Endothelial Dysfunction in Patients with Advanced Heart Failure Treated with Levosimendan Periodic Infusion Compared with Optimal Medical Therapy: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Protocol
2.3. Flow-Mediated Vasodilation
2.4. Cardiac Ultrasonography
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Cortese, F.; Ciccone, M.M.; Gesualdo, M.; Iacoviello, M.; Frigerio, M.; Cipriani, M.; Giannattasio, C.; Maloberti, A.; Giordano, P. Continuous flow left ventricular assist devices do not worsen endothelial function in subjects with chronic heart failure: A pilot study. ESC Heart Fail. 2021, 8, 3587–3593. [Google Scholar] [CrossRef] [PubMed]
- Giannitsi, S.; Maria, B.; Bechlioulis, A.; Naka, K. Endothelial dysfunction and heart failure: A review of the existing bibliography with emphasis on flow mediated dilation. JRSM Cardiovasc. Dis. 2019, 8, 204800401984304. [Google Scholar] [CrossRef] [PubMed]
- Alem, M.M. Endothelial dysfunction in chronic heart failure: Assessment, findings, significance, and potential therapeutic targets. Int. J. Mol. Sci. 2019, 13, 3198. [Google Scholar] [CrossRef] [PubMed]
- Corretti, M.C.; Anderson, T.J.; Benjamin, E.J.; Celermajer, D.; Charbonneau, F.; Creager, M.A.; Deanfield, J.; Drexler, H.; Gerhard-Herman, M.; Herrington, D.; et al. International Brachial Artery Reactivity Task Force. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the international brachial artery reactivity task force. J. Am. Coll. Cardiol. 2002, 2, 257–265. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Bruno, R.M.; van Mil, A.C.C.M.; Holder, S.M.; Faita, F.; Greyling, A.; Zock, P.L.; Taddei, S.; Deanfield, J.E.; Luscher, T.; et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019, 40, 2534–2547. [Google Scholar] [CrossRef]
- Shahin, Y.; Khan, J.A.; Samuel, N.; Chetter, I. Angiotensin converting enzyme inhibitors effect on endothelial dysfunction: A meta-analysis of randomised controlled trials. Atherosclerosis 2011, 1, 7–16. [Google Scholar] [CrossRef]
- Souza-Barbosa, L.A.; Ferreira-Melo, S.E.; Ubaid-Girioli, S.; Arantes Nogueira, E.; Yugar-Toledo, J.C.; Moreno, H. Endothelial vascular function in hypertensive patients after renin-angiotensin system blockade. J. Clin. Hypertens. 2006, 11, 803–811. [Google Scholar] [CrossRef]
- Erbs, S.; Beck, E.B.; Linke, A.; Adams, V.; Gielen, S.; Kränkel, N.; Möbius-Winkler, S.; Höllriegel, R.; Thiele, H.; Hambrecht, R.; et al. High-dose rosuvastatin in chronic heart failure promotes vasculogenesis, corrects endothelial function, and improves cardiac remodeling—Results from a randomized, double-blind, and placebo-controlled study. Int. J. Cardiol. 2011, 146, 56–63. [Google Scholar] [CrossRef]
- Tousoulis, D.; Andreou, I.; Tsiatas, M.; Miliou, A.; Tentolouris, C.; Siasos, G.; Papageorgiou, N.; Papadimitriou, C.A.; Dimopoulos, M.A.; Stefanadis, C. Effects of rosuvastatin and allopurinol on circulating endothelial progenitor cells in patients with congestive heart failure: The impact of inflammatory process and oxidative stress. Atherosclerosis 2011, 214, 151–157. [Google Scholar] [CrossRef]
- Oikonomou, E.; Siasos, G.; Zaromitidou, M.; Hatzis, G.; Mourouzis, K.; Chrysohoou, C.; Zisimos, K.; Mazaris, S.; Tourikis, P.; Athanasiou, D.; et al. Atorvastatin treatment improves endothelial function through endothelial progenitor cells mobilization in ischemic heart failure patients. Atherosclerosis 2015, 2, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Bonadei, I.; Sciatti, E.; Vizzardi, E.; Fabbricatore, D.; Pagnoni, M.; Rossi, L.; Carubelli, V.; Lombardi, C.M.; Metra, M. Effects of ivabradine on endothelial function, aortic properties and ventricular-arterial coupling in chronic systolic heart failure patients. Cardiovasc. Ther. 2018, 3, e12323. [Google Scholar] [CrossRef] [PubMed]
- Alem, M.M. Allopurinol and endothelial function: A systematic review with meta-analysis of randomized controlled trials. Cardiovasc. Ther. 2018, 4, e12432. [Google Scholar] [CrossRef] [PubMed]
- Dell'Oro, R.; Maloberti, A.; Nicoli, F.; Villa, P.; Gamba, P.; Bombelli, M.; Mancia, G.; Grassi, G. Long-term Saxagliptin Treatment Improves Endothelial Function but not Pulse Wave Velocity and Intima-Media Thickness in Type 2 Diabetic Patients. High Blood Press Cardiovasc. Prev. 2017, 4, 393–400. [Google Scholar] [CrossRef]
- Ammirati, E.; Musca, F.; Oliva, F.; Garascia, A.; Pacher, V.; Verde, A.; Cipriani, M.; Moreo, A.; Martinelli, L.; Frigerio, M. Levosimendan reverted severe pulmonary hypertension in one patient on waiting list for heart transplantation. Int. J. Cardiol. 2013, 4, 4518–4519. [Google Scholar] [CrossRef]
- Bouchez, S.; Fedele, F.; Giannakoulas, G.; Gustafsson, F.; Harjola, V.P.; Karason, K.; Kivikko, M.; von Lewinski, D.; Oliva, F.; Papp, Z.; et al. Levosimendan in Acute and Advanced Heart Failure: An Expert Perspective on Posology and Therapeutic Application. Cardiovasc. Drugs Ther. 2018, 6, 617–624. [Google Scholar] [CrossRef]
- Oliva, F.; Perna, E.; Marini, M.; Nassiacos, D.; Cirò, A.; Malfatto, G.; Morandi, F.; Caico, I.; Perna, G.; Meloni, S.; et al. The RELEVANT-HF multicentre collaboration. Int. J. Cardiol. 2018, 272, 255–259. [Google Scholar] [CrossRef]
- Grossini, E.; Molinari, C.; Caimmi, P.P.; Uberti, F.; Vacca, G. Levosimendan induces NO production through p38 MAPK.; ERK and Akt in porcine coronary endothelial cells: Role for mitochondrial K ATP channel. Br. J. Pharmacol. 2009, 2, 250–261. [Google Scholar] [CrossRef]
- Papp, Z.; Édes, I.; Fruhwald, S.; De Hert, S.G.; Salmenperä, M.; Leppikangas, H.; Mebazaa, A.; Landoni, G.; Grossini, E.; Caimmi, P.; et al. Levosimendan: Molecular mechanisms and clinical implications: Consensus of experts on the mechanisms of action of levosimendan. Int. J. Cardiol. 2012, 2, 82–87. [Google Scholar] [CrossRef]
- Parissis, J.T.; Karavidas, A.; Bistola, V.; Arapi, S.; Paraskevaidis, I.A.; Farmakis, D.; Korres, D.; Filippatos, G.; Matsakas, E.; Kremastinos, D.T. Effects of levosimendan on flow-mediated vasodilation and soluble adhesion molecules in patients with advanced chronic heart failure. Atherosclerosis 2008, 1, 278–282. [Google Scholar] [CrossRef]
- Sangalli, F.; Avalli, L.; Laratta, M.; Formica, F.; Maggioni, E.; Caruso, R.; Cristina Costa, M.; Guazzi, M.; Fumagalli, R. Effects of Levosimendan on Endothelial Function and Hemodynamics During Weaning From Veno-Arterial Extracorporeal Life Support. J. Cardiothorac Vasc. Anesth. 2016, 6, 1449–1453. [Google Scholar] [CrossRef] [PubMed]
- Maytin, M.; Colucci, W.S. Cardioprotection: A new paradigm in the management of acute heart failure syndromes. Am. J. Cardiol. 2005, 6, 26–31. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, B. Evidence for Mitochondrial K+ Channels and Their Role in Cardioprotection. Circ. Res. 2004, 4, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Giannattasio, C.; Achilli, F.; Grappiolo, A.; Failla, M.; Meles, E.; Gentile, G.; Calchera, I.; Capra, A.; Baglivo, J.; Vincenzi, A.; et al. Radial artery flow-mediated dilatation in heart failure patients: Effects of pharmacological and nonpharmacological treatment. Hypertension 2001, 38, 1451–1455. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tarro Genta, F.; Eleuteri, E.; Temporelli, P.L.; Comazzi, F.; Tidu, M.; Bouslenko, Z.; Bertolin, F.; Vigorito, C.; Giannuzzi, P.; Giallauria, F. Flow-mediated dilation normalization predicts outcome in chronic heart failure patients. J. Card. Fail. 2013, 4, 260–267. [Google Scholar] [CrossRef]
- Shechter, M.; Matetzky, S.; Arad, M.; Feinberg, M.S.; Freimark, D. Vascular endothelial function predicts mortality risk in patients with advanced ischaemic chronic heart failure. Eur. J. Heart Fail. 2009, 6, 588–593. [Google Scholar] [CrossRef]
- Meyer, B.; Mörtl, D.; Strecker, K.; Hülsmann, M.; Kulemann, V.; Neunteufl, T.; Pacher, R.; Berger, R. Flow. Flow-mediated vasodilation predicts outcome in patients with chronic heart failure: Comparison with B-type natriuretic peptide. J. Am. Coll. Cardiol. 2005, 6, 1011–1018. [Google Scholar] [CrossRef]
- Hryniewicz, K.; Dimayuga, C.; Hudaihed, A.; Androne, A.S.; Zheng, H.; Jankowski, K.; Katz, S.D. Inhibition of angiotensin-converting enzyme and phosphodiesterase type 5 improves endothelial function in heart failure. Clin. Sci. 2005, 108, 331–338. [Google Scholar] [CrossRef]
- Anderson, T.J.; Elstein, E.; Haber, H.; Charbonneau, F. Comparative study of ACE-inhibition, angiotensin II antagonism, and calcium channel blockade on flow-mediated vasodilation in patients with coronary disease (BANFF study). J. Am. Coll. Cardiol. 2000, 35, 60–66. [Google Scholar] [CrossRef]
- Bots, M.L.; Remme, W.J.; Lüscher, T.F.; Fox, K.M.; Bertrand, M.; Ferrari, R.; Simoons, M.L.; Grobbee, D.E.; EUROPA-PERFECT Investigators. ACE Inhibition and Endothelial Function: Main Findings of PERFEC.T, a Sub-Study of the EUROPA Trial. Cardiovasc. Drugs Ther. 2007, 21, 269–279. [Google Scholar] [CrossRef]
- Okutucu, S.; Yetis Sayin, B.; Aksoy, H.; Oto, A. Angiotensin Receptor Neprilysin Inhibition Improves Flow Mediated Dilatation of Brachial Artery in Heart Failure Patients With Reduced Ejection Fraction. Circulation 2018, 138, A10968. [Google Scholar]
- Nathaniel, S.; McGinty, S.; Witman, M.A.; Edwards, D.G.; Farquhar, W.B.; Hosmane, V.; Wenner, M.M. Impact of angiotensin receptor–neprilysin inhibition on vascular function in heart failure with reduced ejection fraction: A pilot study. Physiol. Rep. 2022, 10, e15209. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.W.; Rogers, J.G. Evolution of left ventricular assist device therapy for advanced heart failure: A review. JAMA Cardiol. 2018, 3, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Estep, J.D.; Trachtenberg, B.H.; Loza, L.P.; Bruckner, B.A. Continuous flow left ventricular assist devices: Shared care goals of monitoring and treating patients. Methodist Debakey Cardiovasc. J. 2015, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Peyton, K.J.; Durante, W. Physiological cyclic strain promotes endothelial cell survival via the induction of heme oxygenase-1. Am. J. Physiol. Heart Circ. Physiol. 2013, 304, H1634–H1643. [Google Scholar] [CrossRef] [PubMed]
- Morgan, N.; Warner, P.; Kiernan, M.; Al-Quthami, A.; Rahban, Y.; Pham, D.T.; DeNofrio, D.; Karas, R.; Kuvin, J. Arterial stiffness and vascular endothelial function in patients with long-term continuous-flow left ventricular assist devices. J. Card. Fail. 2013, 19, S18. [Google Scholar] [CrossRef]
- Symons, J.D.; Deeter, L.; Deeter, N.; Bonn, T.; Cho, J.M.; Ferrin, P.; McCreath, L.; Diakos, N.A.; Taleb, I.; Alharethi, R.; et al. Effect of continuous-flow left ventricular assist device support on coronary artery endothelial function in ischemic and nonischemic cardiomyopathy. Circ. Heart Fail. 2019, 12, e006085. [Google Scholar] [CrossRef]
- Hasin, T.; Matsuzawa, Y.; Guddeti, R.R.; Aoki, T.; Kwon, T.G.; Schettle, S.; Lennon, R.J.; Chokka, R.G.; Lerman, A.; Kushwaha, S.S. Attenuation in peripheral endothelial function after continuous flow left ventricular assist device therapy is asso ciated with cardiovascular adverse events. Circ. J. 2015, 79, 770–777. [Google Scholar] [CrossRef]
- Adam, M.; Meyer, S.; Knors, H.; Klinke, A.; Radunski, U.K.; Rudolph, T.K.; Rudolph, V.; Spin, J.M.; Tsao, P.S.; Costard-Jäckle, A.; et al. Levosimendan displays anti-inflammatory effects and decreases MPO bioavailability in patients with severe heart failure. Sci. Rep. 2015, 5, 1–9. [Google Scholar] [CrossRef]
- Ananth, C.V.; Schisterman, E.F. Hidden biases in observational epidemiology: The case of unmeasured confounding. BJOG 2018, 125, 644–646. [Google Scholar] [CrossRef]
- Zhang, X.; Stamey, J.D.; Mathur, M.B. Assessing the impact of unmeasured confounders for credible and reliable real-world evidence. Pharmacoepidemiol. Drug Saf. 2020, 29, 1219–1227. [Google Scholar] [CrossRef] [PubMed]

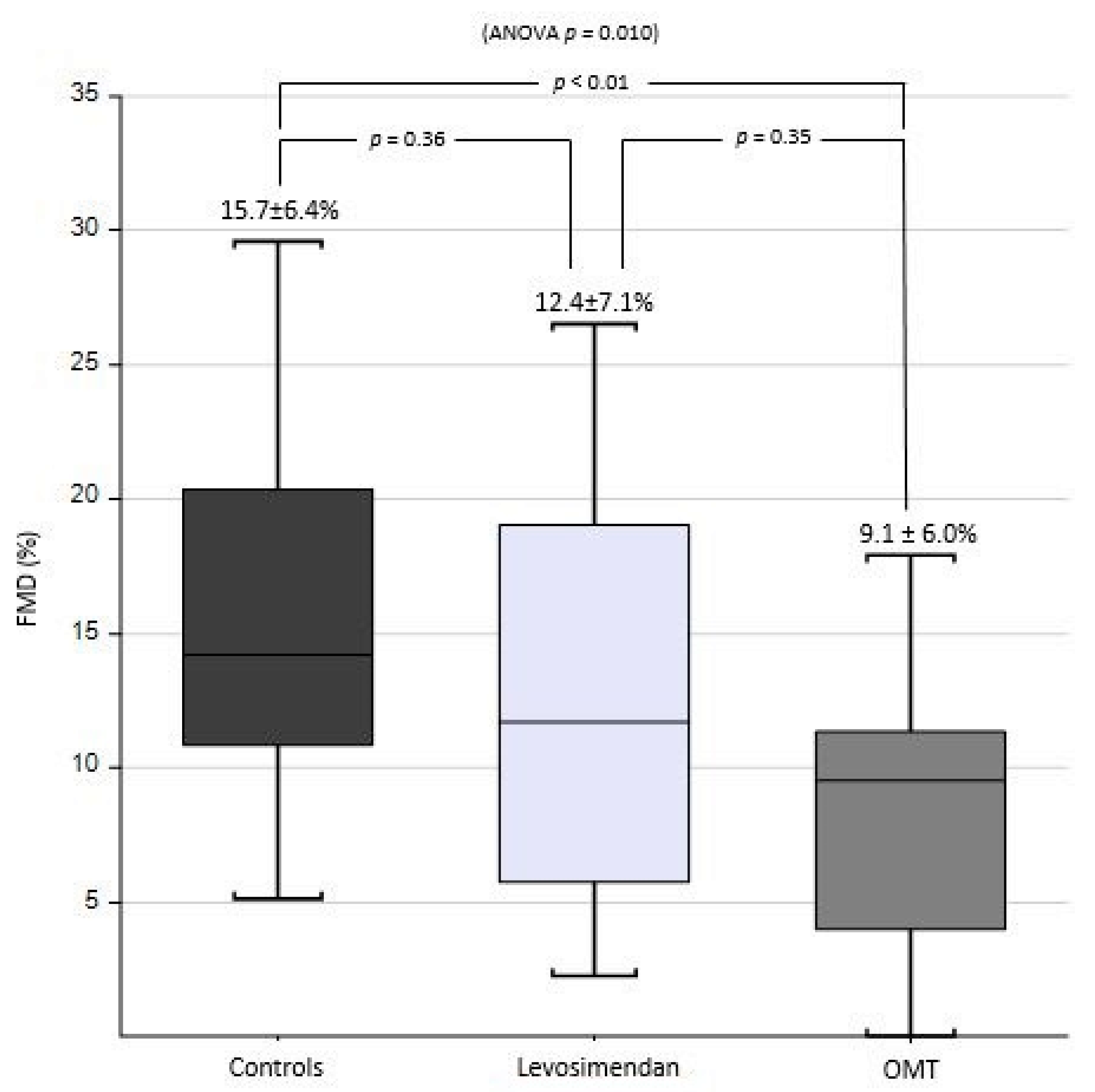
| OMT | Levosimendan | Healthy Control | p Value (ANOVA) | p Value (OMT vs. LEVO) | |
|---|---|---|---|---|---|
| Number | 20 | 20 | 20 | - | - |
| Clinical and anamnestic variables | |||||
| Age (years) | 56.9 ± 8.3 | 59.1 ± 5.2 | 56.3 ± 11.2 | 0.561 | 0.975 |
| Sex (male) | 16 (80) | 17 (85) | 10 (50) | 0.029 | 0.785 |
| BMI (kg/m2) | 26.1 ± 4.0 | 24.8 ± 3.2 | 26.4 ± 4.1 | 0.356 | 0.796 |
| SBP (mmHg) | 97.0 ± 12.2 * | 102.0 ± 12.4 * | 118.0 ± 18.6 | <0.001 | 0.875 |
| DBP (mmHg) | 61.5 ± 6.3 * | 67.9 ± 8.8 | 73.3 ± 11.6 | 0.001 | 0.095 |
| HR (bpm) | 65.5 ± 8.6 | 69.3 ± 8.2 | 71.7 ± 13.7 | 0.178 | 0.787 |
| NYHA Class (%) | - | - | - | - | 0.001 |
| 2 | 11 (55) | 1 (5) | - | - | - |
| 3 | 9 (45) | 17 (85) | - | - | - |
| 4 | 0 (0) | 2 (10) | - | - | - |
| Etiology (%) | - | - | - | - | <0.001 |
| Ischaemic | 8 (40) | 12 (60) | - | - | - |
| Nonischaemic | 12 (60) | 8 (40) | - | - | - |
| Disease duration (months) | 132.6 ± 92.1 | 132.1 ± 147.3 | - | - | 0.24 |
| Time from first levosimendan infusion (months) | - | 22 ± 26 | - | - | - |
| Smoke (%) | - | - | - | 0.028 | 0.135 |
| Nonsmoker | 9 (45) | 10 (50) | 15 (75) | - | - |
| Current smoker | 1 (5) | 2 (10) | 4 (20) | - | - |
| Former smoker | 10 (50) | 8 (40) | 1 (5) | - | - |
| Hypertension (%) | 2 (10) | 3 (15) | 6 (30) | 0.235 | 0.334 |
| Dyslipidaemia (%) | 3 (15) | 2 (10) | 6 (30) | 0.259 | 0.472 |
| DM (%) | 3 (15) | 5 (25) | 1 (5) | 0.208 | 0.361 |
| CKD (%) | 4 (20) | 10 (50) | 0 (0) | 0.001 | 0.067 |
| Laboratory analysis | |||||
| Creatinine (mg/dL) | 1.21 ± 0.36 | 1.48 ± 0.50 | - | - | 0.055 |
| NT-proBNP (ng/L) | 755.5 (540.7–1001) | 4217 (2102.7–6329.7) | - | - | 0.005 |
| Glycemia (mg/dL) | 91.9 ± 11.3 | 116.0 ± 24.8 | - | - | <0.001 |
| Total Cholesterol (mg/dL) | 154.0 ±34.7 | 165.3± 51.8 | - | - | 0.422 |
| LDL-C (mg/dL) | 87.0 ± 29.0 | 96.6 ± 37.9 | - | - | 0.374 |
| HDL-C (mg/dL) | 42.7 ± 13.0 | 43.2 ± 17.5 | - | - | 0.919 |
| Triglyceride (mg/dL) | 132.5 (85.7–169.2) | 115.5 (86–158.5) | - | - | 0.349 |
| Echocardiographic data | |||||
| LVEF (%) | 27.8 ± 7.8 | 23.5 ± 6.1 | - | - | 0.057 |
| E and A | 1.5 ± 1.2 | 3.2 ± 1.8 | - | - | 0.032 |
| MV DecT (ms) | 171.0 ± 44.6 | 156.8 ± 31.5 | - | - | 0.521 |
| E/e’ | 12.8 ± 7.2 | 19.2 ± 3.7 | - | - | 0.034 |
| LA Volume Indexed by BSA (mL/m2) | 53.0 ± 20.9 | 73.0 ± 15.8 | - | - | 0.003 |
| TAPSE (mm) | 16.9 ± 29.6 | 15.3 ± 3.2 | - | - | 0.152 |
| Tricuspid lateral s’ (cm/s) | 8.3 ± 1.4 | 7.3 ± 1.5 | - | - | 0.134 |
| sPAP (mmHg) | 32.7 ± 7.9 | 42.8 ± 12.9 | - | - | 0.007 |
| Right Heart Catheterization data | |||||
| PAPm (mmHg) | 22.9± 7.3 | 33.4 ± 11.2 | - | - | 0.004 |
| WP (mmHg) | 22.5 ± 7.09 | 15.1 ± 6.4 | - | - | 0.005 |
| CI (L/min/m2) | 2 ± 0.3 | 1.8 ± 0.4 | - | - | 0.142 |
| PVRI (woods unit/m2) | 3.9 ± 1.2 | 6.7 ± 5.2 | - | - | 0.038 |
| OMT | Levosimendan | p-Value | |
|---|---|---|---|
| Number | 20 | 20 | |
| ASA (%) | 10 (50) | 8 (40) | 0.751 |
| Warfarin (%) | 6 (30) | 12 (60) | 0.191 |
| NAOC (%) | 3 (15) | 0 (0) | - |
| Beta-blocker (%) | 20 (100) | 16 (80) | 0.106 |
| Mean bisoprolol dose | 6.6 ± 2.8 | 5.0 ± 1.5 | 0.645 |
| ACE-I (%) | 1 (5) | 10 (50) | 0.003 |
| ARB (%) | 1 (5) | 5 (25) | 0.182 |
| ARNI (%) | 18 (90) | 3 (15) | <0.001 |
| Mean sacubitril dose | 51.4 ± 27.1 | 32.3 ± 14.4 | 0.264 |
| MR antagonist (%) | 17 (85) | 17 (85) | 1.000 |
| Mean spironolactone dose | 35.1 ± 20.7 | 42.9 ± 19.3 | 0.280 |
| Loop diuretics (%) | 18 (90) | 19 (95) | 1.000 |
| Mean furosemide dose | 62.5 ± 61.9 | 148.6 ± 91.7 | 0.002 |
| Amiodarone (%) | 5 (25) | 12 (60) | 0.054 |
| Digoxin (%) | 1 (5) | 6 (30) | 0.091 |
| Ivabradine (%) | 4 (20) | 1 (5) | 0.342 |
| Statins (%) | 12 (60) | 10 (50) | 0.751 |
| Antidiabetic (%) | 3 (15) | 3 (15) | 1.000 |
| β | 95% Confidence Interval | p-Value | ||
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| Age (years) | 0.068 | −0.141 | 0.251 | 0.575 |
| Sex (female) | 0.179 | −1.176 | 6.646 | 0.167 |
| CKD (present) | −0.302 | −9.333 | −0.494 | 0.030 |
| LEVO group (reference) | - | - | - | - |
| OMT group | −0.324 | −8.925 | −0.537 | 0.028 |
| Healthy controls group | 0.002 | −0.141 | 0.251 | 0.575 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maloberti, A.; Sun, J.; Zannoni, J.; Occhi, L.; Bassi, I.; Fabbri, S.; Colombo, V.; Gualini, E.; Algeri, M.; Varrenti, M.; et al. Endothelial Dysfunction in Patients with Advanced Heart Failure Treated with Levosimendan Periodic Infusion Compared with Optimal Medical Therapy: A Pilot Study. Life 2022, 12, 1322. https://doi.org/10.3390/life12091322
Maloberti A, Sun J, Zannoni J, Occhi L, Bassi I, Fabbri S, Colombo V, Gualini E, Algeri M, Varrenti M, et al. Endothelial Dysfunction in Patients with Advanced Heart Failure Treated with Levosimendan Periodic Infusion Compared with Optimal Medical Therapy: A Pilot Study. Life. 2022; 12(9):1322. https://doi.org/10.3390/life12091322
Chicago/Turabian StyleMaloberti, Alessandro, Jinwei Sun, Jessica Zannoni, Lucia Occhi, Ilaria Bassi, Saverio Fabbri, Valentina Colombo, Elena Gualini, Michela Algeri, Marisa Varrenti, and et al. 2022. "Endothelial Dysfunction in Patients with Advanced Heart Failure Treated with Levosimendan Periodic Infusion Compared with Optimal Medical Therapy: A Pilot Study" Life 12, no. 9: 1322. https://doi.org/10.3390/life12091322
APA StyleMaloberti, A., Sun, J., Zannoni, J., Occhi, L., Bassi, I., Fabbri, S., Colombo, V., Gualini, E., Algeri, M., Varrenti, M., Masciocco, G., Perna, E., Oliva, F., Cipriani, M., Frigerio, M., & Giannattasio, C. (2022). Endothelial Dysfunction in Patients with Advanced Heart Failure Treated with Levosimendan Periodic Infusion Compared with Optimal Medical Therapy: A Pilot Study. Life, 12(9), 1322. https://doi.org/10.3390/life12091322






