Mdivi-1 Induced Mitochondrial Fusion as a Potential Mechanism to Enhance Stress Tolerance in Wheat
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
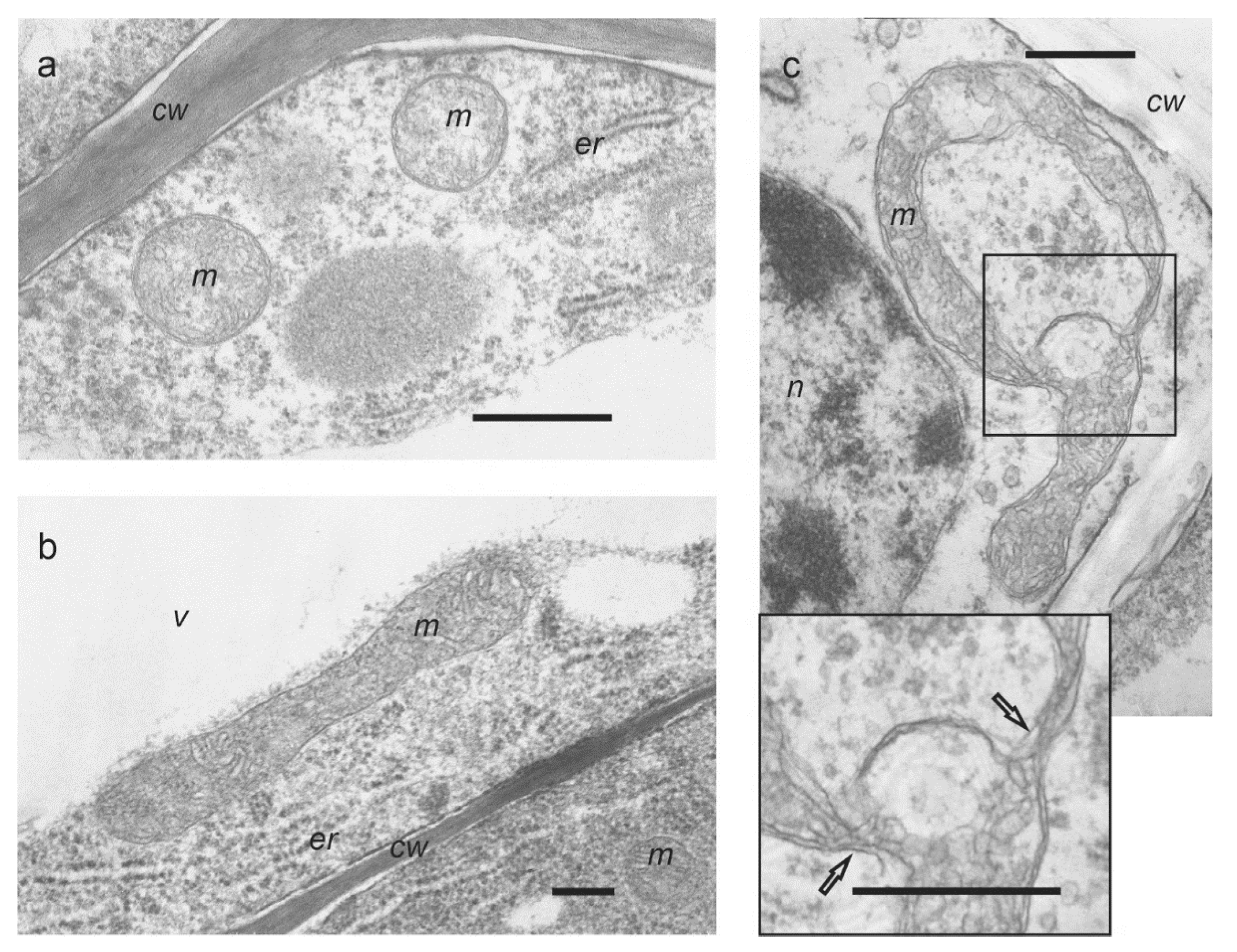
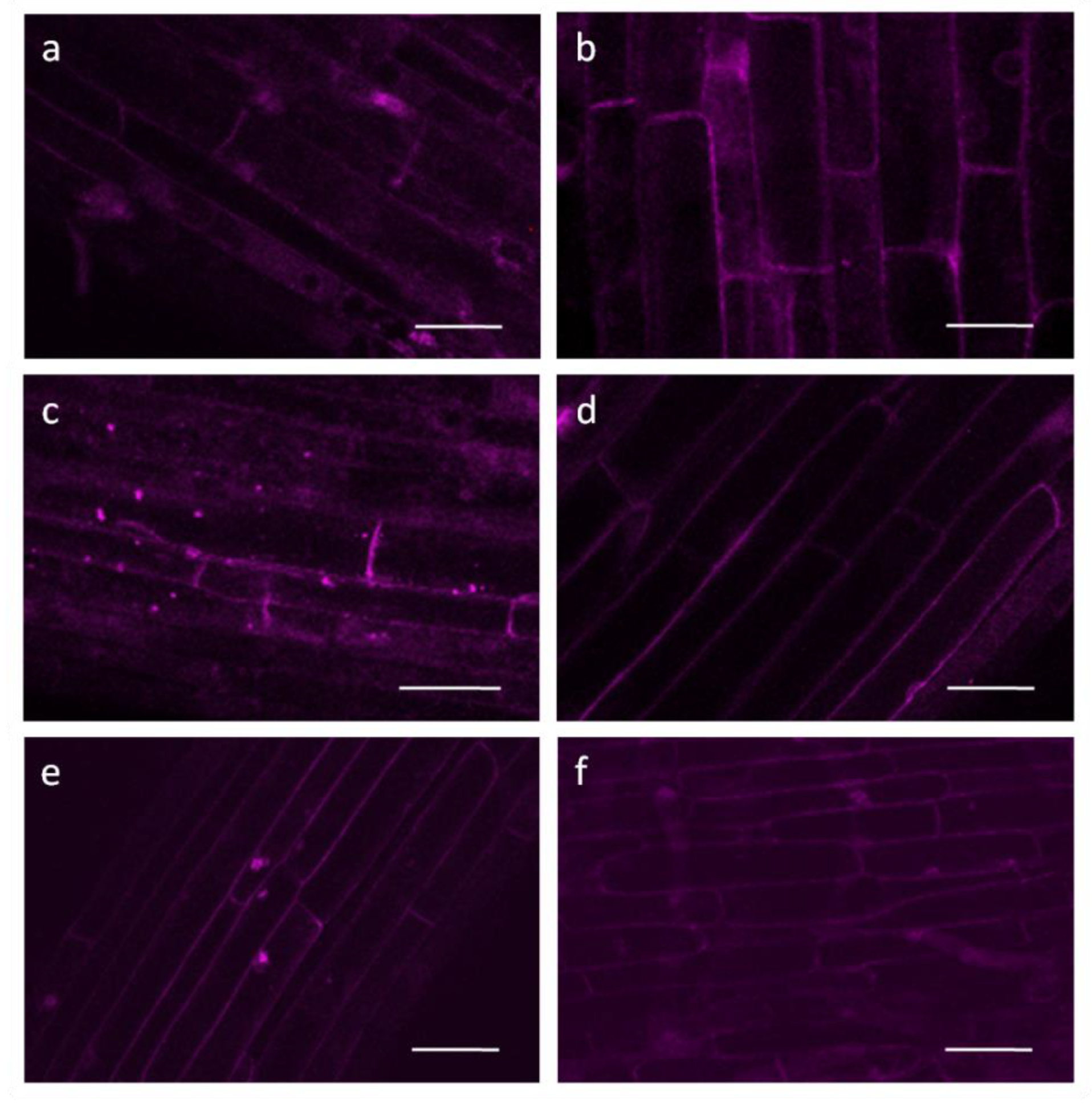
2.2. Confocal and Electron Microscopy
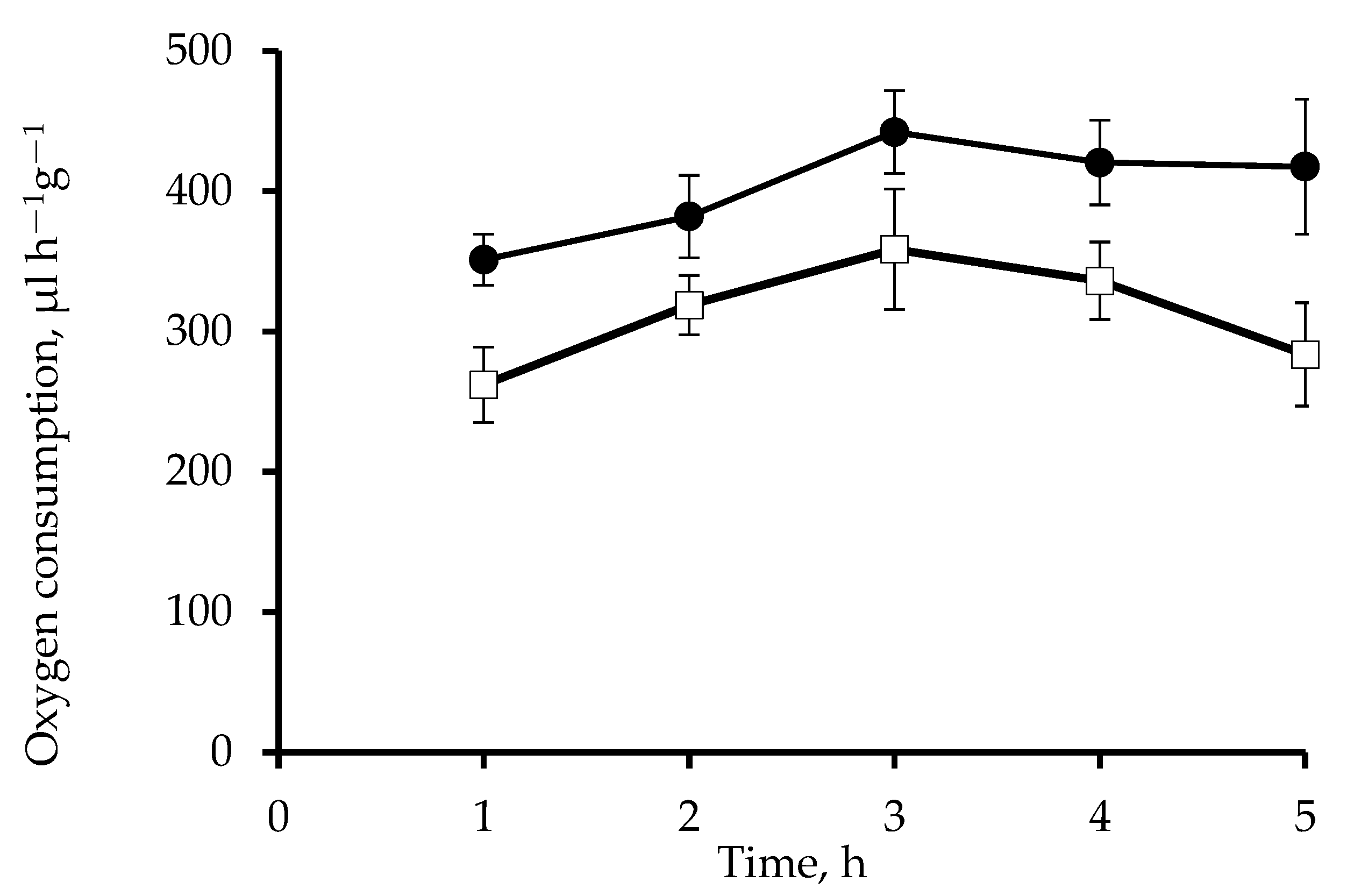
2.3. Respiration Rates
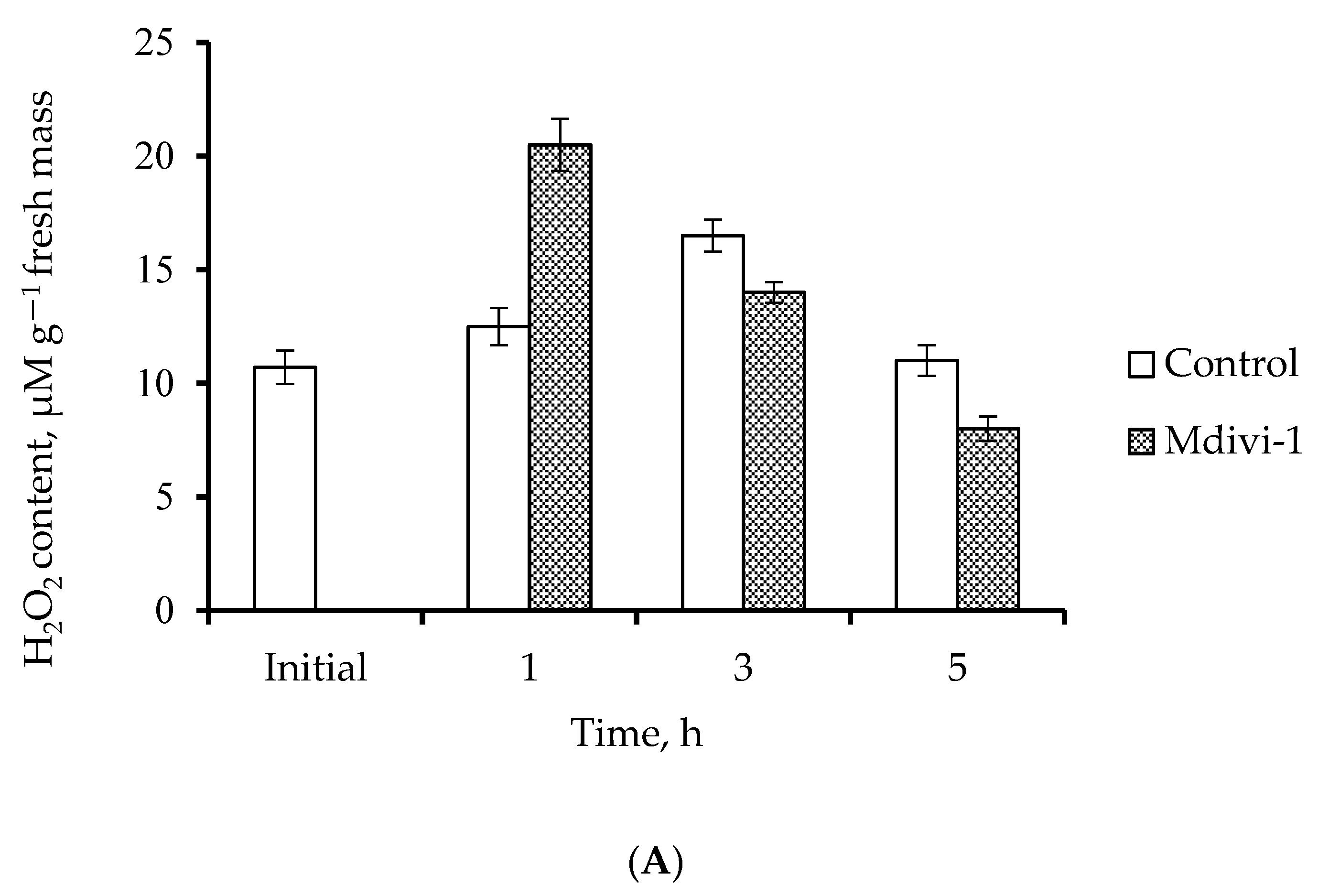
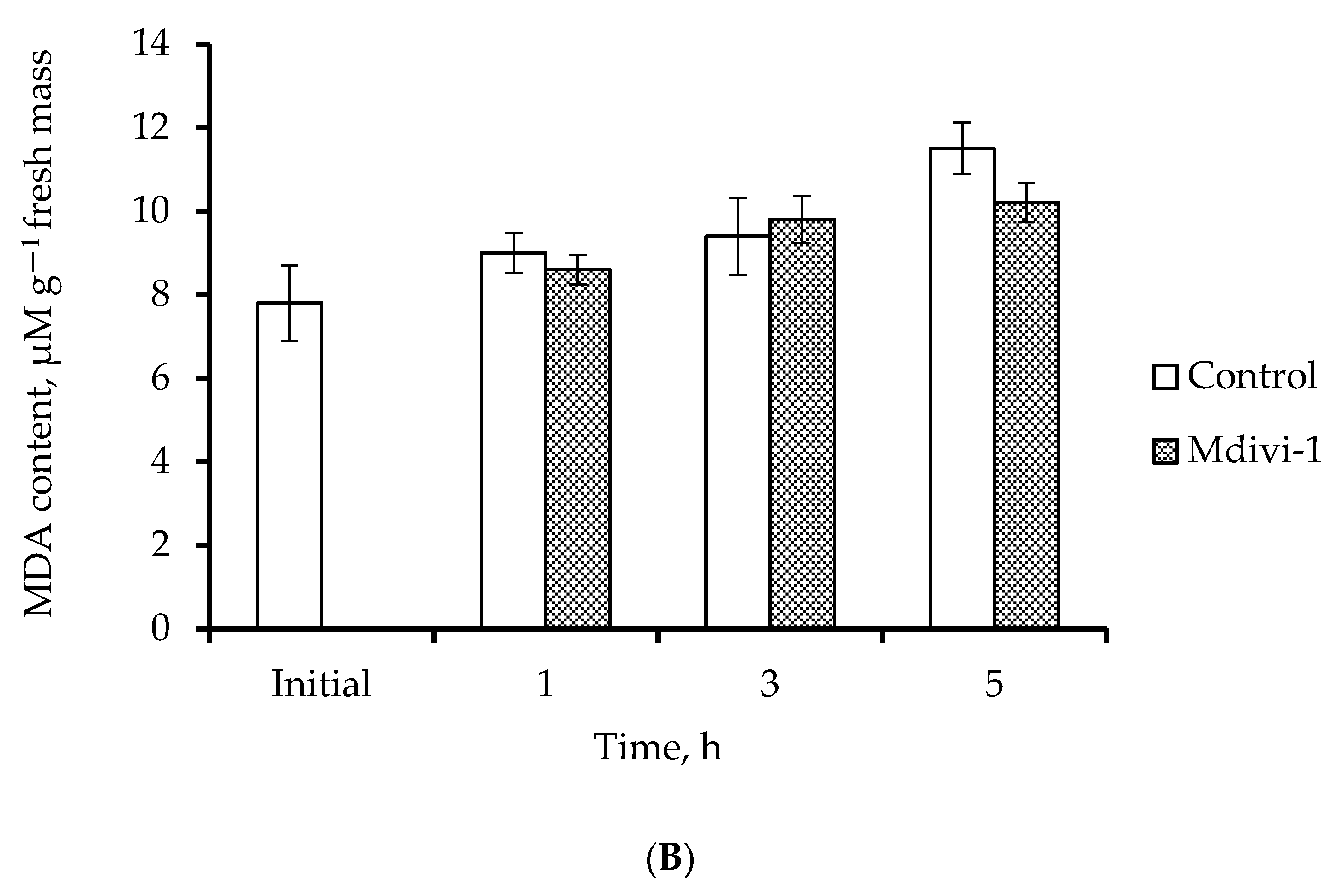
2.4. H2O2 Content and Level of Lipid Peroxidation
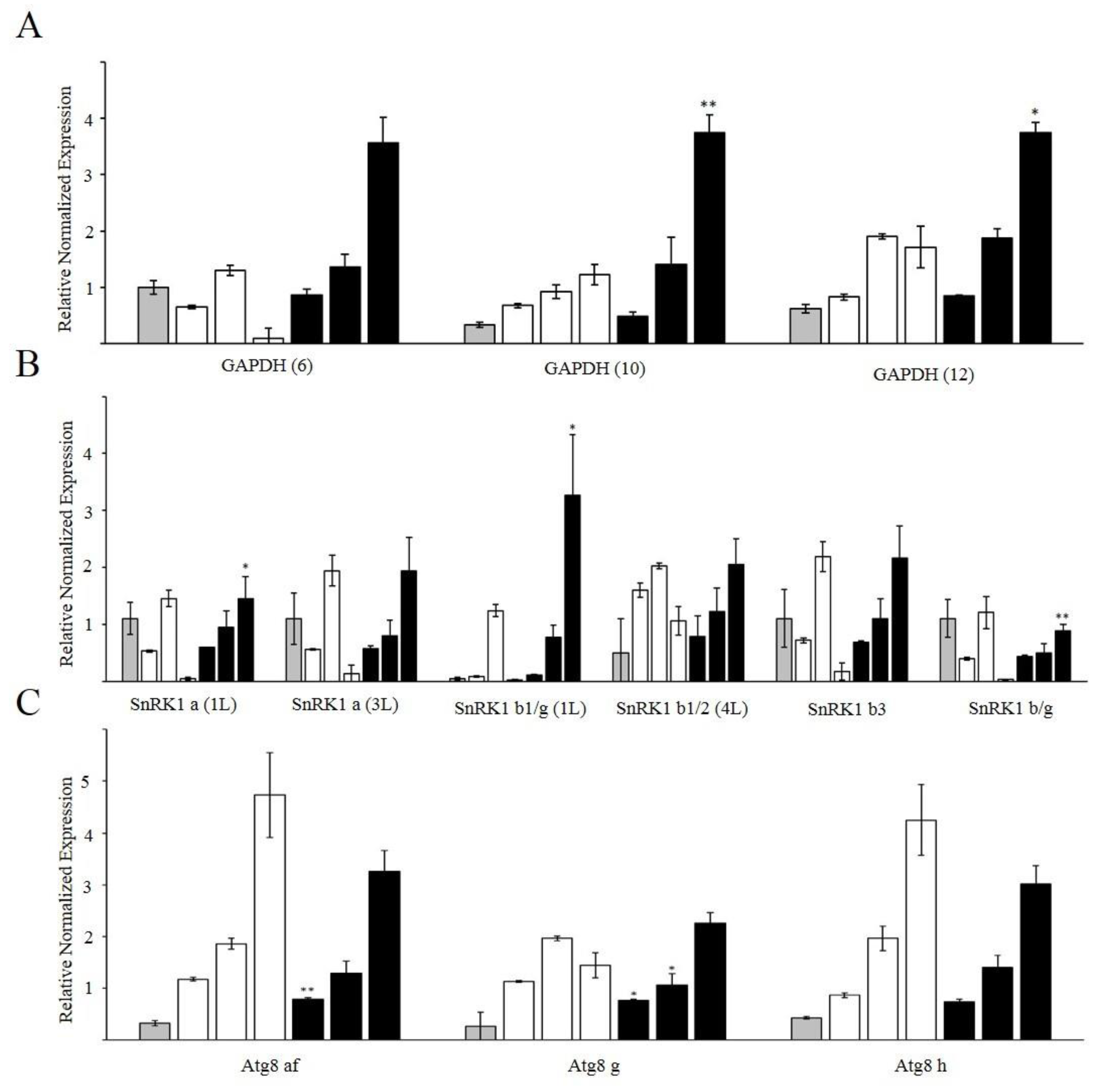
2.5. Gene Expression by Quantitative Real-Time PCR
2.6. Statistics
3. Results
4. Discussion
4.1. Mdivi-1 Changes the Ultrastructure and Energy Status of Mitochondria
4.2. Mdivi-1 Treatment Reduces Oxidative Stress
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, G.; Gallo, G. To Mdivi-1 or Not to Mdivi-1: Is that the Question? Dev. Neurobiol. 2017, 77, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Arimura, S.-I.; Yamamoto, J.; Aida, G.P.; Nakazono, M.; Tsutsumi, N. Frequent Fusion and Fission of Plant Mitochondria with Unequal Nucleoid Distribution. Proc. Natl. Acad. Sci. USA 2004, 101, 7805–7808. [Google Scholar] [CrossRef] [PubMed]
- Labbe, K.; Murley, A.; Nunnari, J. Determinants and Functions of Mitochondrial Behavior. Ann. Rev. Cell. Dev. Biol. 2014, 30, 357–391. [Google Scholar] [CrossRef] [PubMed]
- Flippo, K.H.; Strack, S. Mitochondrial Dynamics in Neuronal Injury, Development and Plasticity. J. Cell Sci. 2017, 130, 671–681. [Google Scholar] [CrossRef]
- Furt, F.; Moreau, P. Importance of Lipid Metabolism for Intracellular and Mitochondrial Membrane Fusion/Fission Prosses. Int. J. Biochem. Cell Biol. 2009, 41, 1828–1836. [Google Scholar] [CrossRef]
- Patrushev, M.V.; Mazunin, I.O.; Vinogradova, E.N.; Kamenski, P.A. Mitochondrial Fission and Fusion. Biochemistry 2015, 80, 1457–1464. [Google Scholar] [CrossRef]
- Liu, X.; Weaver, D.; Shirihai, O.; Hajnóczky, G. Mitochondrial ‘Kiss-and-Run’: Interplay Between Mitochondrial Motility and Fusion-Fission Dynamics. EMBO J. 2009, 28, 3074–3089. [Google Scholar] [CrossRef]
- Twig, G.; Hyde, B.; Shirihai, O.S. Mitochondrial Fusion Fission and Autophagy as a Quality Control Axis: The Bioenergetic View. Biochim. Biophys. Acta 2008, 1777, 1092–1109. [Google Scholar] [CrossRef]
- Rose, R.J. Contribution of Massive Mitochondrial Fusion and Subsequent Fission in the Plant Life Cycle to the Integrity of the Mitochondrion and Its Genome. Int. J. Mol. Sci. 2021, 22, 5429. [Google Scholar] [CrossRef]
- Benard, G.; Rossignol, R. Ultrastructure of the Mitochondrion and Its Bearing on Function and Bioenergetics. Antioxid. Redox Signal. 2008, 10, 1313–1342. [Google Scholar] [CrossRef]
- Ishihara, N.; Jofuku, A.; Eura, Y.; Mihara, K. Regulation of Mitochondrial Morphology by Membrane Potential, And Drp1-Dependent Division and Fzo1-Dependent Fusion Reaction in Mammalian Cells. Biochem. Biophys. Res. Commun. 2013, 301, 891–898. [Google Scholar] [CrossRef]
- Benard, G.; Bellance, N.; James, D.; Parrone, P.; Fernandez, H.; Letellier, T.; Rossignol, R. Mitochondrial Bioenergetics and Structural Network Organization. J. Cell Sci. 2007, 120, 838–848. [Google Scholar] [CrossRef]
- Liot, G.; Bossy, B.; Lubitz, S.; Kushnareva, Y.; Sejbuk, N.; Bossy-Wetzel, E. Complex II inhibition by 3-NP Causes Mitochondrial Fragmentation and Neuronal Cell Death Via An NMDA- And ROS-Dependent Pathway. Cell Death Differ. 2009, 16, 899–909. [Google Scholar] [CrossRef]
- Meeusen, S.; DeVay, R.; Block, J.; Cassidy-Stone, A.; Wayson, S.; McCaffery, J.M.; Nunnari, J. Mitochondrial Inner-Membrane Fusion and Crista Maintenance Requires the Dynamin-Related GTPase Mgm1. Cell 2006, 127, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Galloway, C.A.; Lee, H.; Yoon, Y. Mitochondrial Morphology-Emerging Role in Bioenergetics. Free Radic. Biol. Med. 2012, 53, 2218–2228. [Google Scholar] [CrossRef]
- Jaipargas, E.A.; Barton, K.A.; Mathur, N.; Mathur, J. Mitochondrial Pleomorphy in Plant Cells Is Driven by Contiguous ER Dynamics. Front. Plant. Sci. 2015, 6, 783. [Google Scholar] [CrossRef]
- Fukushima, S.; Akita, K.; Takagi, T.; Kobayashi, K.; Moritoki, N.; Sugaya, H.; Arimura, S.-I.; Kuroiwa, H.; Kuroiwa, T.; Nagata, N. Existence of Giant Mitochondria-Containing Sheet Structures Lacking Cristae and Matrix in the Etiolated Cotyledon of Arabidopsis thaliana. Protoplasma 2022, 259, 731–742. [Google Scholar] [CrossRef]
- Foissner, I. Inhibitor Studies on Formation of Giant Mitochondria in Nitella flexilis. Phyton 1983, 23, 19–29. [Google Scholar]
- Rakhmatullina, D.; Ponomareva, A.; Gazizova, N.; Minibayeva, F. Mitochondrial Morphology and Dynamics in Triticum aestivum Roots in Response to Rotenone and Antimycin A. Protoplasma 2016, 253, 1299–1308. [Google Scholar] [CrossRef]
- Van Gestel, K.; Verbelen, J.P. Giant Mitochondria Are a Response to Low Oxygen Pressure in Cells of Tobacco (Nicotiana tabacum L.). J. Exp. Bot. 2002, 53, 1215–1218. [Google Scholar] [CrossRef]
- White, R.R.; Lin, C.; Leaves, I.; Castro, I.G.; Metz, J.; Bateman, B.C.; Botchway, S.W.; Ward, A.D.; Ashwin, P.; Sparkes, I. Miro2 Tethers the ER to Mitochondria to Promote Mitochondrial Fusion in Tobacco Leaf Epidermal Cells. Commun. Biol. 2020, 3, 161. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Jeong, S.Y.; Karbowski, M.; Smith, C.L.; Youle, R.J. Roles of the Mammalian Mitochondrial Fission and Fusion Mediators Fis1, Drp1, and Opa1 in Apoptosis. Mol. Biol. Cell. 2004, 15, 5001–5011. [Google Scholar] [CrossRef]
- Westermann, B. Bioenergetic Role of Mitochondrial Fusion and Fission. Biochim. Biophys. Acta 2012, 1817, 1833–1838. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C. Fusion and Fission: Interlinked Processes Critical for Mitochondrial Health. Ann. Rev. Genet. 2012, 46, 265–287. [Google Scholar] [CrossRef]
- Hoppins, S. The Regulation of Mitochondrial Dynamics. Curr. Opin. Cell Biol. 2014, 29, 46–52. [Google Scholar] [CrossRef]
- Wang, F.; Liu, P.; Zhang, Q.; Zhu, J.; Chen, T.; Arimura, S.-I.; Tsutsumi, N.; Lin, J. Phosphorylation and Ubiquitination of Dynamin-Related Proteins (AtDRP3A/3B) Synergically Regulate Mitochondrial Proliferation During Mitosis. Plant J. 2012, 72, 43–56. [Google Scholar] [CrossRef]
- Arimura, S.-I.; Fujimoto, M.; Doniwa, Y.; Kadoya, N.; Nakazono, M.; Sakamoto, W.; Tsutsumi, N. Arabidopsis ELONGATED MITOCHONDRIA1 Is Required for Localization of DYNAMIN-RELATED PROTEIN3A to Mitochondrial Fission Sites. Plant Cell 2008, 20, 1555–1566. [Google Scholar] [CrossRef]
- Pan, R.; Hu, J. The Conserved Fission Complex on Peroxisomes and Mitochondria. Plant Signal. Behav. 2011, 6, 870–872. [Google Scholar] [CrossRef]
- Gao, D.; Yang, J.; Wu, Y.; Wang, Q.; Wang, Q.; Lai, E.Y.; Zhu, J. Targeting Dynamin 2 As A Novel Pathway to Inhibit Cardiomyocyte Apoptosis Following Oxidative Stress. Cell Physiol. Biochem. 2016, 39, 2121–2134. [Google Scholar] [CrossRef]
- Mueller, S.J.; Reski, R. Mitochondrial Dynamics and the ER: The Plant Perspective. Front. Cell. Dev. Biol. 2015, 3, 78. [Google Scholar] [CrossRef]
- Lackner, L.; Nunnari, J. Small Molecule Inhibitors of Mitochondrial Division: Tools That Translate Basic Biological Research into Medicine. Chem. Biol. 2010, 17, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Cassidy-Stone, A.; Chipuk, J.E.; Ingerman, E.; Song, C.; Yoo, C.; Kuwana, T.; Kurth, M.J.; Shaw, J.T.; Hinshaw, J.E.; Green, D.R.; et al. Chemical Inhibition of the Mitochondrial Division Dynamin Reveals Its Role in Bax/Bak-Dependent Mitochondrial Outer Membrane Permeabilization. Dev. Cell. 2008, 14, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Martin, A.; Cufi, S.; Corominas-Faja, B.; Oliveras-Ferraros, C.; Vellon, L.; Menendez, J.A. Mitochondrial Fusion by Pharmacological Manipulation Impedes Somatic Cell Reprogramming to Pluripotency: New Insight into The Role of Mitophagy in Cell Stemness. Aging 2012, 4, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H. Inhibitors of Mitochondrial Fission as a Therapeutic Strategy for Diseases with Oxidative Stress and Mitochondrial Disfunction. J. Alzheimers Dis. 2014, 40, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Rosdah, A.A.; Holien, K.J.; Delbridge, L.M.; Dusting, G.J.; Lim, S.Y. Mitochondrial fission—A Drug Target for Cytoprotection or Cytodestruction? Pharmacol. Res. Perspect. 2016, 4, e00235. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S. Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol. 1994, 233, 182–189. [Google Scholar]
- Kumar, G.; Knowles, N.R. Changes in Lipid Peroxidation and Lipolytic and Free-Radical Scavenging Enzyme Actvities During Aging and Sprouting of Potato (Solanum tuberosum) Seed-Tubers. Plant Physiol. 1993, 102, 115–124. [Google Scholar] [CrossRef]
- Paolacci, A.R.; Tanzarella, O.A.; Porceddu, E.; Ciaffi, M. Identification and Validation of Reference Genes for Quantitative RT-PCR Normalization in Wheat. BMC Mol. Biol. 2009, 10, 11. [Google Scholar] [CrossRef]
- Zeng, L.; Deng, R.; Guo, Z.; Yang, S.; Deng, X. Genome-Wide Identification and Characterization of Glyceraldehyde-3-Phosphate Dehydrogenase Genes Family in Wheat (Triticum aestivum). BMC Genomics 2016, 17, 240. [Google Scholar] [CrossRef]
- Scott, I.; Logan, D.C. Mitochondrial dynamics. In Plant Mitochondria; Kempken, F., Ed.; Springer: New York, NY, USA, 2011; pp. 31–63. [Google Scholar]
- Welchen, E.; Garcia, L.; Mansilla, N.; Gonzalez, D. Coordination of Plant Mitochondrial Biogenesis: Keeping Pace with Cellular Requirements. Front. Plant Sci. 2014, 1, 551. [Google Scholar] [CrossRef]
- Logan, D. The Dynamic Plant Chondriome. Semin. Cell Dev. Biol. 2010, 21, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Virolainen, E.; Blokhina, O.; Fagerstedt, K. Ca2+-Induced High Amplitude Swelling and Cytochrome C Release from Wheat (Triticum aestivum L.) Mitochondria Under Anoxic Stress. Ann. Bot. 2002, 90, 509–516. [Google Scholar] [CrossRef]
- Vartapetian, B.B.; Andreeva, I.N.; Generosova, I.P.; Polyakova, L.I.; Maslova, I.P.; Dolgikh, Y.I.; Stepanova, A.Y. Functional Electron Microscopy in Studies of Plant Response and Adaptation to Anaerobic Stress. Ann. Bot. 2003, 91, 155–172. [Google Scholar] [CrossRef] [PubMed]
- Rurek, M.; Woyda-Ploszczyca, A.M.; Jarmuszkiewicz, W. Biogenesis of Mitochondria in Cauliflower (Brassica Oleracea Var. Botrytis) Curds Subjected to Temperature Stress and Recovery Involves Regulation of the Complexome, Respiratory Chain Activity, Organellar Translation and Ultrastructure. Biochim. Biophys. Acta 2015, 1847, 399–417. [Google Scholar] [CrossRef][Green Version]
- Arpagaus, S.; Rawyler, A.; Braendle, R. Occurrence and Characteristics of the Mitochondrial Permeability Transition in Plants. J. Biol. Chem. 2002, 277, 1780–1787. [Google Scholar] [CrossRef]
- Murphy, C.; Wilson, J.M. Ultrastructural Features of Chilling–Injury in Episcia Reptans. Plant Cell Environ. 1981, 4, 261–265. [Google Scholar]
- Lee, S.H.; Singh, A.P.; Chung, G.C.; Kim, Y.S.; Kong, I. Chilling Root Temperature Causes Rapid Ultrastructural Changes in Cortical Cells in Cucumber (Cucumis sativus L.) Root Tips. J. Exp. Bot. 2002, 53, 2225–2237. [Google Scholar] [CrossRef]
- Arimura, S.-I.; Kurisu, R.; Sugaya, H.; Kadoya, N.; Tsutsumi, N. Cold Treatment Induces Transient Mitochondrial Fragmentation in Arabidopsis Thaliana in a Way That Requires DRP3A But Not ELM1 or an ELM1-Like Homologue ELM2. Int. J. Mol. Sci. 2017, 18, 2161. [Google Scholar] [CrossRef]
- Zick, M.; Rabl, R.; Reichert, A. Cristae formation–Linking Ultrastructure and Function of Mitochondria. Biochem. Biophys. Acta 2009, 1793, 5–19. [Google Scholar] [CrossRef]
- Bordt, E.A.; Clerc, P.; Roelofs, B.A.; Saladino, A.J.; Tretter, L.; Adam-Vizi, V.; Cherok, E.; Khalil, A.; Yadava, N.; Ge, S.X.; et al. The Putative Drp1 Inhibitor Mdivi-1 Is a Reversible Mitochondrial Complex I Inhibitor That Modulates Reactive Oxygen Species. Dev. Cell 2017, 40, 583–594. [Google Scholar] [CrossRef]
- Manczak, M.; Kandimalla, R.; Yin, X.; Reddy, P.H. Mitochondrial Division Inhibitor 1 Reduces Dynamin-Related Protein 1 and Mitochondrial Fission Activity. Hum. Mol. Genet. 2019, 28, 177–199. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.T.; Henson, D.; Nyirenda, M.; Desikan, R.; Harrison, J.; Lewis, M.; Hughes, J.; Neill, S.J. Proteomic Identification of Glyceraldehyde 3-Phosphate Dehydrogenase as an Inhibitory Target of Hydrogen Peroxide in Arabidopsis. Plant Physiol. Biochem. 2005, 43, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Knuesting, J.; Birkholz, O.; Jürgen, J.; Scheibe, H.R. Cytosolic GAPDH as a Redox-Dependent Regulator of Energy Metabolism. BMC Plant Biol. 2018, 18, 184. [Google Scholar] [CrossRef]
- Wurzinger, B.; Nukarinen, E.; Nagele, T.; Weckwerth, W.; Teige, M. The SnRK1 Kinase as a Central Mediator of Energy Signaling Between Different Organelles. Plant Physiol. 2018, 176, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Halford, N.G.; Hey, S. Snf1-Related Protein Kinases (SnRKs) Acts Within an Intricate Network That Links Metabolic and Stress Signaling in Plants. Biochem. J. 2009, 419, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Toyama, E.Q.; Herzig, S.; Courchet, J.; Tommy, L.L.; Losón, O.C.; Hellberg, K.; Young, N.P.; Chen, H.; Polleux, F.; Chan, D.C.; et al. AMP-Activated Protein Kinase Mediates Mitochondrial Fission in Response to Energy Stress. Science 2016, 351, 275–281. [Google Scholar] [CrossRef]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef]
- Minibayeva, F.; Dmitrieva, S.; Ponomareva, A.; Ryabovol, V. Oxidative Stress-Induced Autophagy in Plants: The Role of Mitochondria. Plant Physiol. Biochem. 2012, 59, 11–19. [Google Scholar] [CrossRef]
- Nakatogawa, H.; Ichimura, Y.; Ohsumi, Y. Atg8, a Ubiquitin-Like Protein Required for Autophagosome Formation, Mediates Membrane Tethering and Hemifusion. Cell 2007, 130, 165–178. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rakhmatullina, D.; Mazina, A.; Ponomareva, A.; Dmitrieva, S.; Beckett, R.P.; Minibayeva, F. Mdivi-1 Induced Mitochondrial Fusion as a Potential Mechanism to Enhance Stress Tolerance in Wheat. Life 2022, 12, 1386. https://doi.org/10.3390/life12091386
Rakhmatullina D, Mazina A, Ponomareva A, Dmitrieva S, Beckett RP, Minibayeva F. Mdivi-1 Induced Mitochondrial Fusion as a Potential Mechanism to Enhance Stress Tolerance in Wheat. Life. 2022; 12(9):1386. https://doi.org/10.3390/life12091386
Chicago/Turabian StyleRakhmatullina, Daniya, Anastasia Mazina, Anastasia Ponomareva, Svetlana Dmitrieva, Richard Peter Beckett, and Farida Minibayeva. 2022. "Mdivi-1 Induced Mitochondrial Fusion as a Potential Mechanism to Enhance Stress Tolerance in Wheat" Life 12, no. 9: 1386. https://doi.org/10.3390/life12091386
APA StyleRakhmatullina, D., Mazina, A., Ponomareva, A., Dmitrieva, S., Beckett, R. P., & Minibayeva, F. (2022). Mdivi-1 Induced Mitochondrial Fusion as a Potential Mechanism to Enhance Stress Tolerance in Wheat. Life, 12(9), 1386. https://doi.org/10.3390/life12091386





