Central Venous Access and the Risk for Thromboembolic Events in Patients Undergoing Neoadjuvant Chemotherapy and Radical Cystectomy for Muscle-Invasive Bladder Cancer
Abstract
1. Introduction
2. Materials and Methods
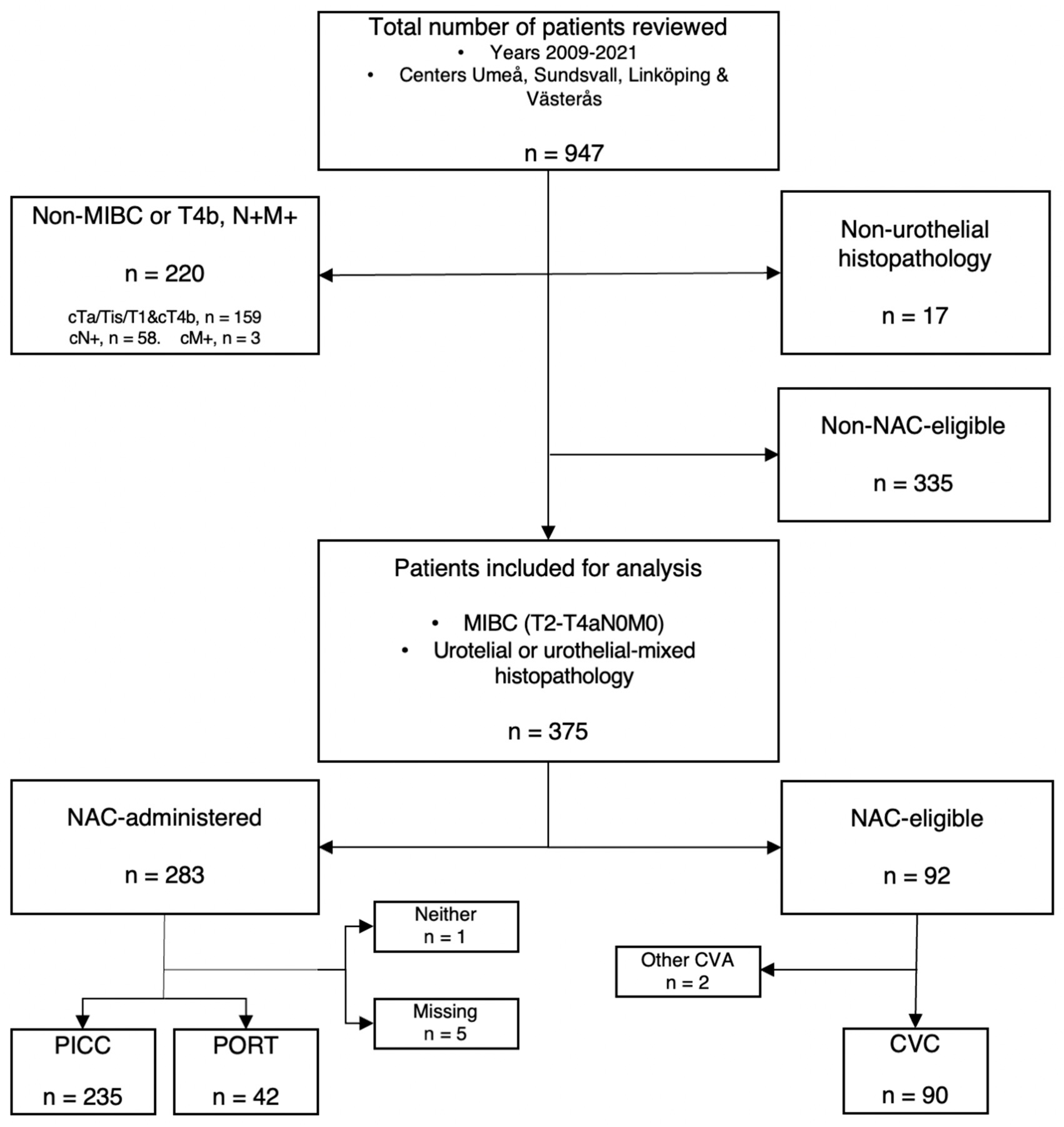
2.1. Patient Population
2.2. NAC-Eligibility
2.3. Data Collection & TEE-Definitions
2.3.1. Statistics
2.3.2. Ethics
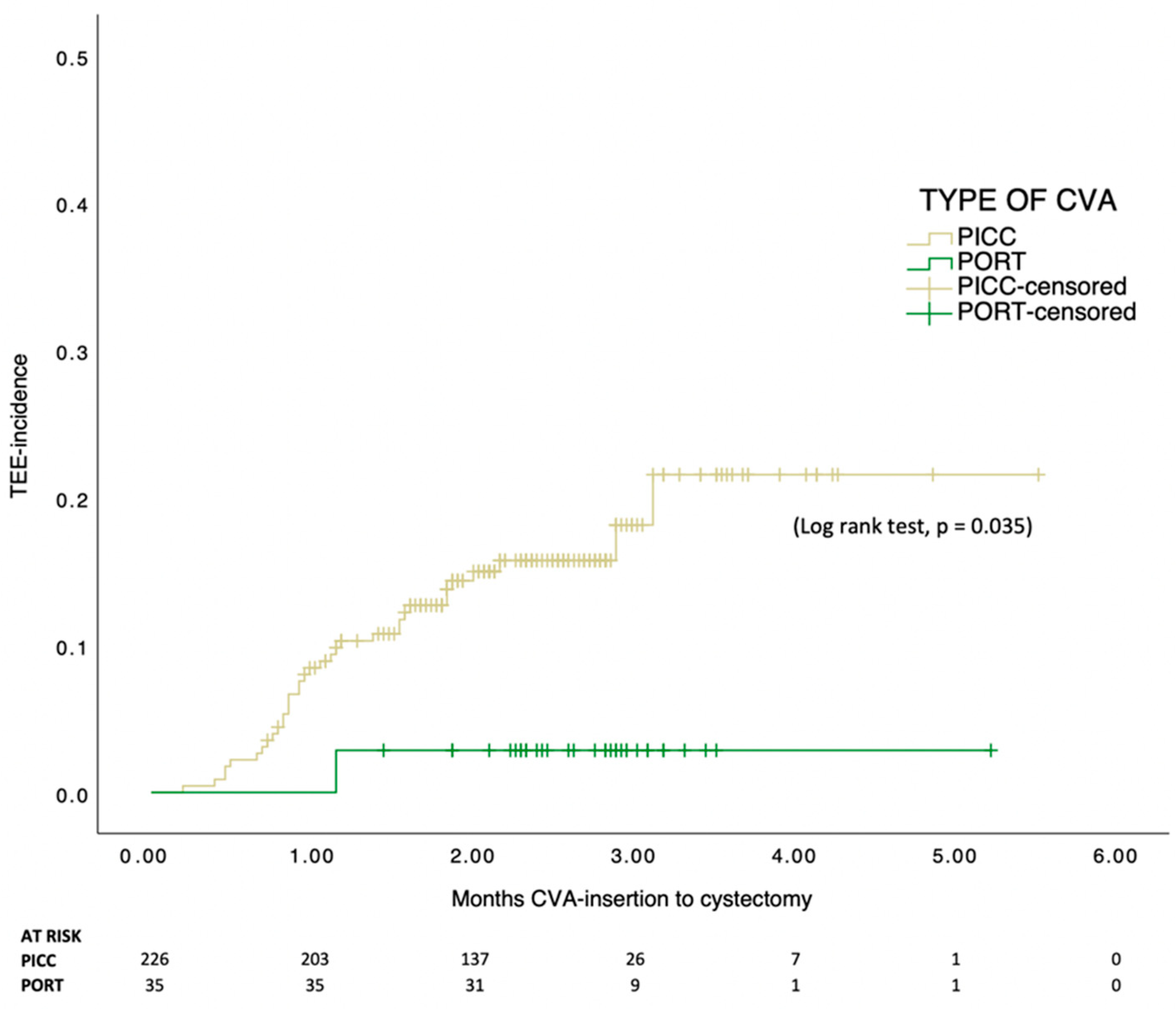
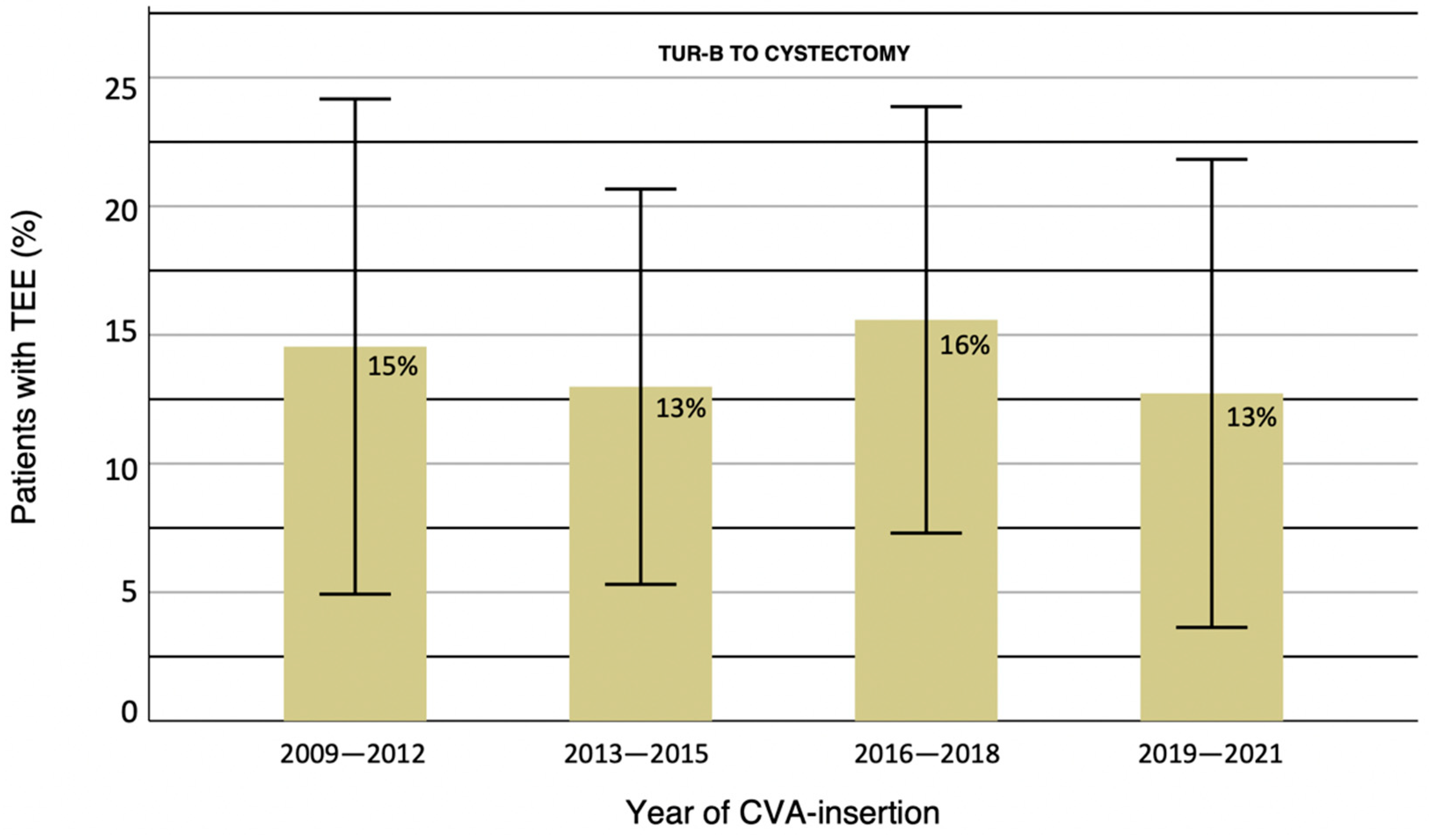
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Burger, M.; Catto, J.W.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; La Vecchia, C.; Shariat, S.; et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241. (In English) [Google Scholar] [CrossRef] [PubMed]
- Antoni, S.; Ferlay, J.; Soerjomataram, I.; Znaor, A.; Jemal, A.; Bray, F. Bladder Cancer Incidence and Mortality: A Global Overview and Recent Trends. Eur. Urol. 2017, 71, 96–108. (In English) [Google Scholar] [CrossRef] [PubMed]
- Alfred Witjes, J.; Lebret, T.; Compérat, E.M.; Cowan, N.C.; De Santis, M.; Bruins, H.M.; Hernández, V.; Espinós, E.L.; Dunn, J.; Rouanne, M.; et al. Updated 2016 EAU Guidelines on Muscle-invasive and Metastatic Bladder Cancer. Eur. Urol. 2017, 71, 462–475. (In English) [Google Scholar] [CrossRef]
- Rosenblatt, R.; Sherif, A.; Rintala, E.; Wahlqvist, R.; Ullén, A.; Nilsson, S.; Malmström, P.U.; Group, N.U.C. Pathologic downstaging is a surrogate marker for efficacy and increased survival following neoadjuvant chemotherapy and radical cystectomy for muscle-invasive urothelial bladder cancer. Eur. Urol. 2012, 61, 1229–1238. (In English) [Google Scholar] [CrossRef] [PubMed]
- Jerlström, T.; Chen, R.; Liedberg, F.; Andrén, O.; Ströck, V.; Aljabery, F.A.S.; Hosseini, A.; Sherif, A.; Malmström, P.U.; Ullén, A.; et al. No increased risk of short-term complications after radical cystectomy for muscle-invasive bladder cancer among patients treated with preoperative chemotherapy: A nation-wide register-based study. World J. Urol. 2020, 38, 381–388. (In English) [Google Scholar] [CrossRef] [PubMed]
- Leiberg, F. Nationellt Vårdprogram Cancer i Urinblåsa, Njurbäcken, Urinledare Och Urinrör. Available online: https://cancercentrum.se/samverkan/cancerdiagnoser/urinblasa-urinvagar/vardprogram/ (accessed on 28 June 2022).
- Bhindi, B.; Frank, I.; Mason, R.J.; Tarrell, R.F.; Thapa, P.; Cheville, J.C.; Costello, B.A.; Pagliaro, L.C.; Karnes, R.J.; Thompson, R.H.; et al. Oncologic Outcomes for Patients with Residual Cancer at Cystectomy Following Neoadjuvant Chemotherapy: A Pathologic Stage-matched Analysis. Eur. Urol. 2017, 72, 660–664. (In English) [Google Scholar] [CrossRef]
- Lavery, H.J.; Stensland, K.D.; Niegisch, G.; Albers, P.; Droller, M.J. Pathological T0 following radical cystectomy with or without neoadjuvant chemotherapy: A useful surrogate. J. Urol. 2014, 191, 898–906. (In English) [Google Scholar] [CrossRef] [PubMed]
- Martini, A.; Jia, R.; Ferket, B.S.; Waingankar, N.; Plimack, E.R.; Crabb, S.J.; Harshman, L.C.; Yu, E.Y.; Powles, T.; Rosenberg, J.E.; et al. Tumor downstaging as an intermediate endpoint to assess the activity of neoadjuvant systemic therapy in patients with muscle-invasive bladder cancer. Cancer 2019, 125, 3155–3163. (In English) [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.P.; Montoya Perez, I.; Klén, R.; Ettala, O.O.; Syvänen, K.T.; Elo, L.L.; Boström, P.J. Adverse Events During Neoadjuvant Chemotherapy for Muscle Invasive Bladder Cancer. Bladder Cancer 2019, 5, 273–279. [Google Scholar] [CrossRef]
- Eriksson, V.; Holmlund, J.; Wiberg, E.; Johansson, M.; Huge, Y.; Alamdari, F.; Svensson, J.; Aljabery, F.; Sherif, A. Adverse Events during neoadjuvant chemotherapy for muscle invasive bladder cancer—A Swedish retrospective multicenter study of a clinical database. 2022; in press. [Google Scholar]
- Zareba, P.; Patterson, L.; Pandya, R.; Margel, D.; Hotte, S.J.; Mukherjee, S.D.; Elavathil, L.; Daya, D.; Shayegan, B.; Pinthus, J.H. Thromboembolic events in patients with urothelial carcinoma undergoing neoadjuvant chemotherapy and radical cystectomy. Urol. Oncol. 2014, 32, 975–980. (In English) [Google Scholar] [CrossRef] [PubMed]
- Moore, R.A.; Adel, N.; Riedel, E.; Bhutani, M.; Feldman, D.R.; Tabbara, N.E.; Soff, G.; Parameswaran, R.; Hassoun, H. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: A large retrospective analysis. J. Clin. Oncol. 2011, 29, 3466–3473. (In English) [Google Scholar] [CrossRef] [PubMed]
- Falanga, A.; Marchetti, M.; Russo, L. The mechanisms of cancer-associated thrombosis. Thromb Res. 2015, 135 (Suppl. 1), S8–S11. (In English) [Google Scholar] [CrossRef]
- Tejedor, S.C.; Tong, D.; Stein, J.; Payne, C.; Dressler, D.; Xue, W.; Steinberg, J.P. Temporary central venous catheter utilization patterns in a large tertiary care center: Tracking the “idle central venous catheter”. Infect. Control Hosp. Epidemiol. 2012, 33, 50–57. (In English) [Google Scholar] [CrossRef] [PubMed]
- Periard, D.; Monney, P.; Waeber, G.; Zurkinden, C.; Mazzolai, L.; Hayoz, D.; Doenz, F.; Zanetti, G.; Wasserfallen, J.B.; Denys, A. Randomized controlled trial of peripherally inserted central catheters vs. peripheral catheters for middle duration in-hospital intravenous therapy. J. Thromb. Haemost. 2008, 6, 1281–1288. (In English) [Google Scholar] [CrossRef]
- Chopra, V.; Flanders, S.A.; Saint, S.; Woller, S.C.; O’Grady, N.P.; Safdar, N.; Trerotola, S.O.; Saran, R.; Moureau, N.; Wiseman, S.; et al. The Michigan Appropriateness Guide for Intravenous Catheters (MAGIC): Results From a Multispecialty Panel Using the RAND/UCLA Appropriateness Method. Ann. Intern. Med. 2015, 163, S1–S40. (In English) [Google Scholar] [CrossRef] [PubMed]
- Sousa, B.; Furlanetto, J.; Hutka, M.; Gouveia, P.; Wuerstlein, R.; Mariz, J.M.; Pinto, D.; Cardoso, F. Central venous access in oncology: ESMO Clinical Practice Guidelines. Ann. Oncol. 2015, 26 (Suppl. 5), v152–v168. (In English) [Google Scholar] [CrossRef]
- Bodenham Chair, A.; Babu, S.; Bennett, J.; Binks, R.; Fee, P.; Fox, B.; Johnston, A.J.; Klein, A.A.; Langton, J.A.; McLure, H.; et al. Association of Anaesthetists of Great Britain and Ireland: Safe vascular access 2016. Anaesthesia 2016, 71, 573–585. (In English) [Google Scholar] [CrossRef] [PubMed]
- Frykholm, P.; Pikwer, A.; Hammarskjöld, F.; Larsson, A.T.; Lindgren, S.; Lindwall, R.; Taxbro, K.; Oberg, F.; Acosta, S.; Akeson, J. Clinical guidelines on central venous catheterisation. Swedish Society of Anaesthesiology and Intensive Care Medicine. Acta Anaesthesiol. Scand. 2014, 58, 508–524. (In English) [Google Scholar] [CrossRef] [PubMed]
- Schiffer, C.A.; Mangu, P.B.; Wade, J.C.; Camp-Sorrell, D.; Cope, D.G.; El-Rayes, B.F.; Gorman, M.; Ligibel, J.; Mansfield, P.; Levine, M. Central venous catheter care for the patient with cancer: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2013, 31, 1357–1370. (In English) [Google Scholar] [CrossRef] [PubMed]
- Garas, S.N.; McAlpine, K.; Ross, J.; Carrier, M.; Bossé, D.; Yachnin, D.; Mallick, R.; Cagiannos, I.; Morash, C.; Breau, R.H.; et al. Venous thromboembolism risk in patients receiving neoadjuvant chemotherapy for bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2022, 40, 381.e1–381.e7. [Google Scholar] [CrossRef] [PubMed]
- Ottosson, K.; Pelander, S.; Johansson, M.; Huge, Y.; Aljabery, F.; Sherif, A. The increased risk for thromboembolism pre-cystectomy in patients undergoing neoadjuvant chemotherapy for muscle-invasive urinary bladder cancer is mainly due to central venous access: A multicenter evaluation. Int. Urol. Nephrol. 2020, 52, 661–669. (In English) [Google Scholar] [CrossRef] [PubMed]
- Dyer, J.; Wyke, S.; Lynch, C. Hospital Episode Statistics data analysis of postoperative venous thromboembolus in patients undergoing urological surgery: A review of 126,891 cases. Ann. R. Coll. Surg. Engl. 2013, 95, 65–69. (In English) [Google Scholar] [CrossRef] [PubMed]
- Duivenvoorden, W.C.; Daneshmand, S.; Canter, D.; Lotan, Y.; Black, P.C.; Abdi, H.; van Rhijn, B.W.; Fransen van de Putte, E.E.; Zareba, P.; Koskinen, I.; et al. Incidence, Characteristics and Implications of Thromboembolic Events in Patients with Muscle Invasive Urothelial Carcinoma of the Bladder Undergoing Neoadjuvant Chemotherapy. J. Urol. 2016, 196, 1627–1633. (In English) [Google Scholar] [CrossRef] [PubMed]
- Moss, J.G.; Wu, O.; Bodenham, A.R.; Agarwal, R.; Menne, T.F.; Jones, B.L.; Heggie, R.; Hill, S.; Dixon-Hughes, J.; Soulis, E.; et al. Central venous access devices for the delivery of systemic anticancer therapy (CAVA): A randomised controlled trial. Lancet 2021, 398, 403–415. (In English) [Google Scholar] [CrossRef]
- Taxbro, K.; Hammarskjöld, F.; Thelin, B.; Lewin, F.; Hagman, H.; Hanberger, H.; Berg, S. Clinical impact of peripherally inserted central catheters vs implanted port catheters in patients with cancer: An open-label, randomised, two-centre trial. Br. J. Anaesth. 2019, 122, 734–741. (In English) [Google Scholar] [CrossRef]
- Taxbro, K.; Hammarskjöld, F.; Juhlin, D.; Hagman, H.; Bernfort, L.; Berg, S. Cost analysis comparison between peripherally inserted central catheters and implanted chest ports in patients with cancer-A health economic evaluation of the PICCPORT trial. Acta Anaesthesiol. Scand. 2020, 64, 385–393. (In English) [Google Scholar] [CrossRef]
- Taxbro, K.; Berg, S.; Hammarskjöld, F.; Hanberger, H.; Malmvall, B.E. A prospective observational study on 249 subcutaneous central vein access ports in a Swedish county hospital. Acta Oncol. 2013, 52, 893–901. (In English) [Google Scholar] [CrossRef]
- Haas, S.K.; Freund, M.; Heigener, D.; Heilmann, L.; Kemkes-Matthes, B.; von Tempelhoff, G.F.; Melzer, N.; Kakkar, A.K. Low-molecular-weight heparin versus placebo for the prevention of venous thromboembolism in metastatic breast cancer or stage III/IV lung cancer. Clin. Appl. Thromb. Hemost. 2012, 18, 159–165. (In English) [Google Scholar] [CrossRef]
- Mehrazin, R.; Piotrowski, Z.; Egleston, B.; Parker, D.; Tomaszweski, J.J.; Smaldone, M.C.; Abbosh, P.H.; Ito, T.; Bloch, P.; Iffrig, K.; et al. Is extended pharmacologic venous thromboembolism prophylaxis uniformly safe after radical cystectomy? Urology 2014, 84, 1152–1156. (In English) [Google Scholar] [CrossRef][Green Version]
- Andersen, C.S.; Thoft Jensen, B.; Nielsen Holck, E.; Kingo, P.S.; Jensen, J.B. Prospective versus retrospective recordings of comorbidities and complications in bladder cancer patients undergoing radical cystectomy—A randomized controlled trial. Scand. J. Urol. 2022, 56, 6–11. (In English) [Google Scholar] [CrossRef]
| CVA-Group | CVA-Group | CVA-Group | ||
|---|---|---|---|---|
| PICC n = 235 | Port n = 42 | Neither n = 90 | ||
| Variable | Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 67 (7) | 69 (7) | 66 (7) | |
| BMI | 26 (4) | 26 (3) | 26 (4) | |
| CACI | 5 (1) | 5 (1) | 4 (1) | |
| no. NAC-cycles | 3 (1) | 3 (1) | 0 (0) | |
| n (%) | n (%) | n (%) | ||
| Sex | ||||
| Female | 57 (24) | 9 (21) | 18 (20) | |
| Male | 178 (76) | 33 (78) | 72 (80) | |
| Center | ||||
| Umeå | 135 (57) | 5 (12) | 22 (24) | |
| Sundsvall | 39 (17) | 1 (1) | 9 (4) | |
| Västerås | 5 (2) | 32 (76) | 10 (11) | |
| Linköping | 56 (24) | 4 (10) | 49 (54) | |
| ASA | ||||
| 1 | 34 (12) | 3 (7) | 22 (24) | |
| 2 | 140 (60) | 28 (67) | 54 (60) | |
| 3 | 61 (26) | 11 (26) | 14 (16) | |
| Current Smoker | 24 (10) | 3 (7) | 8 (9) | |
| cT-Stage | ||||
| T2 | 147 (63) | 23 (55) | 59 (66) | |
| T3 | 73 (31) | 16 (38) | 30 (33) | |
| T4a | 15 (6) | 3 (7) | 1 (1) | |
| Thrombosis Prophylaxis | ||||
| Anticoagulant | 16 (7) | 4 (10) | 4 (5) | |
| Antiplatelet | 40 (17) | 5 (12) | 14 (16) | |
| Variable | CVA Group PICC n (%) n = 235 | CVA Group Port n (%) n = 42 | CVA Group Neither n (%) n = 90 | |
|---|---|---|---|---|
| Patients with TEE | 38 (16) | 1 (2) | 3 (3) | |
| Type of TEE | ||||
| DVT | 3 (7) | 0 (0) | 2 (50) | |
| Thrombophlebitis | 7 (16) | 0 (0) | 0 (0) | |
| PE | 21 (47) | 1 (50) | 2 (50) | |
| From CVA | 13 (29) | 1 (50) | 0 (0) | |
| Stroke/TIA | 0 (0) | 0 (0) | 0 (0) | |
| Angina/MI | 1 (2) | 0 (0) | 0 (0) | |
| Total amount | 45 (100) | 2 (100) | 4 (100) | |
| CTCAE | 1 | 0 (0) | 0 (0) | 0 (0) |
| 2 | 13 (29) | 0 (0) | 0 (0) | |
| 3 | 29 (64) | 2 (100) | 3 (75) | |
| 4 | 3 (7) | 0 (0) | 1 (25) | |
| 5 Total amount | 0 (0) 45 (100) | 0 (0) 2 (100) | 0 (0) 4 (100) |
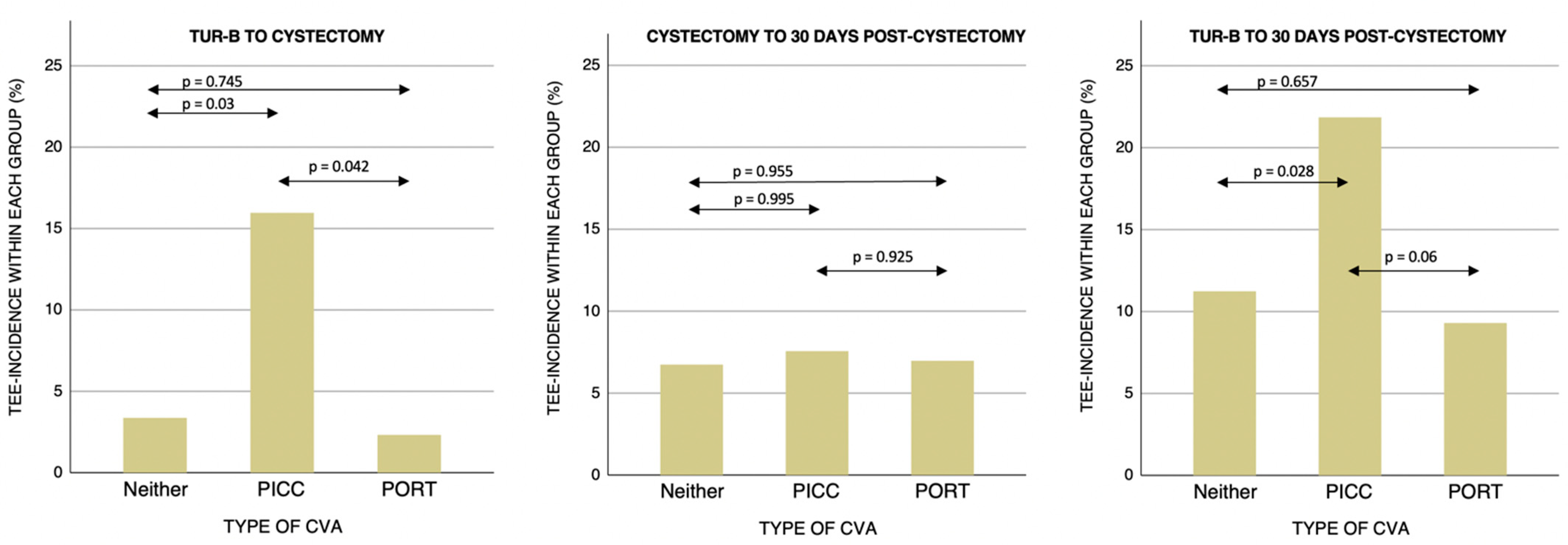
| Time Period | Compared CVA-Groups | p-Value |
|---|---|---|
| TURB-Cystectomy | ||
| PICC-PORT | 0.042 | |
| PICC-Neither | 0.03 | |
| PORT-Neither | 0.745 | |
| Cystectomy-30 days post-cystectomy | ||
| PICC-PORT | 0.925 | |
| PICC-Neither | 0.995 | |
| PORT-Neither | 0.955 | |
| TURB-30 days post-cystectomy | ||
| PICC-PORT | 0.06 | |
| PICC-Neither | 0.028 | |
| PORT-Neither | 0.657 |
| No. Planned | ||||||
|---|---|---|---|---|---|---|
| Patient | CVA | NAC-REGIMEN | No. Cycles | Cycles | Type of TEE | Reason for Termination |
| 1 | PICC | MVAC | 3 | 4 | PE | TEE |
| 2 | PORT | MVAC | 2 | 3 | PE, from CVA | TEE |
| 3 | PICC | MVEC | 2 | 3 | from CVA | TEE + impaired general condition |
| 4 | PICC | MVAC | 1 | 3 | from CVA | TEE + impaired general condition |
| 5 | PICC | OTHER | 2 | * | from CVA thrombophlebitis | septic infection |
| 6 | PICC | MVAC | 2 | 3 | PE, from CVA | TEE |
| 7 | PICC | CARBO-GEM | 2 | 3 | DVT, from CVA | TEE + impaired general condition |
| 8 | PICC | MVAC | 1 | 4 | thrombophlebitis | impaired general condition |
| 9 | PICC | CARBO-GEM & OTHER | 2 | * | from CVA | neutropenia |
| 10 | PICC | OTHER | 3 | 4 | thrombophlebitis | reduced kidney function |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rydell, H.; Huge, Y.; Eriksson, V.; Johansson, M.; Alamdari, F.; Svensson, J.; Aljabery, F.; Sherif, A. Central Venous Access and the Risk for Thromboembolic Events in Patients Undergoing Neoadjuvant Chemotherapy and Radical Cystectomy for Muscle-Invasive Bladder Cancer. Life 2022, 12, 1198. https://doi.org/10.3390/life12081198
Rydell H, Huge Y, Eriksson V, Johansson M, Alamdari F, Svensson J, Aljabery F, Sherif A. Central Venous Access and the Risk for Thromboembolic Events in Patients Undergoing Neoadjuvant Chemotherapy and Radical Cystectomy for Muscle-Invasive Bladder Cancer. Life. 2022; 12(8):1198. https://doi.org/10.3390/life12081198
Chicago/Turabian StyleRydell, Harriet, Ylva Huge, Victoria Eriksson, Markus Johansson, Farhood Alamdari, Johan Svensson, Firas Aljabery, and Amir Sherif. 2022. "Central Venous Access and the Risk for Thromboembolic Events in Patients Undergoing Neoadjuvant Chemotherapy and Radical Cystectomy for Muscle-Invasive Bladder Cancer" Life 12, no. 8: 1198. https://doi.org/10.3390/life12081198
APA StyleRydell, H., Huge, Y., Eriksson, V., Johansson, M., Alamdari, F., Svensson, J., Aljabery, F., & Sherif, A. (2022). Central Venous Access and the Risk for Thromboembolic Events in Patients Undergoing Neoadjuvant Chemotherapy and Radical Cystectomy for Muscle-Invasive Bladder Cancer. Life, 12(8), 1198. https://doi.org/10.3390/life12081198







