Real and Simulated Microgravity: Focus on Mammalian Extracellular Matrix
Abstract
1. Introduction
2. Cells in the Local Microenvironment: Functional Unit of Mechanoreception
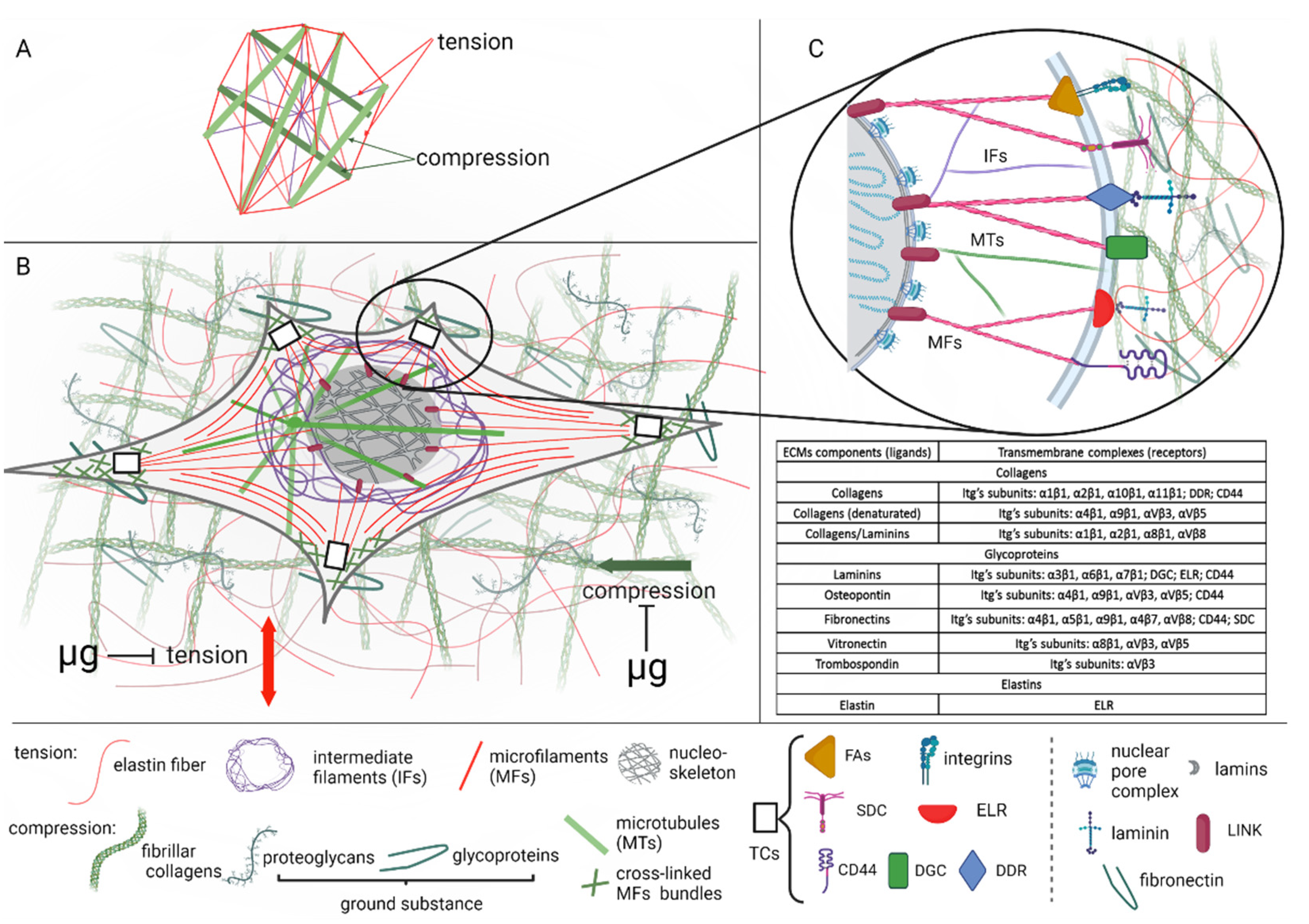
3. Extracellular Matrix Composition and Tensegrity
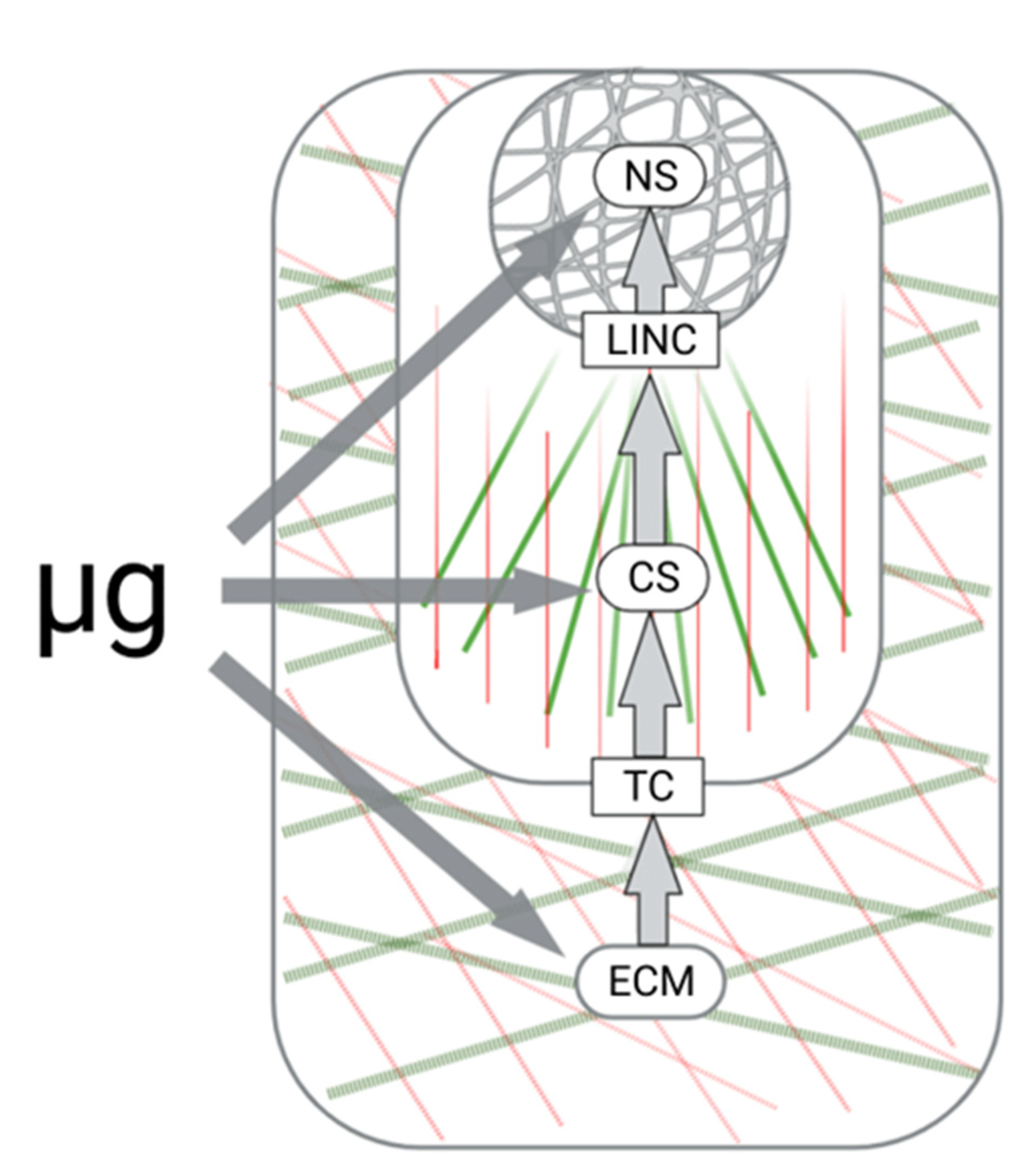
4. Extracellular Matrix and Microgravity
5. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Daley, W.P.; Peters, S.B.; Larsen, M. Extracellular matrix dynamics in development and regenerative medicine. J. Cell. Sci. 2008, 121, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Gattazzo, F.; Urciuolo, A.; Bonaldo, P. Extracellular matrix: A dynamic microenvironment for stem cell niche. Biochim. Biophys. Acta 2014, 1840, 2506–2519. [Google Scholar] [CrossRef] [PubMed]
- White, R.J.; Averner, M. Humans in space. Nature 2001, 409, 1115–1118. [Google Scholar] [CrossRef]
- Oganov, V. Modern analysis of bone loss mechanisms in microgravity. J. Gravit. Physiol. 2004, 11, P143–P146. [Google Scholar]
- Blaber., E.; Marçal, H.; Burns, B.P. Bioastronautics: The influence of microgravity on astronaut health. Astrobiology 2010, 10, 463–473. [Google Scholar] [CrossRef]
- Bandzerewicz, A.; Gadomska-Gajadhur, A. Into the tissues: Extracellular matrix and its artificial substitutes: Cell Signalling Mechanisms. Cells 2022, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.D.; Humphries, M.J. Integrin structure, activation, and interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a004994. [Google Scholar] [CrossRef]
- Haller, S.J.; Dudley, A.T. Extracellular mechanotransduction. J. Gen. Physiol. 2022, 154, e202113026. [Google Scholar] [CrossRef]
- Baker, B.M.; Trappmann, B.; Wang, W.Y.; Sakar, M.S.; Kim, I.L.; Shenoy, V.B.; Burdick, J.A.; Chen, C.S. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat. Mater. 2015, 14, 1262–1268. [Google Scholar] [CrossRef]
- Handorf, A.M.; Zhou, Y.; Halanski, M.A.; Li, W.J. Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis 2015, 11, 1–15. [Google Scholar] [CrossRef]
- Noguera, R.; Nieto, O.A.; Tadeo, I.; Fariñas, F.; Alvaro, T. Extracellular matrix, biotensegrity and tumor microenvironment. An update and overview. Histol. Histopathol. 2012, 27, 693–705. [Google Scholar] [PubMed]
- Ingber, D.E.; Wang, N.; Stamenovic, D. Tensegrity, cellular biophysics, and the mechanics of living systems. Rep. Prog. Phys. 2014, 77, 046603. [Google Scholar] [CrossRef] [PubMed]
- Jansen, K.A.; Donato, D.M.; Balcioglu, H.E.; Schmidt, T.; Danen, E.H.; Koenderink, G.H. A guide to mechanobiology: Where biology and physics meet. Biochim. Biophys. Acta 2015, 1853, 3043–3052. [Google Scholar] [CrossRef]
- Argentati, C.; Morena, F.; Tortorella, I.; Bazzucchi, M.; Porcellati, S.; Emiliani, C.; Martino, S. Insight into Mechanobiology: How stem cells feel mechanical forces and orchestrate biological functions. Int. J. Mol. Sci. 2019, 20, 5337. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Tensegrity: The architectural basis of cellular mechanotransduction. Annu. Rev. Physiol. 1997, 59, 575–599. [Google Scholar] [CrossRef]
- Giannone, G.; Dubin-Thaler, B.J.; Rossier, O.; Cai, Y.; Chaga, O.; Jiang, G.; Beaver, W.; Döbereiner, H.G.; Freund, Y.; Borisy, G. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell 2007, 128, 561–575. [Google Scholar] [CrossRef]
- Leiphart, R.J.; Chen, D.; Peredo, A.P.; Loneker, A.E.; Janmey, P.A. Mechanosensing at cellular interfaces. Langmuir 2019, 35, 7509–7519. [Google Scholar] [CrossRef]
- Venticinque, L.; Jamieson, K.V.; Meruelo, D. Interactions between laminin receptor and the cytoskeleton during translation and cell motility. PLoS ONE 2011, 6, e15895. [Google Scholar]
- Afratis, N.A.; Nikitovic, D.; Multhaupt, H.A.; Theocharis, A.D.; Couchman, J.R.; Karamanos, N.K. Syndecans—Key regulators of cell signaling and biological functions. FEBS J. 2017, 284, 27–41. [Google Scholar] [CrossRef]
- Chua, I.L.S.; Kim, H.-W.; Lee, J.H. Signaling of extracellular matrices for tissue regeneration and therapeutics. Tissue Eng. Regen. Med. 2016, 13, 1–12. [Google Scholar] [CrossRef]
- Goelzer, M.; Goelzer, J.; Ferguson, M.L.; Neu, C.P.; Uzer, G. Nuclear envelope mechanobiology: Linking the nuclear structure and function. Nucleus 2021, 12, 90–114. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Kamm, R.D.; Lee, R.T. Cell mechanics and mechanotransduction: Pathways, probes, and physiology. Am. J. Physiol. (Cell. Physiol.) 2004, 287, C1–C11. [Google Scholar] [CrossRef]
- Discher, D.E.; Janmey, P.; Wang, Y.L. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef]
- DuFort, C.C.; Paszek, M.J.; Weaver, V.M. Balancing forces: Architectural control of mechanotransduction. Nat. Rev. Mol. Cell. Biol. 2011, 12, 308–319. [Google Scholar] [CrossRef]
- Walters, N.J.; Gentleman, E. Evolving insights in cell-matrix interactions: Elucidating how non-soluble properties of the extracellular niche direct stem cell fate. Acta. Biomater. 2015, 11, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Buravkova, L.B.; Gershovich, P.M.; Gershovich, J.G.; Grigor’ev, A.I. Mechanisms of gravitational sensitivity of osteogenic precursor cells. Acta Nat. 2010, 2, 28–36. [Google Scholar] [CrossRef]
- Hughes-Fulford, M. The role of signaling pathways in osteoblast gravity perception. J. Gravit. Physiol. 2002, 9, P257–P260. [Google Scholar] [PubMed]
- Crawford-Young, S.J. Effects of microgravity on cell cytoskeleton and embryogenesis. Int. J. Dev. Biol. 2006, 50, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Ulbrich, C.; Wehland, M.; Pietsch, J.; Aleshcheva, G.; Wise, P.; van Loon, J.; Magnusson, N.; Infanger, M.; Grosse, J.; Eilles, C.; et al. The impact of simulated and real microgravity on bone cells and mesenchymal stem cells. Biomed. Res. Int. 2014, 2014, 928507. [Google Scholar] [CrossRef]
- Vorselen, D.; Roos, W.H.; MacKintosh, F.C.; Wuite, G.J.; van Loon, J.J. The role of the cytoskeleton in sensing changes in gravity by nonspecialized cells. FASEB J. 2014, 28, 536–547. [Google Scholar] [CrossRef]
- Buravkova, L.B.; Romanov, Y.A. The role of cytoskeleton in cell changes under condition of simulated microgravity. Acta Astronaut. 2001, 48, 647–650. [Google Scholar] [CrossRef]
- Corydon, T.J.; Kopp, S.; Wehland, M.; Braun, M.; Schutte, A.; Mayer, T.; Hulsing, T.; Oltmann, H.; Schmitz, B.; Hemmersbach, R.; et al. Alterations of the cytoskeleton in human cells in space proved by life-cell imaging. Sci. Rep. 2016, 6, 20043. [Google Scholar] [CrossRef]
- Thiel, C.S.; Tauber, S.; Lauber, B.; Polzer, J.; Seebacher, C.; Uhl, R.; Neelam, S.; Zhang, Y.; Levine, H.; Ullrich, O. Rapid morphological and cytoskeletal response to microgravity in human primary macrophages. Int. J. Mol. Sci. 2019, 20, 2402. [Google Scholar] [CrossRef]
- Infanger, M.; Kossmehl, P.; Shakibaei, M.; Bauer, J.; Kossmehl-Zorn, S.; Cogoli, A.; Curcio, F.; Oksche, A.; Wehland, M.; Kreutz, R.; et al. Simulated weightlessness changes the cytoskeleton and extracellular matrix proteins in papillary thyroid carcinoma cells. Cell. Tissue Res. 2006, 324, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Tauber, S.; Lauber, B.A.; Paulsen, K.; Layer, L.E.; Lehmann, M.; Hauschild, S.; Shepherd, N.R.; Polzer, J.; Segerer, J.; Thiel, C.S.; et al. Cytoskeletal stability and metabolic alterations in primary human macrophages in long-term microgravity. PLoS ONE 2017, 12, e0175599. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.L.; Reynolds, J.L.; Cubano, L.A.; Hatton, J.P.; Lawless, B.D.; Piepmeier, E.H. Spaceflight alters microtubules and increases apoptosis in human lymphocytes (Jurkat). FASEB J. 1998, 12, 1007–1018. [Google Scholar] [CrossRef]
- Corydon, T.J.; Mann, V.; Slumstrup, L.; Kopp, S.; Sahana, J.; Askou, A.L.; Magnusson, N.E.; Echegoyen, D.; Bek, T.; Sundaresan, A.; et al. Reduced expression of cytoskeletal and extracellular matrix genes in human adult retinal pigment epithelium cells exposed to simulated microgravity. Cell. Physiol. Biochem. 2016, 40, 1–17. [Google Scholar] [CrossRef]
- Louis, F.; Bouleftour, W.; Rattner, A.; Linossier, M.-T.; Vico, L.; Guignandon, A. Rho-GTPase stimulation is associated with strontium chloride treatment to counter simulated microgravity-induced changes in multipotent cell commitment. NPJ Microgravity 2017, 3, 7. [Google Scholar] [CrossRef]
- Tan, X.; Xu, A.; Zhao, T.; Zhao, Q.; Zhang, J.; Fan, C.; Deng, Y.; Freywald, A.; Genth, H.; Xiang, J. Simulated microgravity inhibits cell focal adhesions leading to reduced melanoma cell proliferation and metastasis via FAK/RhoA-regulated mTORC1 and AMPK pathways. Sci. Rep. 2018, 8, 3769. [Google Scholar] [CrossRef]
- Nassef, M.Z.; Kopp, S.; Wehland, M.; Melnik, D.; Sahana, J.; Kruger, M.; Corydon, T.J.; Oltmann, H.; Schmitz, B.; Schutte, A.; et al. Real microgravity influences the cytoskeleton and focal adhesions in human breast cancer cells. Int. J. Mol. Sci. 2019, 20, 3156. [Google Scholar] [CrossRef]
- Gershovich, P.M.; Gershovich, J.G.; Zhambalova, A.P.; Romanov, Y.A.; Buravkova, L.B. Cytoskeletal proteins and stem cell markers gene expression in human bone marrow mesenchymal stromal cells after different periods of simulated microgravity. Acta Astronaut. 2012, 70, 36–42. [Google Scholar] [CrossRef]
- Grenon, S.M.; Jeanne, M.; Aguado-Zuniga, J.; Conte, M.S.; Hughes-Fulford, M. Effects of gravitational mechanical unloading in endothelial cells: Association between caveolins, inflammation and adhesion molecules. Sci. Rep. 2013, 3, 1494. [Google Scholar] [CrossRef] [PubMed]
- Ratushnyy, A.Y.; Buravkova, L.B. Expression of focal adhesion genes in mesenchymal stem cells under simulated microgravity. Dokl. Biochem. Biophys. 2017, 477, 354–356. [Google Scholar] [CrossRef] [PubMed]
- Guignandon, A.; Akhouayri, O.; Usson, Y.; Rattner, A.; Laroche, N.; Lafage-Proust, M.H.; Alexandre, C.; Vico, L. Focal contact clustering in osteoblastic cells under mechanical stresses: Microgravity and cyclic deformation. Cell. Commun. Adhes. 2003, 10, 69–83. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jo, J.; Abdi Nansa, S.; Kim, D.H. Molecular regulators of cellular mechanoadaptation at cell-material interfaces. Front. Bioeng. Biotechnol. 2020, 8, 608569. [Google Scholar] [CrossRef]
- Ahn, C.B.; Lee, J.H.; Han, D.G.; Kang, H.W.; Lee, S.H.; Lee, J.I.; Son, K.H.; Lee, J.W. Simulated microgravity with floating environment promotes migration of non-small cell lung cancers. Sci. Rep. 2019, 9, 14553. [Google Scholar] [CrossRef]
- Dietz, C.; Infanger, M.; Romswinkel, A.; Strube, F.; Kraus, A. Apoptosis induction and alteration of cell adherence in human lung cancer cells under simulated microgravity. Int. J. Mol. Sci. 2019, 20, 3601. [Google Scholar] [CrossRef]
- Lombardi, M.L.; Jaalouk, D.E.; Shanahan, C.M.; Burke, B.; Roux, K.J.; Lammerding, J. The interaction between nesprins and sun proteins at the nuclear envelope is critical for force transmission between the nucleus and cytoskeleton. J. Biol. Chem. 2011, 286, 26743–26753. [Google Scholar] [CrossRef]
- Fedorchak, G.R.; Kaminski, A.; Lammerding, J. Cellular mechanosensing: Getting to the nucleus of it all. Prog. Biophys. Mol. Biol. 2014, 115, 76–92. [Google Scholar] [CrossRef]
- Navarro, A.; Collins, M.; Folker, E. The nucleus is a conserved mechanosensation and mechanoresponse organelle. Cytoskeleton 2016, 73, 59–67. [Google Scholar] [CrossRef]
- Belaadi, N.; Aureille, J.; Guilluy, C. Under pressure: Mechanical stress management in the nucleus. Cells 2016, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Clause, K.C.; Barker, T.H. Extracellular matrix signaling in morphogenesis and repair. Curr. Opin. Biotechnol. 2013, 24, 830–833. [Google Scholar] [CrossRef]
- Hynes, R.O.; Naba, A. Overview of the matrisome—An inventory of extracellular matrix constituents and functions. Cold Spring Harb. Perspect. Biol. 2012, 4, a004903. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix. Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell. Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef]
- Halper, J.K.M. Basic components of connective tissues and extracellular matrix: Elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv. Exp. Med. Biol. 2014, 802, 31–47. [Google Scholar]
- Zollinger, A.J.; Smith, M.L. Fibronectin, the extracellular glue. Matrix. Biol. 2017, 60–61, 27–37. [Google Scholar] [CrossRef]
- Ruoslahti, E. RGD and other recognition sequences for integrins. Annu. Rev. Cell Dev. Biol. 1996, 12, 697–715. [Google Scholar] [CrossRef]
- Uitto, J.; Christiano, A.M.; Kahari, V.M.; Bashir, M.M.; Rosenbloom, J. Molecular biology and pathology of human elastin. Biochem. Soc. Trans. 1991, 19, 824–829. [Google Scholar] [CrossRef]
- Swee, M.H.; Parks, W.C.; Pierce, R.A. Developmental regulation of elastin production. Expression of tropoelastin pre-mRNA persists after down-regulation of steady-state mRNA levels. J. Biol. Chem. 1995, 270, 14899–14906. [Google Scholar] [CrossRef]
- Petersen, E.; Wagberg, F.; Angquist, K.A. Serum concentrations of elastin-derived peptides in patients with specific manifestations of atherosclerotic disease. Eur. J. Vasc. Endovasc. Surg. 2002, 24, 440–444. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth factors, matrices, and forces combine and control stem cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef]
- Rahman, S.; Patel, Y.; Murray, J.; Patel, K.V.; Sumathipala, R.; Sobel, M.; Wijelath, E.S. Novel hepatocyte growth factor (HGF) binding domains on fibronectin and vitronectin coordinate a distinct and amplified Met-integrin induced signalling pathway in endothelial cells. BMC Cell Biol. 2005, 6, 8. [Google Scholar] [CrossRef]
- Martino, M.M.; Brkic, S.; Bovo, E.; Burger, M.; Schaefer, D.J.; Wolff, T.; Gurke, L.; Briquez, P.S.; Larsson, H.M.; Gianni-Barrera, R.; et al. Extracellular matrix and growth factor engineering for controlled angiogenesis in regenerative medicine. Front. Bioeng. Biotechnol. 2015, 3, 45. [Google Scholar] [CrossRef]
- Bellon, G.; Martiny, L.; Robinet, A. Matrix metalloproteinases and matrikines in angiogenesis. Crit. Rev. Oncol./Hematol. 2004, 49, 203–220. [Google Scholar] [CrossRef]
- Adair-Kirk, T.L.; Senior, R.M. Fragments of extracellular matrix as mediators of inflammation. Int. J. Biochem. Cell. Biol. 2008, 40, 1101–1110. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Ding, H.; Whittaker, C.A.; Carr, S.A.; Hynes, R.O. The extracellular matrix: Tools and insights for the “omics” era. Matrix. Biol. 2016, 49, 10–24. [Google Scholar] [CrossRef]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef]
- Mustaţă, T.; Rusu, V. Mechanotransduction and tensegrity (I). Rev. Med. Chir. Soc. Med. Nat. Iasi. 1998, 102, 25–35. [Google Scholar] [PubMed]
- Huang, C.; Miyazaki, K.; Akaishi, S.; Watanabe, A.; Hyakusoku, H.; Ogawa, R. Biological effects of cellular stretch on human dermal fibroblasts. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, e351–e361. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; Sasso, G.R.d.S.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.K.; Aloia, J.F.; Tierney, J.M.; Sprintz, S. Effect of treadmill exercise on vertebral and tibial bone mineral content and bone mineral density in the aged adult rat: Determined by dual energy X-ray absorptiometry. Calcif. Tissue Int. 1993, 52, 234–238. [Google Scholar] [CrossRef]
- Raisz, L.G. Physiology and pathophysiology of bone remodeling. Clin. Chem. 1999, 45, 1353–1358. [Google Scholar]
- Reijnders, K.; Coppes, M.; van Hulzen, A.; Gravendeel, J.; Ginkel, R.J.; Hoekstra, H. Image guided surgery: New technology for surgery of soft tissue and bone sarcomas. Eur. J. Surg. Oncol. 2007, 33, 390–398. [Google Scholar] [CrossRef]
- Zerath, E. Gravitational microenvironment and bone tissue: Lessions from space studies. In Adaptation Biology and Medicine; Moravec, J., Takeda, N., Singai, P.K., Eds.; Narosa Publishing House: New Deli, India, 2002; pp. 202–210. [Google Scholar]
- Vico, L.; Hinsenkamp, M.; Jones, D.; Marie, P.J.; Zallone, A.; Cancedda, R. Osteobiology, strain, and microgravity. Part II: Studies at the tissue level. Calcif. Tissue Int. 2001, 68, 1–10. [Google Scholar] [CrossRef]
- Grimm, D.; Grosse, J.; Wehland, M.; Mann, V.; Reseland, J.E.; Sundaresan, A.; Corydon, T.J. The impact of microgravity on bone in humans. Bone 2016, 87, 44–56. [Google Scholar] [CrossRef]
- Sibonga, J.; Evans, H.J.; Sung, H.G.; Spector, E.; Lang, T.; Oganov, V.S.; Bakulin, A.V.; Shackelford, L.; LeBlanc, A.D. Recovery of spaceflight-induced bone loss: Bone mineral density after long-duration missions as fitted with an exponential function. Bone 2008, 41, 973–978. [Google Scholar] [CrossRef]
- Nagaraja, M.P.; Risin, D. The current state of bone loss research: Data from spaceflight and microgravity simulators. J. Cell. Biochem. 2013, 114, 1001–1008. [Google Scholar] [CrossRef]
- Smith, S.; Heer, M.; Shackelford, L.; Sibonga, J.; Spatz, J.; Pietrzyk, R.; Hudson, E.; Zwart, S. Bone metabolism and renal stone risk during International Space Station missions. Bone 2015, 81, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Jee, W.S.; Wronski, T.J.; Morey, E.R.; Kimmel, D.B. Effects of spaceflight on trabecular bone in rats. Am. J. Physiol. 1983, 244, R310–R314. [Google Scholar] [CrossRef] [PubMed]
- Wronski, T.J.; Morey, E.R. Effect of spaceflight on periosteal bone formation in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1983, 244, R305–R309. [Google Scholar] [CrossRef]
- Zernicke, R.F.; Vailas, A.C.; Salem, G.J. Biomechanical response of bone to weightlessness. Exerc. Sport. Sci. Rev. 1990, 18, 167–192. [Google Scholar] [CrossRef]
- Pedrini-Mille, A.; Maynard, J.A.; Durnova, G.N.; Kaplansky, A.S.; Pedrini, V.A.; Chung, C.B.; Fedler-Troester, J. Effects of microgravity on the composition of the intervertebral disk. J. Appl. Physiol. 1992, 73, S26–S32. [Google Scholar] [CrossRef] [PubMed]
- Durnova, G.; Kaplansky, A.; Morey-Holton, E. Histomorphometric study of tibia of rats exposed aboard American Spacelab Life Sciences 2 Shuttle Mission. J. Gravit. Physiol. 1996, 3, 80–81. [Google Scholar] [PubMed]
- Ilyin, E. Historical overview of the Bion project. J. Gravit. Physiol. 2000, 7, S1–S8. [Google Scholar]
- Arnaud, S.B.; Buckendahl, P.; Durnova, G.; Bromage, T.; Yamauchi, M. Bone biochemistry in rat femoral diaphysis after space flight. J. Gravit. Physiol. 2000, 7, 7–15. [Google Scholar]
- Evans, G.L.; Morey-Holton, E.; Turner, R.T. Spaceflight has compartment- and gene-specific effects on mRNA levels for bone matrix proteins in rat femur. J. Appl. Physiol. 1998, 84, 2132–2137. [Google Scholar] [CrossRef]
- Van Loon, J.J.; Bervoets, D.J.; Burger, E.H.; Dieudonne, S.C.; Hagen, J.W.; Semeins, C.M.; Doulabi, B.Z.; Veldhuijzen, J.P. Decreased mineralization and increased calcium release in isolated fetal mouse long bones under near weightlessness. J. Bone Miner. Res. 1995, 10, 550–557. [Google Scholar] [CrossRef]
- Fitzgerald, J.; Endicott, J.; Hansen, U.; Janowitz, C. Articular cartilage and sternal fibrocartilage respond differently to extended microgravity. NPJ Microgravity 2019, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Chatani, M.; Morimoto, H.; Takeyama, K.; Mantoku, A.; Tanigawa, N.; Kubota, K.; Suzuki, H.; Uchida, S.; Tanigaki, F.; Shirakawa, M.; et al. Acute transcriptional up-regulation specific to osteoblasts/osteoclasts in medaka fish immediately after exposure to microgravity. Sci. Rep. 2016, 6, 39545. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Goldsmith, M.; Crooks, S.D.; Condon, S.F.; Morris, M.; Komarova, S.V. Bone health in spacefaring rodents and primates: Systematic review and meta-analysis. NPJ Microgravity 2021, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Globus, R.K.; Morey-Holton, E. Hindlimb unloading: Rodent analog for microgravity. J. Appl. Physiol. 2016, 120, 1196–1206. [Google Scholar] [CrossRef]
- Carmeliet, G.; Nys, G.; Bouillon, R. Microgravity reduces the differentiation of human osteoblastic MG-63 cells. JBMR 1997, 12, 786–794. [Google Scholar] [CrossRef]
- Bikle, D.D.; Harris, J.; Halloran, B.P.; Morey-Holton, E. Altered skeletal pattern of gene expression in response to spaceflight and hindlimb elevation. Am. J. Physiol. Endocrinol. Metab. 1994, 267, E822–E827. [Google Scholar] [CrossRef]
- Landis, W.J.; Hodgens, K.J.; Block, D.; Toma, C.D.; Gerstenfeld, L.C. Spaceflight effects on cultured embryonic chick bone cells. JBMR 2000, 15, 1099–1112. [Google Scholar] [CrossRef]
- Harris, S.A.; Zhang, M.; Kidder, L.S.; Evans, G.L.; Spelsberg, T.C.; Turner, R.T. Effects of orbital spaceflight on human osteoblastic cell physiology and gene expression. Bone 2000, 26, 325–331. [Google Scholar] [CrossRef]
- Bradbury, P.; Wu, H.; Choi, J.U.; Rowan, A.E.; Zhang, H.; Poole, K.; Lauko, J.; Chou, J. Modeling the impact of microgravity at the cellular level: Implications for human disease. Front. Cell. Dev. Biol. 2020, 8, 96. [Google Scholar] [CrossRef]
- Buken, C.; Sahana, J.; Corydon, T.; Melnik, D.; Bauer, J.; Wehland, M.; Krüger, M.; Balk, S.; Abuagela, N.; Infanger, M.; et al. Morphological and molecular changes in juvenile normal human fibroblasts exposed to simulated microgravity. Sci. Rep. 2019, 9, 11882. [Google Scholar] [CrossRef]
- Ebnerasuly, F.; Hajebrahimi, Z.; Tabaie, S.M.; Darbouy, M. Simulated microgravity condition alters the gene expression of some ECM and adhesion molecules in adipose derived stem cells. Int. J. Mol. Cell. Med. 2018, 7, 146–157. [Google Scholar] [PubMed]
- Makihira, S.; Kawahara, Y.; Yuge, L.; Mine, Y.; Nikawa, H. Impact of the microgravity environment in a 3-dimensional clinostat on osteoblast- and osteoclast-like cells. Cell. Biol. Int. 2008, 32, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Pardo, S.; Patel, M.; Sykes, M.; Platt, M.; Boyd, N.; Sorescu, G.; Xu, M.; Loon, J.; Wang, M.; Jo, H. Simulated microgravity using the Random Positioning Machine inhibits differentiation and alters gene expression profiles of 2T3 preosteoblasts. Am. J. Physiol. Cell. Physiol. 2005, 288, C1211–C1221. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wang, R.; Ling, S.; Wan, Y.M.; Li, Y. Simulated microgravity inhibits the proliferation and osteogenesis of rat bone marrow mesenchymal stem cells. Cell. Prolif. 2008, 41, 375. [Google Scholar] [CrossRef]
- Zhivodernikov, I.V.; Ratushnyy, A.Y.; Matveeva, D.K.; Buravkova, L.B. Extracellular matrix proteins and transcription of matrix-associated genes in mesenchymal stromal cells during modeling of the effects of microgravity. Bull. Exp. Biol. Med. 2020, 170, 230–232. [Google Scholar] [CrossRef]
- Uddin, S.M.Z.; Qin, Y.X. Enhancement of osteogenic differentiation and proliferation in human mesenchymal stem cells by a modified low intensity ultrasound stimulation under simulated microgravity. PLoS ONE 2013, 8, e73914. [Google Scholar]
- Urbanczyk, M.; Layland, S.L.; Schenke-Layland, K. The role of extracellular matrix in biomechanics and its impact on bioengineering of cells and 3D tissues. Matrix. Biol. 2020, 85–86, 1–14. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreeva, E.; Matveeva, D.; Zhidkova, O.; Zhivodernikov, I.; Kotov, O.; Buravkova, L. Real and Simulated Microgravity: Focus on Mammalian Extracellular Matrix. Life 2022, 12, 1343. https://doi.org/10.3390/life12091343
Andreeva E, Matveeva D, Zhidkova O, Zhivodernikov I, Kotov O, Buravkova L. Real and Simulated Microgravity: Focus on Mammalian Extracellular Matrix. Life. 2022; 12(9):1343. https://doi.org/10.3390/life12091343
Chicago/Turabian StyleAndreeva, Elena, Diana Matveeva, Olga Zhidkova, Ivan Zhivodernikov, Oleg Kotov, and Ludmila Buravkova. 2022. "Real and Simulated Microgravity: Focus on Mammalian Extracellular Matrix" Life 12, no. 9: 1343. https://doi.org/10.3390/life12091343
APA StyleAndreeva, E., Matveeva, D., Zhidkova, O., Zhivodernikov, I., Kotov, O., & Buravkova, L. (2022). Real and Simulated Microgravity: Focus on Mammalian Extracellular Matrix. Life, 12(9), 1343. https://doi.org/10.3390/life12091343





