Recent Progress in the Diagnosis and Management of Type 2 Diabetes Mellitus in the Era of COVID-19 and Single Cell Multi-Omics Technologies
Abstract
:1. Introduction
2. Prevalence of T2DM
3. Economic Burden of T2DM
4. Signs and Symptoms of T2DM
5. Non-Pharmacological Treatments of T2DM: Exercise and Diet
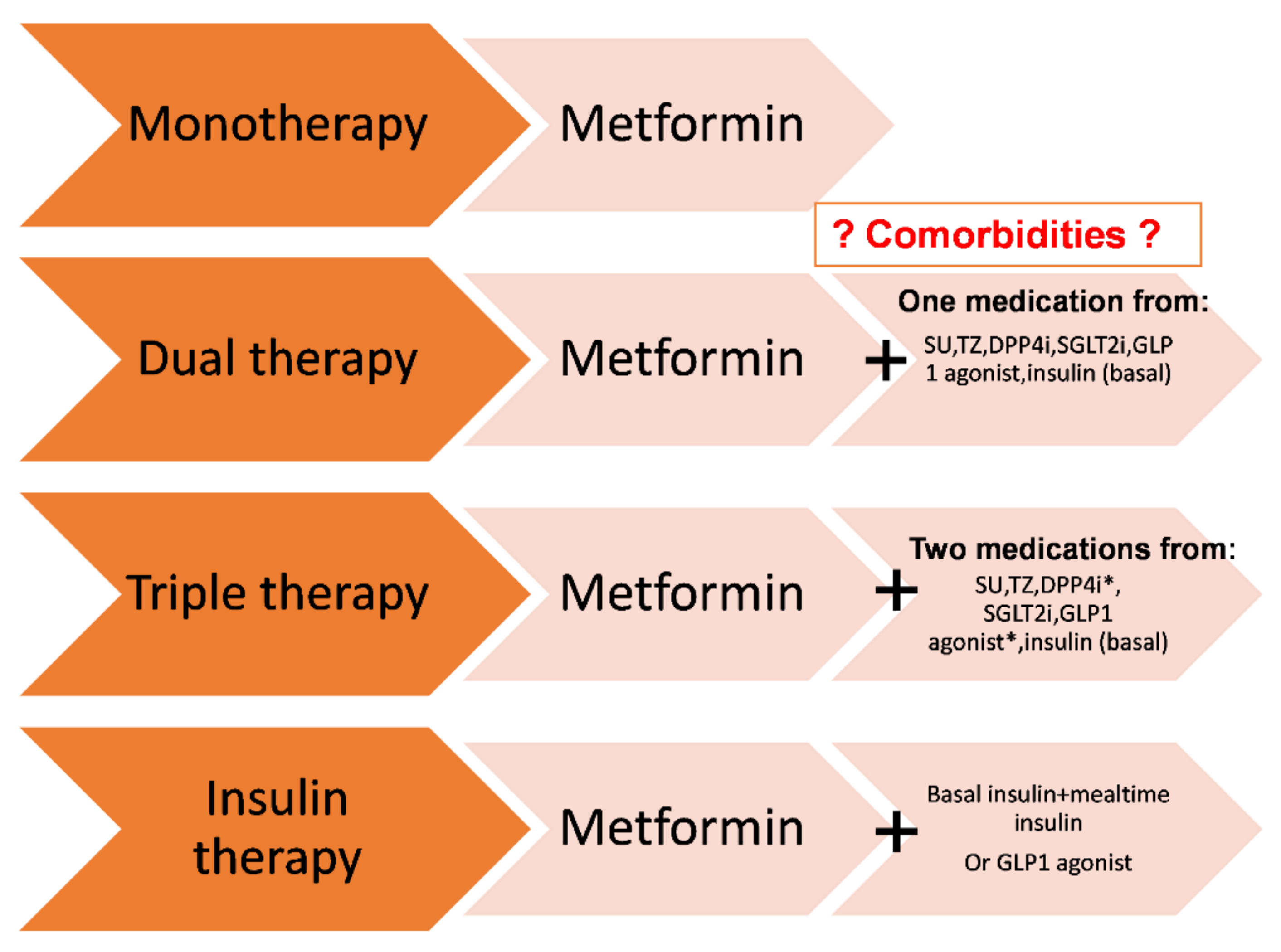
6. Pharmacological Treatment of T2DM
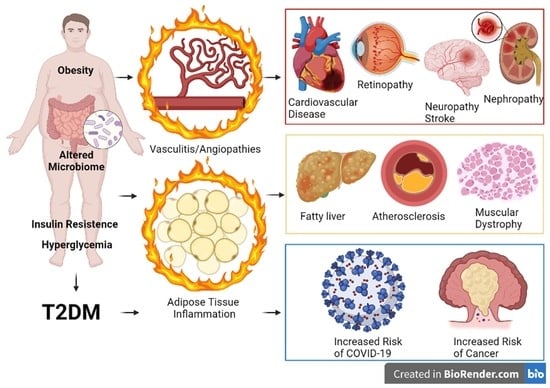
7. Clinical Complications of T2DM
8. Pathophysiology of T2DM and COVID-19
9. Prognosis of T2DM Patients with COVID-19
10. The Potential Role of Multi-Omics and Single Cell-Based Technologies in the Current Research of T2DM
| Omics/Field | Measures | Results | Assay | References |
|---|---|---|---|---|
| Genomics | 16S rRNA on microbiome analysis | Smoking and/or HIV lowers microbiome diversity in T2DM | NGS | [168] |
| Genomics | 16S rRNA on microbiome analysis | Metformin helps to normalize microbiome with the support of Blautia spp. | NGS | [169] |
| Genomics | Analysis of SNPs | SNPs in the CAT, FTO and UCP1 genes associated with retinopathy and nephropathy | Sequenom platform | [170] |
| Genomics | Genome sequencing | Heritability of T2DM is approximately 10–15% | GWAS | [15,16,17,171] |
| Epigenomics | CpGs methylation pattern | CpG methylation of ABCG1, LOXL2, TXNIP, SLC1A5 and SREBF1 is associated with T2DM | EWAS, Illumina 450K methylation array | [174] |
| Epigenomics | Alpha or beta cell-specific open chromatin landscape | Alpha cell-specific ATAC-seq peaks: ISL1 and MAFB; beta cell-specific: SMAD2 | ATAC-seq | [175] |
| Epigenomics Genomics | Open chromatin regions/SNPs | Thousands of pancreatic islet-specific enhancer–target gene pairs | Hi-C, ATAC-seq, ChIP-seq | [176] |
| Transcriptomics | Gene expression | T2DM-specific gene expression signatures in alpha, beta and delta cells | scRNA-seq | [177] |
| Transcriptomics | Gene expression, regulatory networks | Increased OTUD7B, PPRC1, ARRB2, C17orf96, NME2, and E2F1 or four markers with decreased PageRank centrality (FBXW7, CXCL8, FHL1, and CELF4) | scRNA-seq | [178] |
| Epigenomics Genomics | scRNA-seq and deep learning approaches | T2DM-associated SNPs were significantly enriched in beta cell-specific and common islet-specific open chromatin | scRNA-seq and deep learning approaches | [179] |
| Transcriptomics | Gene expression, pathway analysis | T2DM-associated genes responsible for energy metabolism, immune homeostasis, and autophagy | Meta-analysis of scRNA-seq data | [180] |
| Transcriptomics |
Whole transcriptome analysis | Top DEGs in peripheral fat of Asian Indians associated with T2DM: HOXB3, RSPO3, HOXA5, GREM1, ORMDL1, C7, TRIM23, CLDN11, ABCA10, ETV5, TRIM2, TP53INP1, ST6GAL1, THBS2, ERAP1, OGT, RARRES1, CTDSPL and TBCC | Affymetrix GeneChip PrimeView Human Gene Expression Array | [182] |
| Transcriptomics |
Whole transcriptome analysis | Altered lipid, glucose, and protein metabolism; adipogenesis defect; and inflammation in peripheral fat of Asian Indians associated with T2DM | Bulk RNAseq | [183] |
| Genomics | Analysis of SNPs | s2241766-G (ADIPOQ), rs6494730-T (FEM1B), rs1799817-A, rs2059806-T (INSR), rs11745088-C (FST), rs9939609-A, and rs9940128-A (FTO) were associated with T2DM in southern Asian Indians | AGENA MassARRAYiPLEX™ platform | [184] |
| Proteomics | Protein concentrations | Osteopontin and osteoprotegerin are elevated in T2DM | Milliplex Luminex assay | [185] |
| Proteomics | Protein concentrations | High KIM-1 and β2-B2M are associated with renal failure | Luminex Multiplex ELISA Luminex assay | [186] |
| Proteomics | Protein concentration | High KIM-1 is associated with low GFR | Multiplex Luminex Panel | [187] |
| Proteomics | Immune cell infiltration | High HLA-DR+ macrophages and HLA-DR+ CD8+ T-cells in the islets of pancreata of T2DM patients | Single-cell imaging mass cytometry | [188] |
| Lipidomics | Lipid composition | High TAGs, DAGs, PEs: high risk for T2DM High LPs, PC–PLs, SMs, CEs: low risk for T2DM | Mass spectrometry (MS) | [190] |
| Lipidomics | Lipid composition | High TAGs, DAGs and Low PC–PLs: high risk for T2DM | Ultra-performance liquid chromatography and MS | [191] |
Author Contributions
Funding
Conflicts of Interest
References
- Mathew, T.K.; Tadi, P. Blood Glucose Monitoring. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Sims, E.K.; Bundy, B.N.; Stier, K. Addendum. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, 2182. [Google Scholar] [CrossRef]
- Schleicher, E.; Gerdes, C.; Petersmann, A.; Muller-Wieland, D.; Muller, U.A.; Freckmann, G.; Heinemann, L.; Nauck, M.; Landgraf, R. Definition, Classification and Diagnosis of Diabetes mellitus: Update 2021. Diabetol. Stoffwechs. 2021, 16, S110–S118. [Google Scholar] [CrossRef]
- Horber, S.; Kaiser, P.; Achenbach, P.; Schleicher, E.; Peter, A. Novel classification of diabetes mellitus—requirements for laboratory parameters. Diabetol. Stoffwechs. 2021, 16, 63–69. [Google Scholar]
- Nauck, M.; Gerdes, C.; Petersmann, A.; Muller-Wieland, D.; Muller, U.A.; Freckmann, G.; Heinemann, L.; Schleicher, E.; Landgraf, R. Definition, classification and diagnosis of diabetes mellitus: Update 2020. Diabetologe 2021, 17, 404–410. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee; Draznin, B.; Aroda, V.R.; Bakris, G.; Benson, G.; Brown, F.M.; Freeman, R.; Green, J.; Huang, E.; Isaacs, D.; et al. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintanilla Rodriguez, B.S.; Mahdy, H. Gestational Diabetes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Petersmann, A.; Muller-Wieland, D.; Muller, U.A.; Landgraf, R.; Nauck, M.; Freckmann, G.; Heinemann, L.; Schleicher, E. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2019, 127, S1–S7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Nolan, C.J.; Damm, P.; Prentki, M. Type 2 diabetes across generations: From pathophysiology to prevention and management. Lancet 2011, 378, 169–181. [Google Scholar] [CrossRef]
- Kahn, S.E.; Hull, R.L.; Utzschneider, K.M. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 2006, 444, 840–846. [Google Scholar] [CrossRef]
- Dendup, T.; Feng, X.; Clingan, S.; Astell-Burt, T. Environmental Risk Factors for Developing Type 2 Diabetes Mellitus: A Systematic Review. Int J. Environ. Res. Public Health 2018, 15, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Yu, W.; Hu, C. Genetics of type 2 diabetes: Insights into the pathogenesis and its clinical application. Biomed. Res. Int 2014, 2014, 926713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mambiya, M.; Shang, M.; Wang, Y.; Li, Q.; Liu, S.; Yang, L.; Zhang, Q.; Zhang, K.; Liu, M.; Nie, F.; et al. The Play of Genes and Non-genetic Factors on Type 2 Diabetes. Front. Public Health 2019, 7, 349. [Google Scholar] [CrossRef] [PubMed]
- DeForest, N.; Majithia, A.R. Genetics of Type 2 Diabetes: Implications from Large-Scale Studies. Curr. Diabetes Rep. 2022, 22, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Haedersdal, S.; Lund, A.; Knop, F.K.; Vilsboll, T. The Role of Glucagon in the Pathophysiology and Treatment of Type 2 Diabetes. Mayo Clin. Proc. 2018, 93, 217–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Unger, R.H. Glucagon and the insulin: Glucagon ratio in diabetes and other catabolic illnesses. Diabetes 1971, 20, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Back, S.H.; Kaufman, R.J. Endoplasmic reticulum stress and type 2 diabetes. Annu. Rev. Biochem. 2012, 81, 767–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinti, M.V.; Fink, G.K.; Hathaway, Q.A.; Durr, A.J.; Kunovac, A.; Hollander, J.M. Mitochondrial dysfunction in type 2 diabetes mellitus: An organ-based analysis. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E268–E285. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shao, J.; Bian, Y.; Wu, H.; Shi, L.; Zeng, L.; Li, W.; Dong, J. Prevalence of type 2 diabetes mellitus among inland residents in China (2000–2014): A meta-analysis. J. Diabetes Investig. 2016, 7, 845–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef]
- Suh, D.C.; Kim, C.M.; Choi, I.S.; Plauschinat, C.A. Comorbid conditions and glycemic control in elderly patients with type 2 diabetes mellitus, 1988 to 1994 to 1999 to 2004. J. Am. Geriatr. Soc. 2008, 56, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Liu, B.; Sun, Y.; Du, Y.; Snetselaar, L.G.; Hu, F.B.; Bao, W. Prevalence of diagnosed type 1 and type 2 diabetes among US adults in 2016 and 2017: Population based study. BMJ 2018, 362, k1497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, A.; Vollenweider, P.; Waeber, G.; Marques-Vidal, P. Prevalence, awareness and treatment of type 2 diabetes mellitus in Switzerland: The CoLaus study. Diabet. Med. 2012, 29, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Lascar, N.; Brown, J.; Pattison, H.; Barnett, A.H.; Bailey, C.J.; Bellary, S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018, 6, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Bommer, C.; Heesemann, E.; Sagalova, V.; Manne-Goehler, J.; Atun, R.; Barnighausen, T.; Vollmer, S. The global economic burden of diabetes in adults aged 20–79 years: A cost-of-illness study. Lancet Diabetes Endocrinol. 2017, 5, 423–430. [Google Scholar] [CrossRef]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Economic Burden of Cardiovascular Disease in Type 2 Diabetes: A Systematic Review. Value Health 2018, 21, 881–890. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef]
- O’Connell, J.M.; Manson, S.M. Understanding the Economic Costs of Diabetes and Prediabetes and What We May Learn About Reducing the Health and Economic Burden of These Conditions. Diabetes Care 2019, 42, 1609–1611. [Google Scholar] [CrossRef] [Green Version]
- Ismail, L.; Materwala, H.; Al Kaabi, J. Association of risk factors with type 2 diabetes: A systematic review. Comput. Struct. Biotechnol. J. 2021, 19, 1759–1785. [Google Scholar] [CrossRef]
- Reed, J.; Bain, S.; Kanamarlapudi, V. A Review of Current Trends with Type 2 Diabetes Epidemiology, Aetiology, Pathogenesis, Treatments and Future Perspectives. Diabetes Metab. Syndr. Obes. 2021, 14, 3567–3602. [Google Scholar] [CrossRef]
- Tian, J.; Olcott, A.P.; Kaufman, D.L. Antigen-based immunotherapy drives the precocious development of autoimmunity. J. Immunol. 2002, 169, 6564–6569. [Google Scholar] [CrossRef] [Green Version]
- Andrade-Oliveira, V.; Camara, N.O.; Moraes-Vieira, P.M. Adipokines as drug targets in diabetes and underlying disturbances. J. Diabetes Res. 2015, 2015, 681612. [Google Scholar] [CrossRef] [PubMed]
- Toth, M.E.; Dukay, B.; Peter, M.; Balogh, G.; Szucs, G.; Zvara, A.; Szebeni, G.J.; Hajdu, P.; Sarkozy, M.; Puskas, L.G.; et al. Male and Female Animals Respond Differently to High-Fat Diet and Regular Exercise Training in a Mouse Model of Hyperlipidemia. Int. J. Mol. Sci. 2021, 22, 4198. [Google Scholar] [CrossRef]
- Rodriguez, A.J.; Nunes Vdos, S.; Mastronardi, C.A.; Neeman, T.; Paz-Filho, G.J. Association between circulating adipocytokine concentrations and microvascular complications in patients with type 2 diabetes mellitus: A systematic review and meta-analysis of controlled cross-sectional studies. J. Diabetes Complicat. 2016, 30, 357–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsiki, N.; Mikhailidis, D.P.; Banach, M. Leptin, cardiovascular diseases and type 2 diabetes mellitus. Acta Pharmacol. Sin. 2018, 39, 1176–1188. [Google Scholar] [CrossRef] [Green Version]
- Hamasaki, H. Daily physical activity and type 2 diabetes: A review. World J. Diabetes 2016, 7, 243–251. [Google Scholar] [CrossRef]
- Swinburn, B.A.; Kraak, V.I.; Allender, S.; Atkins, V.J.; Baker, P.I.; Bogard, J.R.; Brinsden, H.; Calvillo, A.; De Schutter, O.; Devarajan, R.; et al. The Global Syndemic of Obesity, Undernutrition, and Climate Change: The Lancet Commission report. Lancet 2019, 393, 791–846. [Google Scholar] [CrossRef]
- Dai, H.; Alsalhe, T.A.; Chalghaf, N.; Ricco, M.; Bragazzi, N.L.; Wu, J. The global burden of disease attributable to high body mass index in 195 countries and territories, 1990–2017: An analysis of the Global Burden of Disease Study. PLoS Med. 2020, 17, e1003198. [Google Scholar] [CrossRef] [PubMed]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014, 384, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Pineda, E.; Sanchez-Romero, L.M.; Brown, M.; Jaccard, A.; Jewell, J.; Galea, G.; Webber, L.; Breda, J. Forecasting Future Trends in Obesity across Europe: The Value of Improving Surveillance. Obes. Facts 2018, 11, 360–371. [Google Scholar] [CrossRef]
- Pan, X.R.; Li, G.W.; Hu, Y.H.; Wang, J.X.; Yang, W.Y.; An, Z.X.; Hu, Z.X.; Lin, J.; Xiao, J.Z.; Cao, H.B.; et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: The Da Qing IGT and diabetes study. Diabetes Care 1997, 20, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Knowler, W.C.; Barrett-Connor, E.; Fowler, S.E.; Hamman, R.F.; Lachin, J.M.; Walker, E.A.; Nathan, D.M.; Diabetes Prevention Program Research, G. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002, 346, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, K.; Noda, M.; Kuzuya, T. Prevention of type 2 diabetes by lifestyle intervention: A Japanese trial in IGT males. Diabetes Res. Clin. Pract. 2005, 67, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and exercise in the prevention and treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Grontved, A.; Rimm, E.B.; Willett, W.C.; Andersen, L.B.; Hu, F.B. A prospective study of weight training and risk of type 2 diabetes mellitus in men. Arch. Intern. Med. 2012, 172, 1306–1312. [Google Scholar] [CrossRef] [Green Version]
- Umpierre, D.; Ribeiro, P.A.; Kramer, C.K.; Leitao, C.B.; Zucatti, A.T.; Azevedo, M.J.; Gross, J.L.; Ribeiro, J.P.; Schaan, B.D. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: A systematic review and meta-analysis. JAMA 2011, 305, 1790–1799. [Google Scholar] [CrossRef] [Green Version]
- Karstoft, K.; Pedersen, B.K. Exercise and type 2 diabetes: Focus on metabolism and inflammation. Immunol. Cell Biol. 2016, 94, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and management of type 2 diabetes: Dietary components and nutritional strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef] [Green Version]
- Sami, W.; Ansari, T.; Butt, N.S.; Hamid, M.R.A. Effect of diet on type 2 diabetes mellitus: A review. Int. J. Health Sci. 2017, 11, 65–71. [Google Scholar]
- Khazrai, Y.M.; Defeudis, G.; Pozzilli, P. Effect of diet on type 2 diabetes mellitus: A review. Diabetes Metab. Res. Rev. 2014, 30, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Menotti, A.; Karvonen, M.J.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S.; Fidanza, F.; Keys, M.H.; et al. The Diet and 15-Year Death Rate in the 7 Countries Study. Am. J. Epidemiol. 1986, 124, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Kastorini, C.M.; Panagiotakos, D.B. Mediterranean diet and diabetes prevention: Myth or fact? World J. Diabetes 2010, 1, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Adam-Perrot, A.; Clifton, P.; Brouns, F. Low-carbohydrate diets: Nutritional and physiological aspects. Obes. Rev. 2006, 7, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Gloede, L.; Green, A.; Ferdowsian, H. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: A randomized, controlled, 74-wk clinical trial. Am. J. Clin. Nutr. 2009, 89, 1588S–1596S. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Misra, A.; Mohan, V.; Taylor, R.; Yancy, W. Dietary and nutritional approaches for prevention and management of type 2 diabetes. BMJ 2018, 361, k2234. [Google Scholar] [CrossRef] [Green Version]
- Matthews, D.R.; Paldanius, P.M.; Proot, P.; Chiang, Y.; Stumvoll, M.; Del Prato, S.; VERIFY study group. Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): A 5-year, multicentre, randomised, double-blind trial. Lancet 2019, 394, 1519–1529. [Google Scholar] [CrossRef]
- Grant, J.S.; Graven, L.J. Progressing from Metformin to Sulfonylureas or Meglitinides. Workplace Health Saf. 2016, 64, 433–439. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.C.; Wu, V.C.; Lin, C.J.; Pan, C.F.; Chen, C.Y.; Huang, T.M.; Wu, C.H.; Chen, L.; Wu, C.J.; Consortium, N.K. Meglitinides increase the risk of hypoglycemia in diabetic patients with advanced chronic kidney disease: A nationwide, population-based study. Oncotarget 2017, 8, 78086–78095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luna, B.; Feinglos, M.N. Oral agents in the management of type 2 diabetes mellitus. Am. Fam. Physician 2001, 63, 1747–1756. [Google Scholar]
- Gourgari, E.; Wilhelm, E.E.; Hassanzadeh, H.; Aroda, V.R.; Shoulson, I. A comprehensive review of the FDA-approved labels of diabetes drugs: Indications, safety, and emerging cardiovascular safety data. J. Diabetes Complicat. 2017, 31, 1719–1727. [Google Scholar] [CrossRef]
- Sanchez-Rangel, E.; Inzucchi, S.E. Metformin: Clinical use in type 2 diabetes. Diabetologia 2017, 60, 1586–1593. [Google Scholar] [CrossRef]
- Baker, C.; Retzik-Stahr, C.; Singh, V.; Plomondon, R.; Anderson, V.; Rasouli, N. Should metformin remain the first-line therapy for treatment of type 2 diabetes? Ther. Adv. Endocrinol. Metab. 2021, 12, 2042018820980225. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, H.; Yu, X.; Wu, Y.; Sui, D. Metformin ameliorates diabetic nephropathy in a rat model of low-dose streptozotocin-induced diabetes. Exp. Ther. Med. 2017, 14, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Al Za’abi, M.; Ali, B.H.; Al Suleimani, Y.; Adham, S.A.; Ali, H.; Manoj, P.; Ashique, M.; Nemmar, A. The Effect of Metformin in Diabetic and Non-Diabetic Rats with Experimentally-Induced Chronic Kidney Disease. Biomolecules 2021, 11, 814. [Google Scholar] [CrossRef]
- Gurung, M.; Li, Z.; You, H.; Rodrigues, R.; Jump, D.B.; Morgun, A.; Shulzhenko, N. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine 2020, 51, 102590. [Google Scholar] [CrossRef] [Green Version]
- Hu, N.; Zhang, Q.; Wang, H.; Yang, X.; Jiang, Y.; Chen, R.; Wang, L. Comparative Evaluation of the Effect of Metformin and Insulin on Gut Microbiota and Metabolome Profiles of Type 2 Diabetic Rats Induced by the Combination of Streptozotocin and High-Fat Diet. Front. Pharmacol. 2021, 12, 794103. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee; Draznin, B.; Aroda, V.R.; Bakris, G.; Benson, G.; Brown, F.M.; Freeman, R.; Green, J.; Huang, E.; Isaacs, D.; et al. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S125–S143. [Google Scholar] [CrossRef]
- Mende, C.W. Chronic Kidney Disease and SGLT2 Inhibitors: A Review of the Evolving Treatment Landscape. Adv. Ther. 2022, 39, 148–164. [Google Scholar] [CrossRef]
- Hao, Z.; Zhang, Y. Dapagliflozin and Empagliflozin in Heart Failure with Reduced Ejection Fraction: A Retrospective Study. Int. J. Gen. Med. 2022, 15, 5915–5918. [Google Scholar] [CrossRef]
- Sposito, A.C.; Berwanger, O.; de Carvalho, L.S.F.; Saraiva, J.F.K. GLP-1RAs in type 2 diabetes: Mechanisms that underlie cardiovascular effects and overview of cardiovascular outcome data. Cardiovasc. Diabetol. 2018, 17, 157. [Google Scholar] [CrossRef]
- Hinnen, D. Glucagon-Like Peptide 1 Receptor Agonists for Type 2 Diabetes. Diabetes Spectr. 2017, 30, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Gallwitz, B. Clinical Use of DPP-4 Inhibitors. Front. Endocrinol. 2019, 10, 389. [Google Scholar] [CrossRef]
- Nauck, M.A.; Quast, D.R.; Wefers, J.; Meier, J.J. GLP-1 receptor agonists in the treatment of type 2 diabetes–state-of-the-art. Mol. Metab. 2021, 46, 101102. [Google Scholar] [CrossRef]
- Anderson, S.L.; Beutel, T.R.; Trujillo, J.M. Oral semaglutide in type 2 diabetes. J. Diabetes Complicat. 2020, 34, 107520. [Google Scholar] [CrossRef]
- Cornell, S. A review of GLP-1 receptor agonists in type 2 diabetes: A focus on the mechanism of action of once-weekly agents. J. Clin. Pharm. Ther. 2020, 45, 17–27. [Google Scholar] [CrossRef]
- de Mortanges, A.P.; Sinaci, E.; Salvador, D., Jr.; Bally, L.; Muka, T.; Wilhelm, M.; Bano, A. GLP-1 Receptor Agonists and Coronary Arteries: From Mechanisms to Events. Front. Pharmacol. 2022, 13, 856111. [Google Scholar] [CrossRef]
- Makrilakis, K. The Role of DPP-4 Inhibitors in the Treatment Algorithm of Type 2 Diabetes Mellitus: When to Select, What to Expect. Int. J. Environ. Res. Public Health 2019, 16, 2720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahren, B. DPP-4 Inhibition and the Path to Clinical Proof. Front. Endocrinol. 2019, 10, 376. [Google Scholar] [CrossRef] [PubMed]
- Al-Awar, A.; Almasi, N.; Szabo, R.; Takacs, I.; Murlasits, Z.; Szucs, G.; Torok, S.; Posa, A.; Varga, C.; Kupai, K. Novel Potentials of the DPP-4 Inhibitor Sitagliptin against Ischemia-Reperfusion (I/R) Injury in Rat Ex-Vivo Heart Model. Int. J. Mol. Sci. 2018, 19, 3226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murase, H.; Kuno, A.; Miki, T.; Tanno, M.; Yano, T.; Kouzu, H.; Ishikawa, S.; Tobisawa, T.; Ogasawara, M.; Nishizawa, K.; et al. Inhibition of DPP-4 reduces acute mortality after myocardial infarction with restoration of autophagic response in type 2 diabetic rats. Cardiovasc. Diabetol. 2015, 14, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lago, R.M.; Singh, P.P.; Nesto, R.W. Diabetes and hypertension. Nat. Clin. Pract. Endocrinol. Metab. 2007, 3, 667. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.A.; Narendran, P. Dapagliflozin for the treatment of type 2 diabetes: A review of the literature. Drug Des. Dev. Ther. 2014, 8, 2493–2505. [Google Scholar] [CrossRef] [Green Version]
- Shaffner, J.; Chen, B.; Malhotra, D.K.; Dworkin, L.D.; Gong, R. Therapeutic Targeting of SGLT2: A New Era in the Treatment of Diabetes and Diabetic Kidney Disease. Front. Endocrinol. 2021, 12, 749010. [Google Scholar] [CrossRef]
- Xu, D.; Chandler, O.; Wee, C.; Ho, C.; Affandi, J.S.; Yang, D.; Liao, X.; Chen, W.; Li, Y.; Reid, C.; et al. Sodium-Glucose Cotransporter-2 Inhibitor (SGLT2i) as a Primary Preventative Agent in the Healthy Individual: A Need of a Future Randomised Clinical Trial? Front. Med. 2021, 8, 712671. [Google Scholar] [CrossRef]
- Oelze, M.; Kroller-Schon, S.; Welschof, P.; Jansen, T.; Hausding, M.; Mikhed, Y.; Stamm, P.; Mader, M.; Zinssius, E.; Agdauletova, S.; et al. The sodium-glucose co-transporter 2 inhibitor empagliflozin improves diabetes-induced vascular dysfunction in the streptozotocin diabetes rat model by interfering with oxidative stress and glucotoxicity. PLoS ONE 2014, 9, e112394. [Google Scholar] [CrossRef]
- Steven, S.; Oelze, M.; Hanf, A.; Kroller-Schon, S.; Kashani, F.; Roohani, S.; Welschof, P.; Kopp, M.; Godtel-Armbrust, U.; Xia, N.; et al. The SGLT2 inhibitor empagliflozin improves the primary diabetic complications in ZDF rats. Redox Biol. 2017, 13, 370–385. [Google Scholar] [CrossRef]
- Tsai, K.F.; Chen, Y.L.; Chiou, T.T.; Chu, T.H.; Li, L.C.; Ng, H.Y.; Lee, W.C.; Lee, C.T. Emergence of SGLT2 Inhibitors as Powerful Antioxidants in Human Diseases. Antioxidants 2021, 10, 1166. [Google Scholar] [CrossRef] [PubMed]
- Cummings, M.H.; Cao, D.; Hadjiyianni, I.; Ilag, L.L.; Tan, M.H. Characteristics of insulin-Naive people with type 2 diabetes who successfully respond to insulin glargine U100 after 24 weeks of treatment: A meta-analysis of individual participant data from 3 randomized clinical trials. Clin. Diabetes Endocrinol. 2018, 4, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 487–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryden, L.; Standl, E.; Bartnik, M.; Van den Berghe, G.; Betteridge, J.; de Boer, M.J.; Cosentino, F.; Jonsson, B.; Laakso, M.; Malmberg, K.; et al. Guidelines on diabetes, pre-diabetes, and cardiovascular diseases: Executive summary. The Task Force on Diabetes and Cardiovascular Diseases of the European Society of Cardiology (ESC) and of the European Association for the Study of Diabetes (EASD). Eur. Heart J. 2007, 28, 88–136. [Google Scholar] [CrossRef]
- Baharvand-Ahmadi, B.; Bahmani, M.; Tajeddini, P.; Naghdi, N.; Rafieian-Kopaei, M. An ethno-medicinal study of medicinal plants used for the treatment of diabetes. J. Nephropathol. 2016, 5, 44–50. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Murtaza, G.; Liu, G.; Rahu, N.; Kalhoro, M.S.; Kalhoro, D.H.; Adebowale, T.O.; Mazhar, M.U.; Rehman, Z.U.; et al. Flavonoids and type 2 diabetes: Evidence of efficacy in clinical and animal studies and delivery strategies to enhance their therapeutic efficacy. Pharmacol. Res. 2020, 152, 104629. [Google Scholar] [CrossRef]
- Eid, H.M.; Nachar, A.; Thong, F.; Sweeney, G.; Haddad, P.S. The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Pharmacogn. Mag. 2015, 11, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.G.; Jang, I.S.; Yang, K.E.; Yoon, S.J.; Baek, S.; Lee, J.Y.; Suzuki, T.; Chung, K.Y.; Woo, S.H.; Choi, J.S. Effect of rutin from tartary buckwheat sprout on serum glucose-lowering in animal model of type 2 diabetes. Acta Pharm. 2016, 66, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J. Recent advances in dietary flavonoids for management of type 2 diabetes. Curr. Opin. Food Sci. 2022, 44, 100806. [Google Scholar] [CrossRef]
- Putta, S.; Yarla, N.S.; Kilari, E.K.; Surekha, C.; Aliev, G.; Divakara, M.B.; Santosh, M.S.; Ramu, R.; Zameer, F.; Mn, N.P.; et al. Therapeutic Potentials of Triterpenes in Diabetes and its Associated Complications. Curr. Top. Med. Chem. 2016, 16, 2532–2542. [Google Scholar] [CrossRef]
- Bruzzone, S.; Magnone, M.; Mannino, E.; Sociali, G.; Sturla, L.; Fresia, C.; Booz, V.; Emionite, L.; De Flora, A.; Zocchi, E. Abscisic Acid Stimulates Glucagon-Like Peptide-1 Secretion from L-Cells and Its Oral Administration Increases Plasma Glucagon-Like Peptide-1 Levels in Rats. PLoS ONE 2015, 10, e0140588. [Google Scholar] [CrossRef] [PubMed]
- Dabelea, D.; Stafford, J.M.; Mayer-Davis, E.J.; D’Agostino, R., Jr.; Dolan, L.; Imperatore, G.; Linder, B.; Lawrence, J.M.; Marcovina, S.M.; Mottl, A.K.; et al. Association of Type 1 Diabetes vs Type 2 Diabetes Diagnosed During Childhood and Adolescence With Complications During Teenage Years and Young Adulthood. JAMA 2017, 317, 825–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hameed, I.; Masoodi, S.R.; Mir, S.A.; Nabi, M.; Ghazanfar, K.; Ganai, B.A. Type 2 diabetes mellitus: From a metabolic disorder to an inflammatory condition. World J. Diabetes 2015, 6, 598–612. [Google Scholar] [CrossRef] [PubMed]
- Mapanga, R.F.; Essop, M.F. Damaging effects of hyperglycemia on cardiovascular function: Spotlight on glucose metabolic pathways. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H153–H173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papatheodorou, K.; Banach, M.; Bekiari, E.; Rizzo, M.; Edmonds, M. Complications of Diabetes 2017. J. Diabetes Res. 2018, 2018, 3086167. [Google Scholar] [CrossRef]
- Ciemins, E.L.; McKinnon, M.; Hamersky, C.M.; Iyer, N.N.; Powelson, J. Cardiovascular Disease in Patients With Type 2 Diabetes: A Qualitative Analysis of Knowledge, Attitudes, and Beliefs of Health Care Professionals. J. Ambul. Care Manag. 2021, 44, 207–217. [Google Scholar] [CrossRef]
- Ceriello, A.; Catrinoiu, D.; Chandramouli, C.; Cosentino, F.; Dombrowsky, A.C.; Itzhak, B.; Lalic, N.M.; Prattichizzo, F.; Schnell, O.; Seferovic, P.M.; et al. Heart failure in type 2 diabetes: Current perspectives on screening, diagnosis and management. Cardiovasc. Diabetol. 2021, 20, 218. [Google Scholar] [CrossRef]
- Tancredi, M.; Rosengren, A.; Svensson, A.M.; Kosiborod, M.; Pivodic, A.; Gudbjornsdottir, S.; Wedel, H.; Clements, M.; Dahlqvist, S.; Lind, M. Excess Mortality among Persons with Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 1720–1732. [Google Scholar] [CrossRef] [Green Version]
- Elwen, F.R.; Huskinson, A.; Clapham, L.; Bottomley, M.J.; Heller, S.R.; James, C.; Abbas, A.; Baxter, P.; Ajjan, R.A. An observational study of patient characteristics and mortality following hypoglycemia in the community. BMJ Open Diabetes Res. Care 2015, 3, e000094. [Google Scholar] [CrossRef] [Green Version]
- Grant, B.; Sandelson, M.; Agyemang-Prempeh, B.; Zalin, A. Managing obesity in people with type 2 diabetes. Clin. Med. 2021, 21, e231–e327. [Google Scholar] [CrossRef] [PubMed]
- van Diepen, J.A.; Robben, J.H.; Hooiveld, G.J.; Carmone, C.; Alsady, M.; Boutens, L.; Bekkenkamp-Grovenstein, M.; Hijmans, A.; Engelke, U.F.H.; Wevers, R.A.; et al. SUCNR1-mediated chemotaxis of macrophages aggravates obesity-induced inflammation and diabetes. Diabetologia 2017, 60, 1304–1313. [Google Scholar] [CrossRef] [Green Version]
- Wondmkun, Y.T. Obesity, Insulin Resistance, and Type 2 Diabetes: Associations and Therapeutic Implications. Diabetes Metab. Syndr. Obes. 2020, 13, 3611–3616. [Google Scholar] [CrossRef] [PubMed]
- Mokgalaboni, K.; Dludla, P.V.; Nyambuya, T.M.; Yakobi, S.H.; Mxinwa, V.; Nkambule, B.B. Monocyte-mediated inflammation and cardiovascular risk factors in type 2 diabetes mellitus: A systematic review and meta-analysis of pre-clinical and clinical studies. JRSM Cardiovasc. Dis. 2020, 9, 2048004019900748. [Google Scholar] [CrossRef] [PubMed]
- Grandl, G.; Wolfrum, C. Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin. Immunopathol. 2018, 40, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Lingvay, I.; Sumithran, P.; Cohen, R.V.; le Roux, C.W. Obesity management as a primary treatment goal for type 2 diabetes: Time to reframe the conversation. Lancet 2022, 399, 394–405. [Google Scholar] [CrossRef]
- Antza, C.; Kostopoulos, G.; Mostafa, S.; Nirantharakumar, K.; Tahrani, A. The links between sleep duration, obesity and type 2 diabetes mellitus. J. Endocrinol. 2021, 252, 125–141. [Google Scholar] [CrossRef]
- Srinivasan, S.; Singh, P.; Kulothungan, V.; Sharma, T.; Raman, R. Relationship between triglyceride glucose index, retinopathy and nephropathy in Type 2 diabetes. Endocrinol. Diabetes Metab. 2021, 4, e00151. [Google Scholar] [CrossRef] [PubMed]
- Low, S.; Khoo, K.C.J.; Irwan, B.; Sum, C.F.; Subramaniam, T.; Lim, S.C.; Wong, T.K.M. The role of triglyceride glucose index in development of Type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2018, 143, 43–49. [Google Scholar] [CrossRef]
- Jiang, C.; Yang, R.; Kuang, M.; Yu, M.; Zhong, M.; Zou, Y. Triglyceride glucose-body mass index in identifying high-risk groups of pre-diabetes. Lipids Health Dis. 2021, 20, 161. [Google Scholar] [CrossRef]
- Cheng, A.Y.; Sloan, L.; Anderson, J. Management of Type 2 Diabetes in People With Renal Impairment. J. Fam. Pract. 2021, 70, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Triozzi, J.L.; Parker Gregg, L.; Virani, S.S.; Navaneethan, S.D. Management of type 2 diabetes in chronic kidney disease. BMJ Open Diabetes Res. Care 2021, 9, e002300. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, T.; Perkovic, V.; de Galan, B.E.; Zoungas, S.; Pillai, A.; Jardine, M.; Patel, A.; Cass, A.; Neal, B.; Poulter, N.; et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J. Am. Soc. Nephrol. 2009, 20, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- van der Velde, M.; Halbesma, N.; de Charro, F.T.; Bakker, S.J.; de Zeeuw, D.; de Jong, P.E.; Gansevoort, R.T. Screening for albuminuria identifies individuals at increased renal risk. J. Am. Soc. Nephrol. 2009, 20, 852–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson-Stuttard, J.; Papadimitriou, N.; Markozannes, G.; Cividini, S.; Kakourou, A.; Gill, D.; Rizos, E.C.; Monori, G.; Ward, H.A.; Kyrgiou, M.; et al. Type 2 Diabetes and Cancer: An Umbrella Review of Observational and Mendelian Randomization Studies. Cancer Epidemiol. Biomark. Prev. 2021, 30, 1218–1228. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, X.; Ma, Y.; Yuan, C.; Wang, M.; Wu, K.; Tabung, F.K.; Tobias, D.; Hu, F.B.; Giovannucci, E.; et al. Incident Type 2 Diabetes Duration and Cancer Risk: A Prospective Study in Two US Cohorts. J. Natl. Cancer Inst. 2021, 113, 381–389. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, W.; Song, M.; Smith-Warner, S.A.; Yang, J.; Li, Y.; Ma, W.; Hu, Y.; Ogino, S.; Hu, F.B.; et al. Type 2 diabetes and risk of colorectal cancer in two large U.S. prospective cohorts. Br. J. Cancer 2018, 119, 1436–1442. [Google Scholar] [CrossRef] [Green Version]
- Rahman, I.; Athar, M.T.; Islam, M. Type 2 Diabetes, Obesity, and Cancer Share Some Common and Critical Pathways. Front. Oncol. 2020, 10, 600824. [Google Scholar] [CrossRef]
- Shlomai, G.; Neel, B.; LeRoith, D.; Gallagher, E.J. Type 2 Diabetes Mellitus and Cancer: The Role of Pharmacotherapy. J. Clin. Oncol 2016, 34, 4261–4269. [Google Scholar] [CrossRef] [Green Version]
- Cuyas, E.; Buxo, M.; Iglesias, M.J.F.; Verdura, S.; Pemas, S.; Dorca, J.; Alvarez, I.; Martinez, S.; Perez-Garcia, J.M.; Batista-Lopez, N.; et al. The C Allele of ATM rs11212617 Associates With Higher Pathological Complete Remission Rate in Breast Cancer Patients Treated With Neoadjuvant Metformin. Front. Oncol. 2019, 9, 193. [Google Scholar] [CrossRef]
- Durrani, I.A.; Bhatti, A.; John, P. The prognostic outcome of ‘type 2 diabetes mellitus and breast cancer’ association pivots on hypoxia-hyperglycemia axis. Cancer Cell Int. 2021, 21, 351. [Google Scholar] [CrossRef] [PubMed]
- Scully, T.; Ettela, A.; LeRoith, D.; Gallagher, E.J. Obesity, Type 2 Diabetes, and Cancer Risk. Front. Oncol. 2021, 10, 615375. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Pizzocaro, A.; Vena, W.; Rastrelli, G.; Semeraro, F.; Isidori, A.M.; Pivonello, R.; Salonia, A.; Sforza, A.; Maggi, M. Diabetes is most important cause for mortality in COVID-19 hospitalized patients: Systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2021, 22, 275–296. [Google Scholar] [CrossRef] [PubMed]
- Dennis, J.M.; Mateen, B.A.; Sonabend, R.; Thomas, N.J.; Patel, K.A.; Hattersley, A.T.; Denaxas, S.; McGovern, A.P.; Vollmer, S.J. Type 2 Diabetes and COVID-19-Related Mortality in the Critical Care Setting: A National Cohort Study in England, March–July 2020. Diabetes Care 2021, 44, 50–57. [Google Scholar] [CrossRef]
- Gong, Y.; Qin, S.; Dai, L.; Tian, Z. The glycosylation in SARS-CoV-2 and its receptor ACE2. Signal Transduct. Target. Ther. 2021, 6, 396. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef]
- Lim, S.; Bae, J.H.; Kwon, H.S.; Nauck, M.A. COVID-19 and diabetes mellitus: From pathophysiology to clinical management. Nat. Rev. Endocrinol. 2021, 17, 11–30. [Google Scholar] [CrossRef]
- Jandeleit-Dahm, K.; Watson, A.; Soro-Paavonen, A. The AGE/RAGE axis in diabetes-accelerated atherosclerosis. Clin. Exp. Pharmacol. Physiol. 2008, 35, 329–334. [Google Scholar] [CrossRef]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 Diabetes and its Impact on the Immune System. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar] [CrossRef]
- Sestan, M.; Marinovic, S.; Kavazovic, I.; Cekinovic, D.; Wueest, S.; Turk Wensveen, T.; Brizic, I.; Jonjic, S.; Konrad, D.; Wensveen, F.M.; et al. Virus-Induced Interferon-gamma Causes Insulin Resistance in Skeletal Muscle and Derails Glycemic Control in Obesity. Immunity 2018, 49, 164–177.e6. [Google Scholar] [CrossRef] [Green Version]
- Samprathi, M.; Jayashree, M. Biomarkers in COVID-19: An Up-To-Date Review. Front. Pediatr. 2020, 8, 607647. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; the Northwell COVID-19 Research Consortium; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Pechlivani, N.; Ajjan, R.A. Thrombosis and Vascular Inflammation in Diabetes: Mechanisms and Potential Therapeutic Targets. Front. Cardiovasc. Med. 2018, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Zhan, K.; Zhang, X.; Wang, B.; Jiang, Z.; Fang, X.; Yang, S.; Jia, H.; Li, L.; Cao, G.; Zhang, K.; et al. Short- and long-term prognosis of glycemic control in COVID-19 patients with type 2 diabetes. QJM 2022, 115, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Rathmann, W.; Kuss, O.; Kostev, K. Incidence of newly diagnosed diabetes after COVID-19. Diabetologia 2022, 65, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Corrao, S.; Pinelli, K.; Vacca, M.; Raspanti, M.; Argano, C. Type 2 Diabetes Mellitus and COVID-19: A Narrative Review. Front. Endocrinol. 2021, 12, 609470. [Google Scholar] [CrossRef]
- Ortega, E.; Corcoy, R.; Gratacos, M.; Claramunt, F.X.C.; Mata-Cases, M.; Puig-Treserra, R.; Real, J.; Vlacho, B.; Castelblanco, E.; Domingo, P.; et al. Risk factors for severe outcomes in people with diabetes hospitalised for COVID-19: A cross-sectional database study. BMJ Open 2021, 11, e051237. [Google Scholar] [CrossRef]
- Fu, Y.; Hu, L.; Ren, H.W.; Zuo, Y.; Chen, S.; Zhang, Q.S.; Shao, C.; Ma, Y.; Wu, L.; Hao, J.J.; et al. Prognostic Factors for COVID-19 Hospitalized Patients with Preexisting Type 2 Diabetes. Int. J. Endocrinol. 2022, 2022, 9322332. [Google Scholar] [CrossRef]
- Rawshani, A.; Kjolhede, E.A.; Rawshani, A.; Sattar, N.; Eeg-Olofsson, K.; Adiels, M.; Ludvigsson, J.; Lindh, M.; Gisslen, M.; Hagberg, E.; et al. Severe COVID-19 in people with type 1 and type 2 diabetes in Sweden: A nationwide retrospective cohort study. Lancet Reg. Health Eur. 2021, 4, 100105. [Google Scholar] [CrossRef]
- Barron, E.; Bakhai, C.; Kar, P.; Weaver, A.; Bradley, D.; Ismail, H.; Knighton, P.; Holman, N.; Khunti, K.; Sattar, N.; et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: A whole-population study. Lancet Diabetes Endocrinol. 2020, 8, 813–822. [Google Scholar] [CrossRef]
- Gregory, J.M.; Slaughter, J.C.; Duffus, S.H.; Smith, T.J.; LeStourgeon, L.M.; Jaser, S.S.; McCoy, A.B.; Luther, J.M.; Giovannetti, E.R.; Boeder, S.; et al. COVID-19 Severity Is Tripled in the Diabetes Community: A Prospective Analysis of the Pandemic’s Impact in Type 1 and Type 2 Diabetes. Diabetes Care 2021, 44, 526–532. [Google Scholar] [CrossRef]
- Ferrando, C.; Mellado-Artigas, R.; Gea, A.; Arruti, E.; Aldecoa, C.; Bordell, A.; Adalia, R.; Zattera, L.; Ramasco, F.; Monedero, P.; et al. Patient characteristics, clinical course and factors associated to ICU mortality in critically ill patients infected with SARS-CoV-2 in Spain: A prospective, cohort, multicentre study. Rev. Esp. Anestesiol. Reanim. (Engl. Ed.) 2020, 67, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Powers, A.C.; Aronoff, D.M.; Eckel, R.H. COVID-19 vaccine prioritisation for type 1 and type 2 diabetes. Lancet Diabetes Endocrinol. 2021, 9, 140–141. [Google Scholar] [CrossRef]
- Marfella, R.; D’Onofrio, N.; Sardu, C.; Scisciola, L.; Maggi, P.; Coppola, N.; Romano, C.; Messina, V.; Turriziani, F.; Siniscalchi, M.; et al. Does poor glycaemic control affect the immunogenicity of the COVID-19 vaccination in patients with type 2 diabetes: The CAVEAT study. Diabetes Obes. Metab. 2022, 24, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Patel, N.; Vemparala, P.; Krishnamurthy, M. Metformin is associated with favorable outcomes in patients with COVID-19 and type 2 diabetes mellitus. Sci. Rep. 2022, 12, 5553. [Google Scholar] [CrossRef] [PubMed]
- Bielka, W.; Przezak, A.; Pawlik, A. Therapy of Type 2 Diabetes in Patients with SARS-CoV-2 Infection. Int. J. Mol. Sci. 2021, 22, 7605. [Google Scholar] [CrossRef]
- Ceriello, A.; Prattichizzo, F. Pharmacological management of COVID-19 in type 2 diabetes. J. Diabetes Complicat. 2021, 35, 107927. [Google Scholar] [CrossRef]
- Kazakou, P.; Lambadiari, V.; Ikonomidis, I.; Kountouri, A.; Panagopoulos, G.; Athanasopoulos, S.; Korompoki, E.; Kalomenidis, I.; Dimopoulos, M.A.; Mitrakou, A. Diabetes and COVID-19; A Bidirectional Interplay. Front. Endocrinol. 2022, 13, 780663. [Google Scholar] [CrossRef]
- Beyerstedt, S.; Casaro, E.B.; Rangel, E.B. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef]
- Steenblock, C.; Richter, S.; Berger, I.; Barovic, M.; Schmid, J.; Schubert, U.; Jarzebska, N.; von Massenhausen, A.; Linkermann, A.; Schurmann, A.; et al. Viral infiltration of pancreatic islets in patients with COVID-19. Nat. Commun. 2021, 12, 3534. [Google Scholar] [CrossRef]
- Hackler, L., Jr.; Dorman, G.; Kele, Z.; Urge, L.; Darvas, F.; Puskas, L.G. Development of chemically modified glass surfaces for nucleic acid, protein and small molecule microarrays. Mol. Divers. 2003, 7, 25–36. [Google Scholar] [CrossRef]
- Darvas, F.; Dorman, G.; Krajcsi, P.; Puskas, L.G.; Kovari, Z.; Lorincz, Z.; Urge, L. Recent advances in chemical genomics. Curr. Med. Chem. 2004, 11, 3119–3145. [Google Scholar] [CrossRef] [PubMed]
- Nasykhova, Y.A.; Barbitoff, Y.A.; Serebryakova, E.A.; Katserov, D.S.; Glotov, A.S. Recent advances and perspectives in next generation sequencing application to the genetic research of type 2 diabetes. World J. Diabetes 2019, 10, 376–395. [Google Scholar] [CrossRef] [PubMed]
- Dash, N.R.; Al Bataineh, M.T. Metagenomic Analysis of the Gut Microbiome Reveals Enrichment of Menaquinones (Vitamin K2) Pathway in Diabetes Mellitus. Diabetes Metab. J. 2021, 45, 77–85. [Google Scholar] [CrossRef]
- Sarkozy, M.; Szucs, G.; Pipicz, M.; Zvara, A.; Eder, K.; Fekete, V.; Szucs, C.; Barkanyi, J.; Csonka, C.; Puskas, L.G.; et al. The effect of a preparation of minerals, vitamins and trace elements on the cardiac gene expression pattern in male diabetic rats. Cardiovasc. Diabetol. 2015, 14, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoel, H.; Hove-Skovsgaard, M.; Hov, J.R.; Gaardbo, J.C.; Holm, K.; Kummen, M.; Rudi, K.; Nwosu, F.; Valeur, J.; Gelpi, M.; et al. Impact of HIV and Type 2 diabetes on Gut Microbiota Diversity, Tryptophan Catabolism and Endothelial Dysfunction. Sci. Rep. 2018, 8, 6725. [Google Scholar] [CrossRef]
- Tong, X.; Xu, J.; Lian, F.; Yu, X.; Zhao, Y.; Xu, L.; Zhang, M.; Zhao, X.; Shen, J.; Wu, S.; et al. Structural Alteration of Gut Microbiota during the Amelioration of Human Type 2 Diabetes with Hyperlipidemia by Metformin and a Traditional Chinese Herbal Formula: A Multicenter, Randomized, Open Label Clinical Trial. mBio 2018, 9, e02392-17. [Google Scholar] [CrossRef] [Green Version]
- Montesanto, A.; Bonfigli, A.R.; Crocco, P.; Garagnani, P.; De Luca, M.; Boemi, M.; Marasco, E.; Pirazzini, C.; Giuliani, C.; Franceschi, C.; et al. Genes associated with Type 2 Diabetes and vascular complications. Aging 2018, 10, 178–196. [Google Scholar] [CrossRef] [Green Version]
- Kwak, S.H.; Park, K.S. Recent progress in genetic and epigenetic research on type 2 diabetes. Exp. Mol. Med. 2016, 48, e220. [Google Scholar] [CrossRef] [Green Version]
- Ling, C.; Ronn, T. Epigenetics in Human Obesity and Type 2 Diabetes. Cell Metab. 2019, 29, 1028–1044. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Wang, L.; Obayomi, S.M.B.; Wei, Z. Epigenetic Regulation of beta Cell Identity and Dysfunction. Front. Endocrinol. 2021, 12, 725131. [Google Scholar] [CrossRef] [PubMed]
- Walaszczyk, E.; Luijten, M.; Spijkerman, A.M.W.; Bonder, M.J.; Lutgers, H.L.; Snieder, H.; Wolffenbuttel, B.H.R.; van Vliet-Ostaptchouk, J.V. DNA methylation markers associated with type 2 diabetes, fasting glucose and HbA1c levels: A systematic review and replication in a case-control sample of the Lifelines study. Diabetologia 2018, 61, 354–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackermann, A.M.; Wang, Z.; Schug, J.; Naji, A.; Kaestner, K.H. Integration of ATAC-seq and RNA-seq identifies human alpha cell and beta cell signature genes. Mol. Metab. 2016, 5, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Greenwald, W.W.; Chiou, J.; Yan, J.; Qiu, Y.; Dai, N.; Wang, A.; Nariai, N.; Aylward, A.; Han, J.Y.; Kadakia, N.; et al. Pancreatic islet chromatin accessibility and conformation reveals distal enhancer networks of type 2 diabetes risk. Nat. Commun. 2019, 10, 2078. [Google Scholar] [CrossRef]
- Lawlor, N.; George, J.; Bolisetty, M.; Kursawe, R.; Sun, L.; Sivakamasundari, V.; Kycia, I.; Robson, P.; Stitzel, M.L. Single-cell transcriptomes identify human islet cell signatures and reveal cell-type-specific expression changes in type 2 diabetes. Genome Res. 2017, 27, 208–222. [Google Scholar] [CrossRef]
- Iacono, G.; Massoni-Badosa, R.; Heyn, H. Single-cell transcriptomics unveils gene regulatory network plasticity. Genome Biol. 2019, 20, 110. [Google Scholar] [CrossRef] [Green Version]
- Rai, V.; Quang, D.X.; Erdos, M.R.; Cusanovich, D.A.; Daza, R.M.; Narisu, N.; Zou, L.S.; Didion, J.P.; Guan, Y.; Shendure, J.; et al. Single-cell ATAC-Seq in human pancreatic islets and deep learning upscaling of rare cells reveals cell-specific type 2 diabetes regulatory signatures. Mol. Metab. 2020, 32, 109–121. [Google Scholar] [CrossRef]
- Marques, E.S.; Formato, E.; Liang, W.; Leonard, E.; Timme-Laragy, A.R. Relationships between type 2 diabetes, cell dysfunction, and redox signaling: A meta-analysis of single-cell gene expression of human pancreatic alpha- and beta-cells. J. Diabetes 2022, 14, 34–51. [Google Scholar] [CrossRef]
- Abate, N.; Chandalia, M.; Snell, P.G.; Grundy, S.M. Adipose tissue metabolites and insulin resistance in nondiabetic Asian Indian men. J. Clin. Endocrinol. Metab. 2004, 89, 2750–2755. [Google Scholar] [CrossRef] [Green Version]
- Saxena, A.; Tiwari, P.; Wahi, N.; Soni, A.; Bansiwal, R.C.; Kumar, A.; Sharma, B.; Punjabi, P.; Gupta, N.; Malik, B.; et al. Transcriptome profiling reveals association of peripheral adipose tissue pathology with type-2 diabetes in Asian Indians. Adipocyte 2019, 8, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Saxena, A.; Mathur, N.; Tiwari, P.; Mathur, S.K. Whole transcriptome RNA-seq reveals key regulatory factors involved in type 2 diabetes pathology in peripheral fat of Asian Indians. Sci. Rep. 2021, 11, 10632. [Google Scholar] [CrossRef] [PubMed]
- Irgam, K.; Reddy, B.S.; Hari, S.G.; Banapuram, S.; Reddy, B.M. The genetic susceptibility profile of type 2 diabetes and reflection of its possible role related to reproductive dysfunctions in the southern Indian population of Hyderabad. BMC Med. Genom. 2021, 14, 272. [Google Scholar] [CrossRef] [PubMed]
- Barchetta, I.; Ceccarelli, V.; Cimini, F.A.; Bertoccini, L.; Fraioli, A.; Alessandri, C.; Lenzi, A.; Baroni, M.G.; Cavallo, M.G. Impaired bone matrix glycoprotein pattern is associated with increased cardio-metabolic risk profile in patients with type 2 diabetes mellitus. J. Endocrinol. Investig. 2019, 42, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Looker, H.C.; Farran, B.; Hess, S.; Groop, L.; Palmer, C.N.A.; Brosnan, M.J.; Dalton, R.N.; Wong, M.; Turner, C.; et al. Serum kidney injury molecule 1 and beta2-microglobulin perform as well as larger biomarker panels for prediction of rapid decline in renal function in type 2 diabetes. Diabetologia 2019, 62, 156–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinzel, A.; Kammer, M.; Mayer, G.; Reindl-Schwaighofer, R.; Hu, K.; Perco, P.; Eder, S.; Rosivall, L.; Mark, P.B.; Ju, W.; et al. Validation of Plasma Biomarker Candidates for the Prediction of eGFR Decline in Patients With Type 2 Diabetes. Diabetes Care 2018, 41, 1947–1954. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Lee, M.Y.Y.; Bahl, V.; Traum, D.; Schug, J.; Kusmartseva, I.; Atkinson, M.A.; Fan, G.; Consortium, H.; Kaestner, K.H. Single-cell analysis of the human pancreas in type 2 diabetes using multi-spectral imaging mass cytometry. Cell Rep. 2021, 37, 109919. [Google Scholar] [CrossRef]
- Szebeni, G.J.; Tancos, Z.; Feher, L.Z.; Alfoldi, R.; Kobolak, J.; Dinnyes, A.; Puskas, L.G. Real architecture For 3D Tissue (RAFT) culture system improves viability and maintains insulin and glucagon production of mouse pancreatic islet cells. Cytotechnology 2017, 69, 359–369. [Google Scholar] [CrossRef] [Green Version]
- Razquin, C.; Toledo, E.; Clish, C.B.; Ruiz-Canela, M.; Dennis, C.; Corella, D.; Papandreou, C.; Ros, E.; Estruch, R.; Guasch-Ferre, M.; et al. Plasma Lipidomic Profiling and Risk of Type 2 Diabetes in the PREDIMED Trial. Diabetes Care 2018, 41, 2617–2624. [Google Scholar] [CrossRef] [Green Version]
- Suvitaival, T.; Bondia-Pons, I.; Yetukuri, L.; Poho, P.; Nolan, J.J.; Hyotylainen, T.; Kuusisto, J.; Oresic, M. Lipidome as a predictive tool in progression to type 2 diabetes in Finnish men. Metabolism 2018, 78, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Shetty, S.S.; Kumari, S. Fatty acids and their role in type-2 diabetes (Review). Exp. Ther. Med. 2021, 22, 706. [Google Scholar] [CrossRef]
| Main types of diabetes mellitus |
|
| Other specific types |
|
| Unclassified diabetes |
|
| Fasting plasma glucose (8 h no food intake) level ≥126 mg/dL (7.0 mmol/L) |
| 75 g OGTT 2 h value ≥ 200 mg/dL (11.1 mmol/L); OGTT: glucose load containing the equivalent of 75 g anhydrous glucose dissolved in water. |
| Hemoglobin A1c ≥ 6.5% |
| Random plasma glucose ≥200 mg/dL (11.1 mmol/L), sometimes appears as a hyperglycemic crisis |
| Clinical symptoms of diabetes (e.g., thirst, polydipsia, polyuria, weight loss, and dry mouth) |
| Pharmacological Group | Drug | Biochemical Key Factor for Mechanism of Action | Mechanism of Action |
|---|---|---|---|
| Sulfonylureas (SU) | glipizide glyburide gliclazide glimepiride | K-ATP channels of beta cells | Close ATP-dependent potassium channels that depolarize the beta cells, opening calcium channels and causing insulin release |
| Meglitinides | repaglinide nateglinide | K-ATP channels of beta cells | Same as SU |
| Biguanides | metformin | Increase hepatic AMP-activated protein kinase activity | Reduce hepatic gluconeogenesis and lipogenesis, stimulate fatty acid oxidation, and increase insulin-mediated uptake of glucose in muscles |
| Thiazolidinediones (TZD) | rosiglitazone pioglitazone | Activate peroxisome proliferator-activated receptor gamma (PPAR-γ) | Increase insulin sensitivity and stimulate fatty acid oxidation |
| α-Glucosidase inhibitors | acarbose miglitol voglibose | Inhibit alpha-glucosidase enzymes in the intestinal brush border cells | Inhibit polysaccharide reabsorption |
| GLP-1 Receptor Agonists | exenatide BID liraglutide lixisenatide exenatide albiglutide, dulaglutide semaglutide oral semaglutide (Rybelsus) | Stimulate GLP-1 receptors | Lead to the increase in insulin secretion |
| DPP-4 inhibitors | sitagliptin vildagliptin saxagliptin linagliptin alogliptin | Inhibit the enzyme dipeptidyl peptidase 4 (DPP-4) | Decrease glucagon release, thus increasing glucose-dependent insulin release |
| SGLT2 inhibitors | dapagliflozin canagliflozin empagliflozin tofogliflozin | Inhibit sodium–glucose cotransporter 2 (SGLT-2) in the proximal tubules of renal glomerulus | Inhibition of glucose reabsorption, resulting in glycosuria |
| Cycloset | bromocriptine | Dopamine (D2) receptor agonist | Resets the hypothalamic circadian rhythm and improves insulin resistance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kupai, K.; Várkonyi, T.; Török, S.; Gáti, V.; Czimmerer, Z.; Puskás, L.G.; Szebeni, G.J. Recent Progress in the Diagnosis and Management of Type 2 Diabetes Mellitus in the Era of COVID-19 and Single Cell Multi-Omics Technologies. Life 2022, 12, 1205. https://doi.org/10.3390/life12081205
Kupai K, Várkonyi T, Török S, Gáti V, Czimmerer Z, Puskás LG, Szebeni GJ. Recent Progress in the Diagnosis and Management of Type 2 Diabetes Mellitus in the Era of COVID-19 and Single Cell Multi-Omics Technologies. Life. 2022; 12(8):1205. https://doi.org/10.3390/life12081205
Chicago/Turabian StyleKupai, Krisztina, Tamás Várkonyi, Szilvia Török, Viktória Gáti, Zsolt Czimmerer, László G. Puskás, and Gábor J. Szebeni. 2022. "Recent Progress in the Diagnosis and Management of Type 2 Diabetes Mellitus in the Era of COVID-19 and Single Cell Multi-Omics Technologies" Life 12, no. 8: 1205. https://doi.org/10.3390/life12081205
APA StyleKupai, K., Várkonyi, T., Török, S., Gáti, V., Czimmerer, Z., Puskás, L. G., & Szebeni, G. J. (2022). Recent Progress in the Diagnosis and Management of Type 2 Diabetes Mellitus in the Era of COVID-19 and Single Cell Multi-Omics Technologies. Life, 12(8), 1205. https://doi.org/10.3390/life12081205