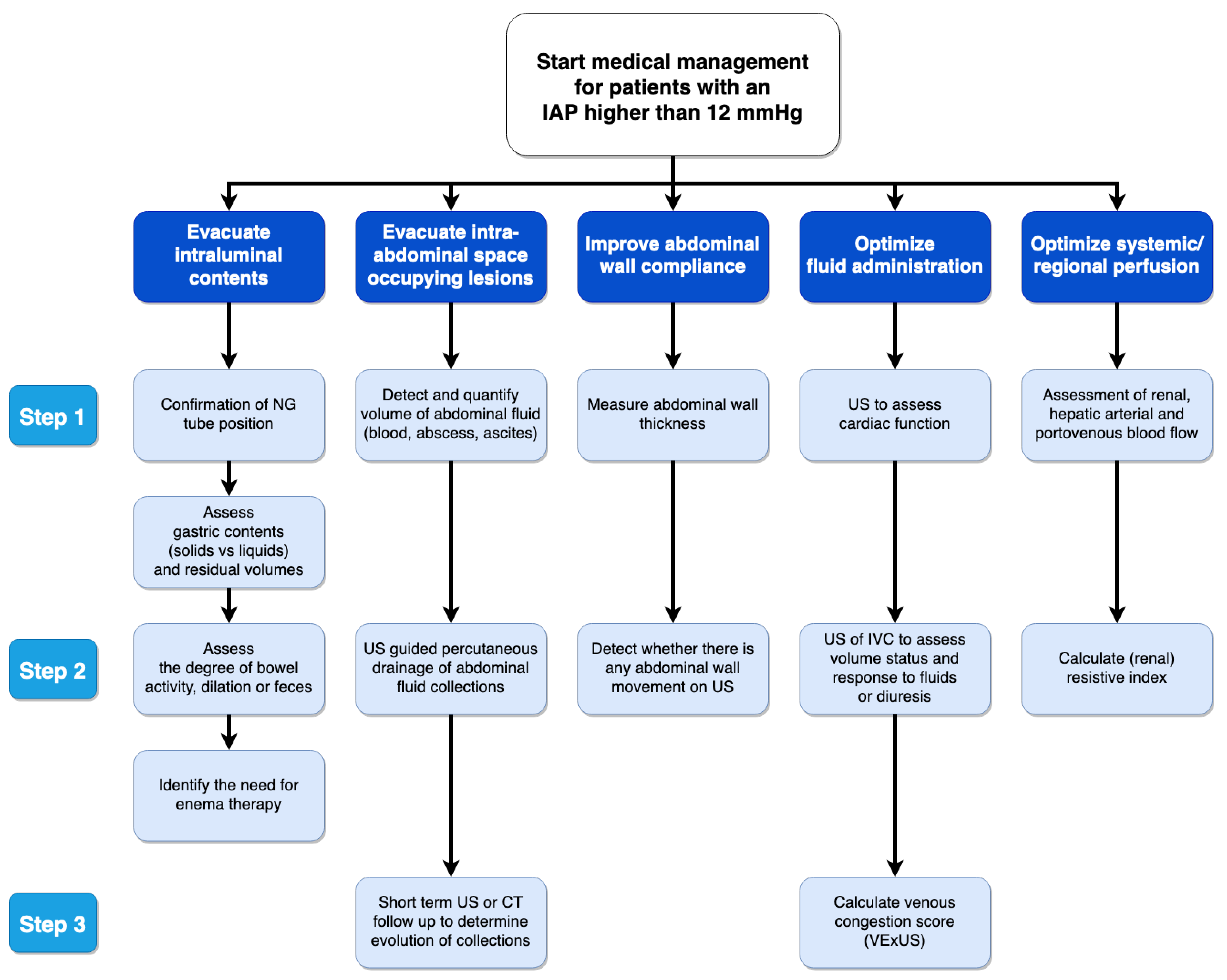
- Pereira, B.M.; Pereira, R.G.; Wise, R.; Sugrue, G.; Zakrison, T.L.; Dorigatti, A.E.; Fiorelli, R.K.; Malbrain, M. The role of point-of-care ultrasound in intra-abdominal hypertension management. Anaesthesiol. Intensive Ther. 2017, 49, 373–381. [Google Scholar] [CrossRef]
- Marik, P.E.; Malbrain, M. The SEP-1 quality mandate may be harmful: How to drown a patient with 30 mL per kg fluid! Anaesthesiol. Intensive Ther. 2017, 49, 323–328. [Google Scholar] [CrossRef]
- O’Connor, M.E.; Prowle, J.R. Fluid Overload. Crit. Care Clin. 2015, 31, 803–821. [Google Scholar] [CrossRef]
- Rizzo, J.A.; Rowan, M.P.; Driscoll, I.R.; Chung, K.K.; Friedman, B.C. Vitamin C in Burn Resuscitation. Crit. Care Clin. 2016, 32, 539–546. [Google Scholar] [CrossRef]
- Tanaka, H.; Matsuda, T.; Miyagantani, Y.; Yukioka, T.; Matsuda, H.; Shimazaki, S. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: A randomized, prospective study. Arch. Surg. 2000, 135, 326–331. [Google Scholar] [CrossRef]
- Smith, J.W.; Garrison, R.N.; Matheson, P.J.; Franklin, G.A.; Harbrecht, B.G.; Richardson, J.D. Direct peritoneal resuscitation accelerates primary abdominal wall closure after damage control surgery. J. Am. Coll Surg 2010, 210, 658–664, 664–657. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jacobs, R.; Wise, R.D.; Myatchin, I.; Vanhonacker, D.; Minini, A.; Mekeirele, M.; Kirkpatrick, A.W.; Pereira, B.M.; Sugrue, M.; De Keulenaer, B.; et al. Fluid Management, Intra-Abdominal Hypertension and the Abdominal Compartment Syndrome: A Narrative Review. Life 2022, 12, 1390. https://doi.org/10.3390/life12091390
Jacobs R, Wise RD, Myatchin I, Vanhonacker D, Minini A, Mekeirele M, Kirkpatrick AW, Pereira BM, Sugrue M, De Keulenaer B, et al. Fluid Management, Intra-Abdominal Hypertension and the Abdominal Compartment Syndrome: A Narrative Review. Life. 2022; 12(9):1390. https://doi.org/10.3390/life12091390
Chicago/Turabian StyleJacobs, Rita, Robert D. Wise, Ivan Myatchin, Domien Vanhonacker, Andrea Minini, Michael Mekeirele, Andrew W. Kirkpatrick, Bruno M. Pereira, Michael Sugrue, Bart De Keulenaer, and et al. 2022. "Fluid Management, Intra-Abdominal Hypertension and the Abdominal Compartment Syndrome: A Narrative Review" Life 12, no. 9: 1390. https://doi.org/10.3390/life12091390
APA StyleJacobs, R., Wise, R. D., Myatchin, I., Vanhonacker, D., Minini, A., Mekeirele, M., Kirkpatrick, A. W., Pereira, B. M., Sugrue, M., De Keulenaer, B., Bodnar, Z., Acosta, S., Ejike, J., Tayebi, S., Stiens, J., Cordemans, C., Van Regenmortel, N., Elbers, P. W. G., Monnet, X., ... Malbrain, M. L. N. G. (2022). Fluid Management, Intra-Abdominal Hypertension and the Abdominal Compartment Syndrome: A Narrative Review. Life, 12(9), 1390. https://doi.org/10.3390/life12091390








