Feeding Our Microbiota: Stimulation of the Immune/Semiochemical System and the Potential Amelioration of Non-Communicable Diseases
Abstract
1. Introduction: The Unstoppable Rise of Obesity
2. Health versus Pollution: The Degraded Microbiome
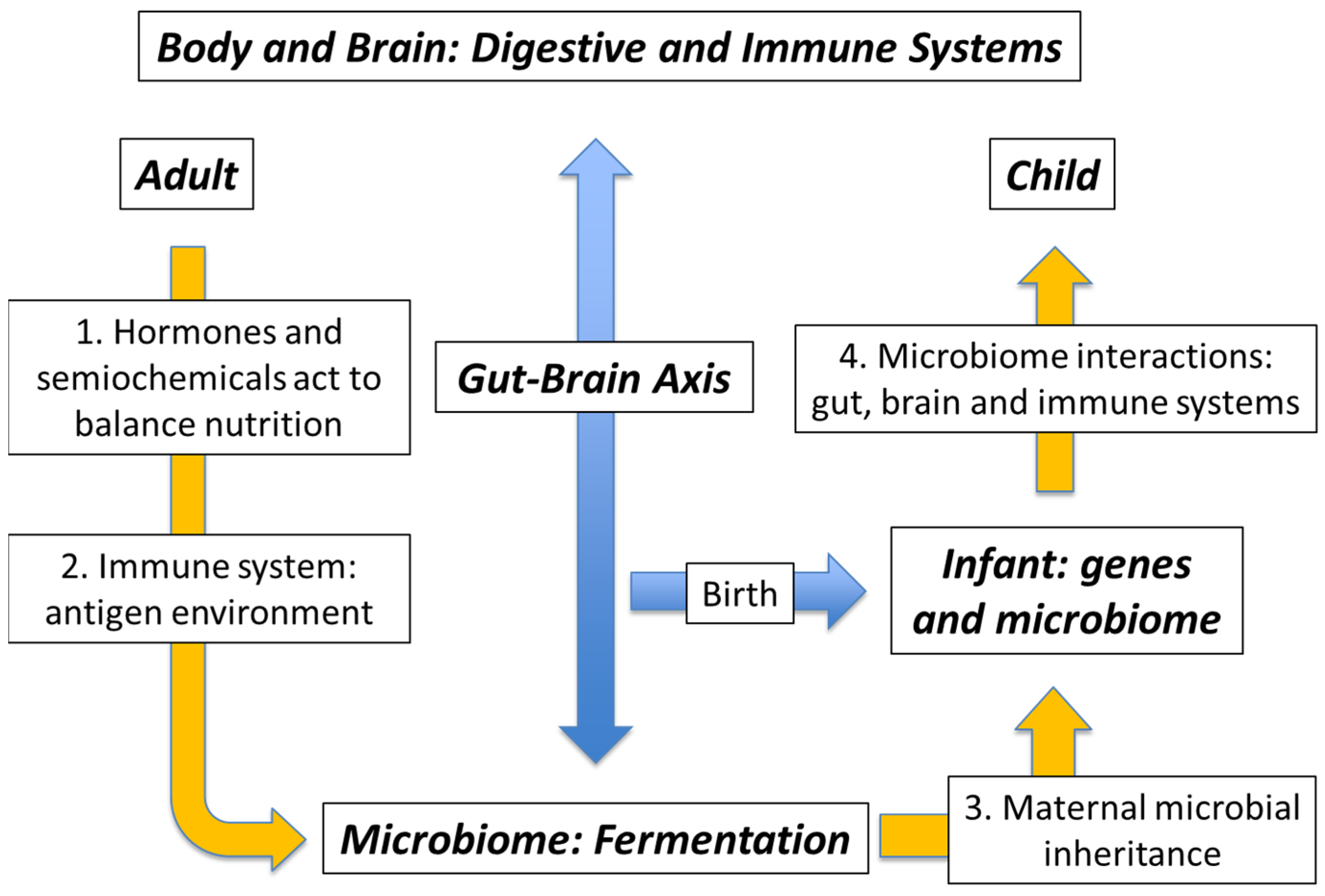
3. Genes versus Environment: Maternal Microbial Inheritance
4. The Mutualistic Microbiome: External Microbes and Their Antigens
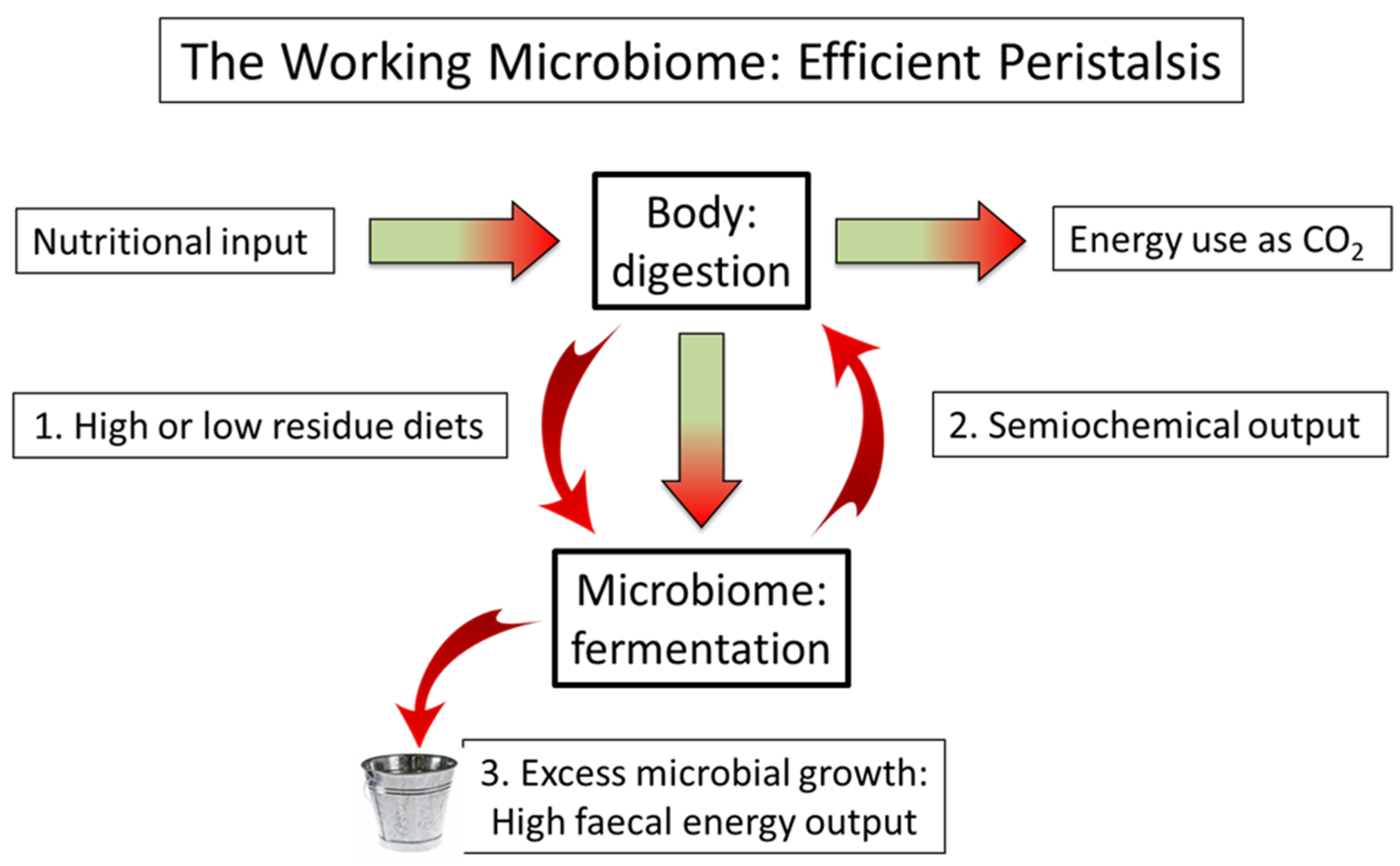
5. The Mutualistic Microbiome: The Immune/Semiochemical Complex
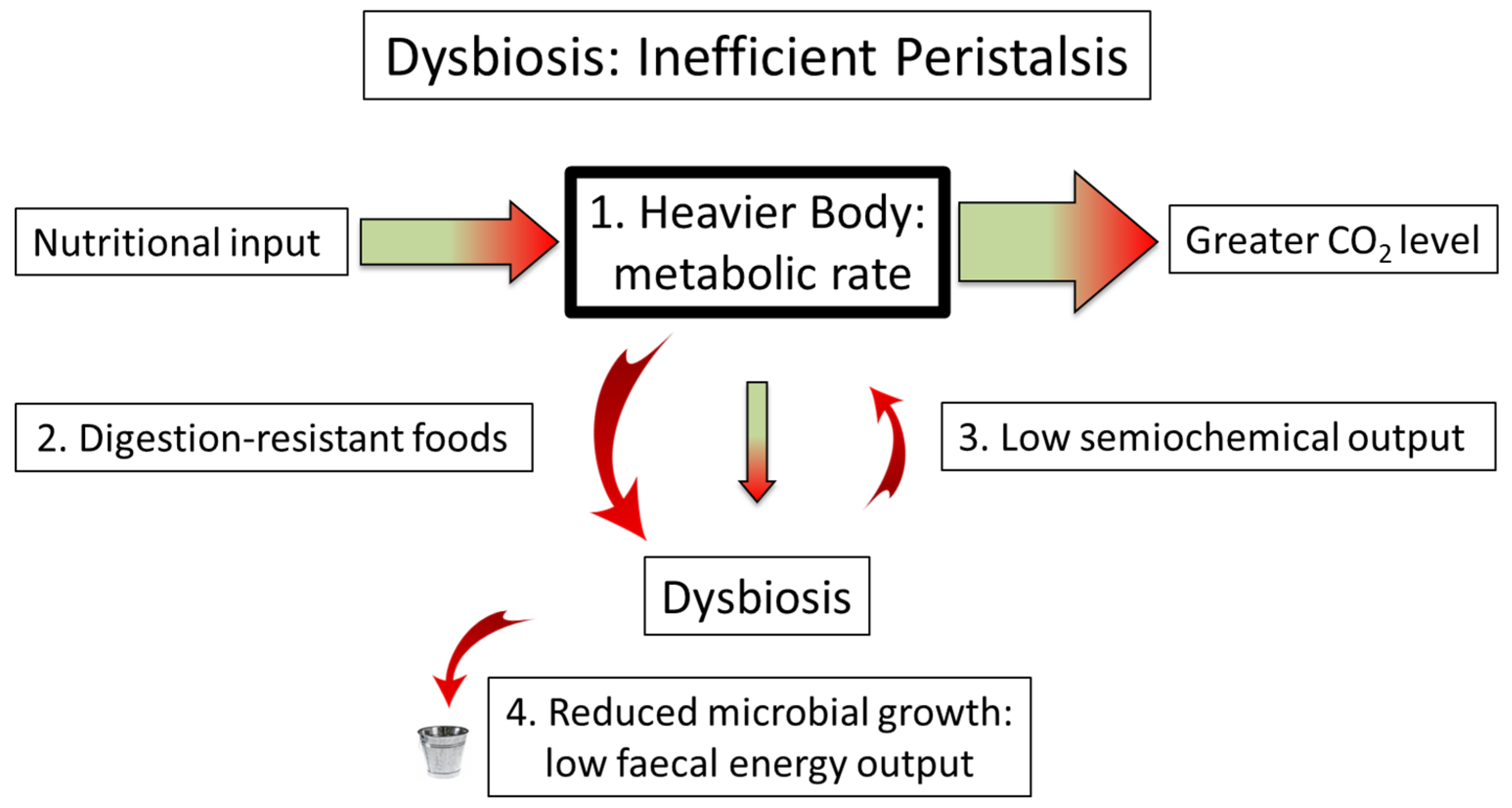
6. Breaking the Contract: Dysbiosis as Microbiome Failure
7. The Partition of Nutrition: The Loss of Semiochemical Control
8. Microbe to Market: Pro- and Pre-Biotics
9. Feeding the Microbiome: Food, Fermentation, FODMAP, and Plant Polyphenols
10. Microbiome-Function Deficiency Disease: Cure, Control, or Prevent?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garner, D.M.; Wooley, S.C. Confronting the failure of behavioral and dietary treatments for obesity. Clin. Psychol. Rev. 1991, 11, 729–780. [Google Scholar] [CrossRef]
- Prentice, A.M.; Jebb, S.A. Obesity in Britain: Gluttony or sloth? BMJ 1995, 311, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R.; Speakman, J.R. Physical activity energy expenditure has not declined since the 1980s and matches energy expenditures of wild mammals. Int. J. Obes. 2008, 32, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Jou, C. The biology and genetics of obesity—A century of inquiries. N. Engl. J. Med. 2014, 370, 1874–1877. [Google Scholar] [CrossRef]
- Casazza, K.; Brown, A.; Astrup, A.; Bertz, F.; Baum, C.; Brown, B.B.; Dawson, J.; Durant, N.; Dutton, G.; Fields, D.A.; et al. Weighing the evidence of common beliefs in obesity research. Crit. Rev. Food Sci. Nutr. 2015, 55, 2014–2053. [Google Scholar] [CrossRef]
- Hruby, A.; Hu, F.B. The epidemiology of obesity: A big picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef]
- Reilly, J.J.; El-Hamdouchi, A.; Diouf, A.; Monyeki, A.; Somda, S.A. Determining the world-wide prevalence of obesity. Lancet 2018, 39, 1773–1774. [Google Scholar] [CrossRef]
- Sandercock, G.R.H.; Cohen, D.D. Temporal trends in muscular fitness of English 10-year-olds 1998–2014: An allometric approach. J. Sci. Med. Sport 2019, 22, 201–205. [Google Scholar] [CrossRef]
- Ðuric, S.; Sember, V.; Starc, G.; Soric, M.; Kovac, M.; Jurak, G. Secular trends in muscular fitness from 1983 to 2014 among Slovenian children and adolescents. Scand. J. Med. Sci. Sports 2021, 31, 1853–1861. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Berthoud, H.-R.; Morrison, C.D.; Münzberg, H. The obesity epidemic in the face of homeostatic body weight regulation: What went wrong and how can it be fixed? Physiol. Behav. 2020, 222, 112959. [Google Scholar] [CrossRef] [PubMed]
- Torres-Fuentes, C.; Schellenkens, H.; Dinan, T.G.; Cryan, J.F. The microbiota-gut-brain axis in obesity. Lancet Gastroenterol. Hepatol. 2017, 2, 747–756. [Google Scholar] [CrossRef]
- Burkitt, D.P. Some diseases characteristic of modern western civilization. BMJ 1973, 1, 274–278. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, S.J. The association between dietary fibre deficiency and high-income lifestyle-associated diseases: Burkitt’s hypothesis revisited. Lancet Gastroenterol. Hepatol. 2019, 4, 984–996. [Google Scholar] [CrossRef]
- Smith, D.; Palacios-Pérez, M.; Jheeta, S. The enclosed intestinal microbiome: Semiochemical signals from the Precambrian and their disruption by heavy metal pollution. Life 2022, 12, 287. [Google Scholar] [CrossRef]
- Smith, D.; Jheeta, S. Microbiome-gut dissociation: Investigating the origins of obesity. Gastrointest. Disord. 2021, 3, 156–172. [Google Scholar] [CrossRef]
- Gill, S.R.; Pop, M.; DeBoy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Huttenhower, C.; Gevers, D.; Knight, R.; Abubucker, S.; Badger, J.H.; Chinwalla, A.T.; Creasy, H.H.; Earl, A.M.; FitzGerald, M.G.; Fulton, R.S.; et al. Structure, Function and Diversity of the Healthy Human Microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Kaelberer, M.M.; Buchanan, K.L.; Klein, M.E.; Barth, B.B.; Montoya, M.M.; Shen, X.; Bohórquez, D.V. A Gut-Brain Neural Circuit for Nutrient Sensory Transduction. Science 2018, 361, eaat5236. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, G.; Marini, E.; Sanchez, W.; Contreras, M.; Estrada, I.; Comandini, O.; Buffa, R.; Magris, M.; Dominguez-Bello, M.G. The nutrition transition in the Venezuelan Amazonia: Increased overweight and obesity with transculturation. Am. J. Hum. Biol. 2014, 26, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.C.; Pehrsson, E.C.; Blaser, M.J.; Sandhu, K.; Gao, Z.; Wang, B.; Magris, M.; Hidalgo, G.; Contreras, M.; Noya-Alarcón, Ó.; et al. The Microbiome of Uncontacted Amerindians. Sci. Adv. 2015, 1, e1500183. [Google Scholar] [CrossRef]
- Kaplan, H.; Thompson, R.C.; Trumble, B.C.; Wann, L.S.; Allam, A.H.; Beheim, B.; Frohlich, B.; Sutherland, M.L.; Sutherland, J.D.; Stieglitz, J.; et al. Coronary atherosclerosis in indigenous South American Tsimane: A cross sectional cohort study. Lancet 2017, 389, 1730–1739. [Google Scholar] [CrossRef]
- Irimia, A.; Chaudhari, N.N.; Robles, D.J.; Rostowsky, K.A.; Maher, A.S.; Chowdhury, N.F.; Calvillo, N.F.; Ngo, V.; Gatz, M.; Mack, W.J.; et al. The indigenous South American Tsimane exhibit relatively modest decrease in brain volume with age despite high systemic inflammation. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 2147–2155. [Google Scholar] [CrossRef]
- Schnorr, S.L.; Candela, M.; Rampelli, S.; Centanni, M.; Consolandi, C.; Basaglia, G.; Turroni, S.; Biagi, E.; Peano, C.; Severgnini, M.; et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 2014, 5, 3654. [Google Scholar] [CrossRef]
- Mizuno, S.; Masaoka, T.; Naganuma, M.; Kishimoto, T.; Kitazawa, M.; Kurokawa, S.; Nakashima, M.; Takeshita, K.; Suda, W.; Mimura, M.; et al. Bifidobacterium-rich fecal donor may be a positive predictor for successful fecal microbiota transplantation in patients with irritable bowel syndrome. Digestion 2017, 96, 29–38. [Google Scholar] [CrossRef]
- Pratt, C.; Campbell, M.D. The effect of bifidobacterium on reducing symptomatic pain in patients with irritable bowel syndrome: A systematic review. Probiotics Antimicrob. Proteins 2020, 12, 834–839. [Google Scholar] [CrossRef]
- Smith, D.; Jheeta, S. The epidemiology of the dysfunctional microbiome in animals and in humans: The propensity for the development of non-communicable disease. EC Gastroenterol. Dig. Syst. 2020, 7, 83–93. [Google Scholar]
- Bouchard, C.; Tremblay, A.; Després, J.-P.; Nadeau, A.; Lupien, P.J.; Thériault, G.; Dussault, J.; Moorjani, S.; Pinault, S.; Fournier, G. The response to long-term overfeeding in identical twins. N. Engl. J. Med. 1990, 322, 1477–1482. [Google Scholar] [CrossRef]
- Barker, D.J. The fetal and infant origins of adult disease. BMJ 1990, 301, 1111. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.G. The fetal origins hypothesis—10 years on. BMJ 2005, 330, 1096–1097. [Google Scholar] [CrossRef] [PubMed]
- Almond, D.; Currie, J. Killing me softly: The fetal origins hypothesis. J. Econ. Perspect. 2011, 25, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Waddington, C.H. Toward a Theoretical Biology; Edinburgh University Press: Edinburgh, UK, 1968; pp. 1–32. [Google Scholar]
- Trerotola, M.; Relli, V.; Simeone, P.; Alberti, S. Epigenetic inheritance and the missing heritability. Hum. Genom. 2015, 9, 17. [Google Scholar] [CrossRef]
- Horsthemke, B. A critical view on transgenerational epigenetic inheritance in humans. Nat. Commun. 2018, 9, 2973. [Google Scholar] [CrossRef]
- Qin, Y.; Wade, P.A. Crosstalk between the microbiome and the epigenome: Messages from bugs. J. Biochem. 2018, 163, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Gaskins, A.J.; Blaine, A.I.; Zhang, C.; Gillman, M.W.; Missmer, S.A.; Field, A.E.; Chavarro, J.E. Association between cesarean birth and risk of obesity in offspring in childhood, adolescence, and early adulthood. JAMA Pediatr. 2016, 170, e162385. [Google Scholar] [CrossRef]
- Zhao, Q.; Elson, C.O. Adaptive Immune Education by Gut Microbiota Antigens. Immunology 2018, 154, 28–37. [Google Scholar] [CrossRef]
- Strachan, D.P. Hay fever, hygiene and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef]
- Rook, G.A.W.; Lowry, C.A.; Raison, C.L. Microbial ‘Old Friends’, immunoregulation and stress resilience. Evol. Med. Public Health 2013, 1, 46–64. [Google Scholar] [CrossRef]
- Banchereau, J.; Briere, F.; Caux, C.; Davoust, J.; Lebecque, S.; Liu, Y.-J.; Pulendran, B.; Palucka, K. Immunobiology of dendritic cells. Annu. Rev. Immunol. 2000, 18, 767–811. [Google Scholar] [CrossRef]
- Jheeta, S.; Smith, D. Seeing the wood for the trees: A new way to view the human intestinal microbiome and its connection with non-communicable disease. Med. Hypotheses 2019, 125, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Tuladhar, E.; Hazeleger, W.C.; Koopmans, M.; Zwietering, M.H.; Beumer, R.R.; Duizer, E. Residual viral and bacterial contamination of surfaces after cleaning and disinfection. Appl. Environ. Microbiol. 2012, 78, 7769–7775. [Google Scholar] [CrossRef] [PubMed]
- Tuladhar, E.; Bouwknegt, M.; Zwietering, M.H.; Koopmans, M.; Duizer, E. Thermal stability of structurally different viruses with proven or potential relevance to food safety. J. Appl. Microbiol. 2012, 112, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Giddings, S.L.; Stevens, A.M.; Leung, D.T. Traveler’s diarrhea. Med. Clin. N. Am. 2016, 100, 317–330. [Google Scholar] [CrossRef]
- Underhill, D.M.; Iliev, I.D. The Mycobiota: Interactions between Commensal Fungi and the Host Immune System. Nat. Rev. Immunol. 2014, 14, 405–416. [Google Scholar] [CrossRef]
- Laforest-Lapointe, I.; Arrieta, M.-C. Microbial eukaryotes: A missing link in gut microbiome studies. mSystems 2018, 3, e00201-17. [Google Scholar] [CrossRef]
- Ward, T.L.; Dominguez-Bello, M.G.; Heisel, T.; Al-Ghalith, G.; Knights, D.; Gale, C.A. Development of the human mycobiome over the first month of life and across body sites. mSystems 2018, 3, e00140. [Google Scholar] [CrossRef]
- Berdoy, M.; Webster, J.P.; Macdonald, D.W. Fatal attraction in rats infected with Toxoplasma gondii. Proc. Royal Soc. B 2000, 267, 1591–1594. [Google Scholar] [CrossRef]
- Scanlan, P.D.; Stensvold, C.R.; Rajilic-Stojanovic, M.; Heilig, H.G.H.J.; De Vos, W.M.; O’Toole, P.W.; Cotter, P.D. The microbial eukaryote Blastocystis is a prevalent and diverse member of the healthy human gut microbiota. FEMS Microbiol. Ecol. 2014, 90, 326–330. [Google Scholar] [CrossRef]
- Noel, C.; Dufemez, F.; Gerbod, G.; Egcomb, V.P.; Delgado-Viscogliosi, P.; Ho, L.-C.; Singh, M.; Wintjens, R.; Soglin, M.L.; Capron, M.; et al. Molecular phylogenies of Blastocystis isolates from different hosts: Implications for genetic diversity, identification of species, and zoonosis. J. Clin. Microbiol. 2005, 43, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Bostock, J. Case of a periodical affection of the eyes and chest. Med. Chir. Trans. 1819, 10, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.; Khan-Wasti, S.; Fletcher, M.; Sheikh, A. Prevalence of hayfever symptoms and diagnosis in UK teenagers. Prim. Care Respir. J. 2005, 14, 270. [Google Scholar] [CrossRef][Green Version]
- Loh, W.; Tang, M.L.K. The epidemiology of food allergy in the global context. Int. J. Environ. Res. Public Health 2018, 15, 2043. [Google Scholar] [CrossRef]
- Hill, D.A.; Spergel, J.M. The atopic march: Critical evidence and clinical relevance. Ann. Allergy Asthma Immunol. 2018, 120, 131–137. [Google Scholar] [CrossRef]
- Bostock, J. Of the catarrhus aestivus or summer catarrh. Med. Chir. Trans. 1828, 14, 437–446. [Google Scholar] [CrossRef]
- Corson, R. Fashions in Makeup: From Ancient to Modern Times; Peter Owen Ltd.: London, UK, 1972. [Google Scholar]
- Tansley, A.G. Sigmund Freud, 1856–1939. Obit. Not. Fellows R. Soc. 1941, 3, 246–275. [Google Scholar]
- Yan, F.; Polk, D.B. Commensal Bacteria in the Gut: Learning Who Our Friends Are. Curr. Opin. Gastroenterol. 2004, 20, 565–571. [Google Scholar] [CrossRef]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.-N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef]
- Kleiman, S.C.; Watson, H.J.; Bulik-Sullivan, E.C.; Huh, E.Y.; Tarantino, L.M.; Bulik, C.M.; Carroll, I.M. The Intestinal Microbiota in Acute Anorexia Nervosa and During Renourishment: Relationship to Depression, Anxiety, and Eating Disorder Psychopathology. Psychosom. Med. 2015, 77, 969–981. [Google Scholar] [CrossRef]
- Vandeputte, D.; Falony, G.; Veira-Silva, S.; Tito, R.; Joossens, M.; Raes, J. Stool consistency is strongly associated with gut microbiota richness and composition, enterotypes and bacterial growth rates. Gut 2016, 65, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N. Biogenic amines: Signals between commensal microbiota and gut physiology. Front. Endocrinol. 2019, 10, 504. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Zhang, H.; Pan, J.; Du, Z.; Zhou, W.; Zhang, Z.; Tian, Z.; Zhou, R.; Bai, L. Peripheral dopamine controlled by gut microbes inhibits invariant natural killer T cell-mediated hepatitis. Front. Immunol. 2018, 9, 2398. [Google Scholar] [CrossRef] [PubMed]
- Ter Horst, K.W.; Lammers, N.M.; Trinko, R.; Opland, D.M.; Figee, M.; Ackermans, M.T.; Booij, J.; van den Munckhof, P.; Schuurman, P.R.; Fliers, E.; et al. Striatal dopamine regulates systemic glucose metabolism in humans and mice. Sci. Transl. Med. 2018, 10, eaar3752. [Google Scholar] [CrossRef]
- LeBlanc, J.G.; Chain, F.; Martin, R.; Bermùndez-Humarán, L.G.; Courau, S.; Langella, P. Beneficial effects on host energy metabolism of short-chain fatty acids and vitamins produced by commensal and probiotic bacteria. Microb. Cell Factories 2017, 16, 79. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F., III; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef]
- Le Chatelier, E.; Nielsen, T.; Qin, J.; Prifti, E.; Hildebrand, F.; Falony, G.; Almeida, M.; Arumugam, M.; Batto, J.-M.; Kennedy, S.; et al. Richness of Human Gut Microbiome Correlates with Metabolic Markers. Nature 2013, 500, 541–546. [Google Scholar] [CrossRef]
- Iyer, L.M.; Aravind, L.; Coon, S.L.; Klein, D.C.; Koonin, E.V. Evolution of cell-cell signaling in animals: Did late horizontal gene transfer from bacteria have a role? Trends Genet. 2004, 20, 292–299. [Google Scholar] [CrossRef]
- Hooks, K.B.; O’Malley, M.A. Dysbiosis and its discontents. mBio 2017, 8, e01492-17. [Google Scholar] [CrossRef]
- Brüssow, H. Problems with the concept of gut microbiota dysbiosis. Microb. Biotechnol. 2019, 13, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Woese, C. On the evolution of cells. Proc. Natl. Acad. Sci. USA 2002, 99, 8742–8747. [Google Scholar] [CrossRef]
- Smith, D.; Palacios-Pérez, M.; Jheeta, S. Microbiome–Gut Dissociation in the Neonate: Obesity and Coeliac Disease as Examples of Microbiome Function Deficiency Disorder. Gastrointest. Disord. 2022, 4, 108–128. [Google Scholar] [CrossRef]
- Margulis, L. Symbiogenesis and symbionticism. In Symbiosis as a Source of Evolutionary Innovation: Speciation and Morphogenesis; Margulis, L., Fester, R., Eds.; MIT Press: Cambridge, MA, USA, 1991; pp. 49–92. [Google Scholar]
- Guerrero, R.; Margulis, L.; Berlanga, M. Symbiogenesis: The holobiont as a unit of evolution. Int. Microbiol. 2013, 16, 133–143. [Google Scholar] [PubMed]
- Simon, J.-C.; Marchesi, J.R.; Mougel, C.; Selosse, M.-A. Host-microbiota interactions: From holobiont theory to analysis. Microbiome 2019, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Mesa, D.M.; Loureiro, B.; Iglesia, I.; Gonzalez, S.F.; Olivé, E.L.; Algar, O.G.; Solana, M.J.; Cabero, M.J.; Sainz, T.; Martinez, L.; et al. The evolving microbiome from pregnancy to early infancy: A comprehensive review. Nutrients 2020, 12, 133. [Google Scholar] [CrossRef]
- Lindfors, K.; Ciacci, C.; Kurppa, K.; Lundin, K.E.A.; Makharia, G.K.; Mearin, M.L.; Murray, J.A.; Verdu, E.F.; Kaukinen, K. Coeliac disease. Nat. Rev. Dis. Primers 2019, 5, 3. [Google Scholar] [CrossRef]
- Gasbarrini, G.; Rickards, O.; Martínez-Labarga, C.; Pacciani, E.; Chilleri, F.; Laterza, L.; Marangi, G.; Scaldaferri, F.; Gasbarrini, A. Origin of celiac disease: How old are predisposing haplotypes? World J. Gastroenterol. 2012, 18, 5300–5304. [Google Scholar]
- Thompson, R.C.; Allam, A.H.; Lombardi, G.G.; Wann, L.S.; Sutherland, M.L.; Sutherland, J.D.; Soliman, M.A.; Frohlich, B.; Mininberg, D.T.; Monge, J.M.; et al. Atherosclerosis across 4000 years of human history: The Horus study of four ancient populations. Lancet 2013, 381, 1211–1222. [Google Scholar] [CrossRef]
- Rose, C.; Parker, A.; Jefferson, B.; Cartmell, E. The characterisation of feces and urine: A review of the literature to inform advanced treatment technology. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1827–1879. [Google Scholar] [CrossRef]
- Tortora, G.J.; Anagnostakos, N.P. Principles of Anatomy and Physiology, 5th ed.; Harper and Row: New York, NY, USA, 1987; p. 624. [Google Scholar]
- Forootan, M.; Bagheri, N.; Darvishi, M. Chronic constipation. Medicine 2018, 97, e10631. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Smits, S.A.; Tikhonov, M.; Higginbottom, S.A.; Wingreen, N.S.; Sonnenburg, J.L. Diet-induced Extinctions in the Gut Microbiota Compound over Generations. Nature 2016, 529, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yakov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized nutrition by prediction of glycaemic responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef] [PubMed]
- Wolever, T. Personalized nutrition by prediction of glycaemic responses: Fact or fantasy? Eur. J. Clin. Nutr. 2016, 70, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C.; Valiere, A. Probiotics and medical nutrition therapy. Nutr. Clin. Care 2004, 7, 56–68. [Google Scholar]
- Hutkins, R.W.; Krumbeck, J.A.; Bindels, L.B.; Cani, P.D.; Fahey, G., Jr.; Goh, Y.J.; Hamaker, B.; Martens, E.C.; Mills, D.A.; Rastal, R.A.; et al. Prebiotics: Why definitions matter. Curr. Opin. Biotechnol. 2016, 37, 1–7. [Google Scholar] [CrossRef]
- Bomba, A.; Nemcova, R.; Gancarcíková, S.; Herich, R. Improvement of the probiotic effect of micro-organisms by their combination with maltodextrins, fructooligosaccharides and polyunsaturated fatty acids. Br. J. Nutr. 2002, 88, S95–S99. [Google Scholar] [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Zhou, L.; Foster, J.A. Psychobiotics and the gut-brain axis: In the pursuit of happiness. Neuropsychiatr. Dis. Treat. 2015, 11, 715–723. [Google Scholar]
- Rijkers, G.T.; de Vos, W.M.; Brummer, R.-J.; Morelli, L. Health benefits and health claims of probiotics: Bridging science and marketing. Br. J. Nutr. 2011, 106, 1291–1296. [Google Scholar] [CrossRef]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef] [PubMed]
- Underwood, M.A.; Salzman, N.H.; Bennett, S.H.; Barman, M.; Mills, D.A.; Marcobal, A.; Tancredi, D.J.; Bevins, C.L.; Sherman, M.P. A randomized placebo-controlled comparison of 2 prebiotic/probiotic combinations in preterm infants: Impact on weight gain, intestinal microbiota, and fecal short-chain fatty acids. J. Paediatr. Gastroenterol. Nutr. 2009, 48, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Petrof, E.O.; Khoruts, A. From Stool Transplants to Next-Generation Microbiota Therapeutics. Gastroenterology 2014, 146, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Tariq, R.; Pardi, D.S.; Bartlett, M.G.; Khanna, S. Low cure rates in controlled trials of fecal microbiota transplantation for recurrent Clostridium difficile infection: A systematic review and meta-analysis. Clin. Infect. Dis. 2019, 68, 1351–1358. [Google Scholar] [CrossRef]
- Wassermann, B.; Müller, H.; Berg, G. An apple a day: Which bacteria do we eat with organic and conventional apples? Front. Microbiol. 2019, 10, 1629. [Google Scholar] [CrossRef]
- Marlowe, F.W.; Berbesque, J.C.; Wood, B.; Crittenden, A.; Porter, C.; Mabulla, A. Honey, Hadza, hunter-gatherers, and human evolution. J. Hum. Evol. 2014, 71, 119–128. [Google Scholar] [CrossRef]
- Olaitan, P.B.; Adeleke, O.E.; Ola, I.O. Honey: A reservoir for microorganisms and an inhibitory agent for microbes. Afr. Health Sci. 2007, 7, 159–165. [Google Scholar]
- McGovern, P.E.; Zhang, J.; Tang, J.; Zhang, Z.; Hall, G.R.; Moreau, R.A.; Nuñez, A.; Butrym, E.D.; Richards, M.P.; Wang, C.S.; et al. Fermented beverages of pre- and proto-historic China. Proc. Natl. Acad. Sci. USA 2004, 101, 17593–17598. [Google Scholar] [CrossRef]
- Rizo, J.; Guillén, D.; Díaz-Ruiz, G.; Wacher, C.; Encarnación, S.; Sánchez, S.; Rodríguez-Sanoja, R. Metaproteomic insights into the microbial community in pozol. Front. Nutr. 2021, 8, 714814. [Google Scholar] [CrossRef]
- Dahl, W.J.; Agro, N.C.; Eliasson, A.M.; Mialki, K.L.; Olivera, J.D.; Rusch, N.C.; Young, C. Heath benefits of fiber fermentation. J. Am. Coll. Nutr. 2017, 36, 127–136. [Google Scholar] [CrossRef]
- Gibson, P.R.; Shepherd, S.J. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J. Gastroenterol. Hepatol. 2010, 25, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Makharia, A.; Catassi, C.; Makharia, G.K. The overlap between irritable bowel syndrome and non-celiac gluten sensitivity: A clinical dilemma. Nutrients 2015, 7, 10417–10426. [Google Scholar] [CrossRef] [PubMed]
- Biesiekierski, J.R.; Iven, J. Non-coeliac gluten sensitivity: Piecing the puzzle together. United Eur. Gastroenterol. 2015, 3, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Whelan, K.; Martin, L.D.; Staudacher, H.M.; Lomer, M.C.E. The low FODMAP diet in the management of irritable bowel syndrome: An evidence-based review of FODMAP restriction, reintroduction and personalisation in clinical practice. J. Hum. Nutr. Diet 2018, 31, 239–255. [Google Scholar] [CrossRef]
- Vandeputte, D.; Joossens, M. Effects of low and high FODMAP diets on human gastrointestinal microbiota composition in adults with intestinal diseases: A systematic review. Microorganisms 2020, 8, 1638. [Google Scholar] [CrossRef]
- Robertson, T.M.; Brown, J.E.; Fielding, B.A.; Hovorka, R.; Robertson, M.D. Resistant starch production and glucose release from pre-prepared chilled food: The SPUD project. Nutr. Bull. 2021, 46, 52–59. [Google Scholar] [CrossRef]
- Yang, X.; Darko, K.O.; Huang, Y.; He, C.; Yang, H.; He, S.; Li, J.; Li, J.; Hocher, B.; Yin, Y. Resistant starch regulates gut microbiota: Structure, biochemistry and cell signalling. Cell. Physiol. Biochem. 2017, 42, 306–318. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Technol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Vari, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Shadnia, H.; Wright, J.S. Understanding the toxicity of phenols: Using quantitative structure-activity relationships and enthalpy changes to discriminate between possible mechanisms. Chem. Res. Toxicol. 2008, 21, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, K.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The essential medicinal chemistry of curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H.; Li, S.; Li, W.-D.; Wang, Y. Consumption of coffee and tea and risk of developing stroke, dementia, and poststroke dementia: A cohort study in the UK Biobank. PLoS Med. 2021, 18, e1003830. [Google Scholar] [CrossRef]
- Ma, G.; Chen, Y. Polyphenol supplementation benefits human health via gut microbiota: A systematic review via meta-analysis. J. Funct. Foods 2020, 66, 103829. [Google Scholar] [CrossRef]
- Falony, G.; Vandeputte, D.; Caenepeel, C.; Vieira-Silva, S.; Daryoush, T.; Vermeire, S.; Raes, J. The Human Microbiome in Health and Disease: Hype or Hope. Acta Clin. Belg. 2019, 74, 53–64. [Google Scholar] [CrossRef]
- Lee-Sarwar, K.A.; Lasky-Su, J.; Kelly, R.S.; Litonjua, A.A.; Weiss, S.T. Metabolome–Microbiome Crosstalk and Human Disease. Metabolites 2020, 10, 181. [Google Scholar] [CrossRef]
- Redondo-Useros, N.; Nova, E.; González-Zancada, N.; Díaz, L.E.; Gómez-Martínez, S.; Marcos, A. Microbiota and Lifestyle: A Special Focus on Diet. Nutrients 2020, 12, 1776. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet Rapidly and Reproducibly Alters the Human Gut Microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Xu, Z.; Knight, R. Dietary Effects on Human Gut Microbiome Diversity. Br. J. Nutr. 2015, 113, S1–S5. [Google Scholar] [CrossRef]
- Yadav, M.; Verma, M.K.; Chauhan, N.S. A Review of Metabolic Potential of Human Gut Microbiome in Human Nutrition. Arch. Microbiol. 2018, 200, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Van Treuren, W.; Dodd, D. Microbial Contribution to the Human Metabolome: Implications for Health and Disease. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 345–369. [Google Scholar] [CrossRef] [PubMed]
- Angelis, M.D.; Garruti, G.; Minervini, F.; Bonfrate, L.; Portincasa, P.; Gobbetti, M. The Food-Gut Human Axis: The Effects of Diet on Gut Microbiota and Metabolome. Curr. Med. Chem. 2019, 26, 3567–3583. [Google Scholar] [CrossRef]
- Capurso, G.; Lahner, E. The Interaction between Smoking, Alcohol and the Gut Microbiome. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Wilmanski, T.; Rappaport, N.; Diener, C.; Gibbons, S.M.; Price, N.D. From Taxonomy to Metabolic Output: What Factors Define Gut Microbiome Health? Gut Microbes 2021, 13, 1907270. [Google Scholar] [CrossRef]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-Level Analysis of Gut Microbiome Variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-Based Metagenomics Analysis Reveals Markers for Gut Microbiome Composition and Diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef]
- Sandhu, K.V.; Sherwin, E.; Schellekens, H.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Feeding the Microbiota-Gut-Brain Axis: Diet, Microbiome, and Neuropsychiatry. Transl. Res. 2017, 179, 223–244. [Google Scholar] [CrossRef]
- Tsai, Y.-L.; Lin, T.-L.; Chang, C.-J.; Wu, T.-R.; Lai, W.-F.; Lu, C.-C.; Lai, H.-C. Probiotics, Prebiotics and Amelioration of Diseases. J. Biomed. Sci. 2019, 26, 3. [Google Scholar] [CrossRef]
- Karu, N.; Deng, L.; Slae, M.; Guo, A.C.; Sajed, T.; Huynh, H.; Wine, E.; Wishart, D.S. A Review on Human Fecal Metabolomics: Methods, Applications and the Human Fecal Metabolome Database. Anal. Chim. Acta 2018, 1030, 1–24. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Butcher, J.; Stintzi, A.; Figeys, D. Advancing Functional and Translational Microbiome Research Using Meta-Omics Approaches. Microbiome 2019, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome Definition Re-Visited: Old Concepts and New Challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Ha, N.; Ou, J.Z.; Berean, K.J. Ingestible Sensors. ACS Sens. 2017, 2, 468–483. [Google Scholar] [CrossRef] [PubMed]
- Traverso, G.; Ciccarelli, G.; Schwartz, S.; Hughes, T.; Boettcher, T.; Barman, R.; Langer, R.; Swiston, A. Physiologic Status Monitoring via the Gastrointestinal Tract. PLoS ONE 2015, 10, e0141666. [Google Scholar] [CrossRef] [PubMed]
- Mimee, M.; Nadeau, P.; Hayward, A.; Carim, S.; Flanagan, S.; Jerger, L.; Collins, J.; McDonnell, S.; Swartwout, R.; Citorik, R.J.; et al. An Ingestible Bacterial-Electronic System to Monitor Gastrointestinal Health. Science 2018, 360, 915–918. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Berean, K.J.; Ha, N.; Chrimes, A.F.; Xu, K.; Grando, D.; Ou, J.Z.; Pillai, N.; Campbell, J.L.; Brkljača, R.; et al. A Human Pilot Trial of Ingestible Electronic Capsules Capable of Sensing Different Gases in the Gut. Nat. Electron. 2018, 1, 79–87. [Google Scholar] [CrossRef]
- Smith, D.; Jheeta, S. Measuring microbiome effectiveness: A role for ingestible sensors. Gastrointest. Disord. 2020, 2, 3–11. [Google Scholar] [CrossRef]
- Burberry, A.; Wells, M.F.; Limone, F.; Couto, A.; Smith, K.S.; Keaney, J.; Gillet, G.; van Gastel, N.; Wang, J.-Y.; Pietilainen, O.; et al. C9orf72 suppresses systemic and neural inflammation induced by gut bacteria. Nature 2020, 582, 89–94. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery Mode Shapes the Acquisition and Structure of the Initial Microbiota across Multiple Body Habitats in Newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Ryan, C.R. Towards an ethics of reciprocity: Ethnobotanical knowledge and medicinal plants as cancer therapies. Humanities 2014, 3, 624–644. [Google Scholar] [CrossRef]
- Inchauspe, J. Glucose Revolution: The Life-Changing Power of Balancing Your Blood Sugar; Simon & Schuster: New York, NY, USA, 2022; ISBN 978-1-982179-41-0. [Google Scholar]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Long-Smith, C.; O’Riordan, K.J.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota-Gut-Brain Axis: New Therapeutic Opportunities. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 477–502. [Google Scholar] [CrossRef] [PubMed]
- Rea, K.; Dinan, T.G.; Cryan, J.F. Gut Microbiota: A Perspective for Psychiatrists. NPS 2020, 79, 50–62. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, D.; Jheeta, S.; Fuentes, H.V.; Palacios-Pérez, M. Feeding Our Microbiota: Stimulation of the Immune/Semiochemical System and the Potential Amelioration of Non-Communicable Diseases. Life 2022, 12, 1197. https://doi.org/10.3390/life12081197
Smith D, Jheeta S, Fuentes HV, Palacios-Pérez M. Feeding Our Microbiota: Stimulation of the Immune/Semiochemical System and the Potential Amelioration of Non-Communicable Diseases. Life. 2022; 12(8):1197. https://doi.org/10.3390/life12081197
Chicago/Turabian StyleSmith, David, Sohan Jheeta, Hannya V. Fuentes, and Miryam Palacios-Pérez. 2022. "Feeding Our Microbiota: Stimulation of the Immune/Semiochemical System and the Potential Amelioration of Non-Communicable Diseases" Life 12, no. 8: 1197. https://doi.org/10.3390/life12081197
APA StyleSmith, D., Jheeta, S., Fuentes, H. V., & Palacios-Pérez, M. (2022). Feeding Our Microbiota: Stimulation of the Immune/Semiochemical System and the Potential Amelioration of Non-Communicable Diseases. Life, 12(8), 1197. https://doi.org/10.3390/life12081197







