Screening of Bioactive Compounds from Endophytic Marine-Derived Fungi in Saudi Arabia: Antimicrobial and Anticancer Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Endophytic Fungi
2.2. Endophytic Fungi Isolation and Cultivation
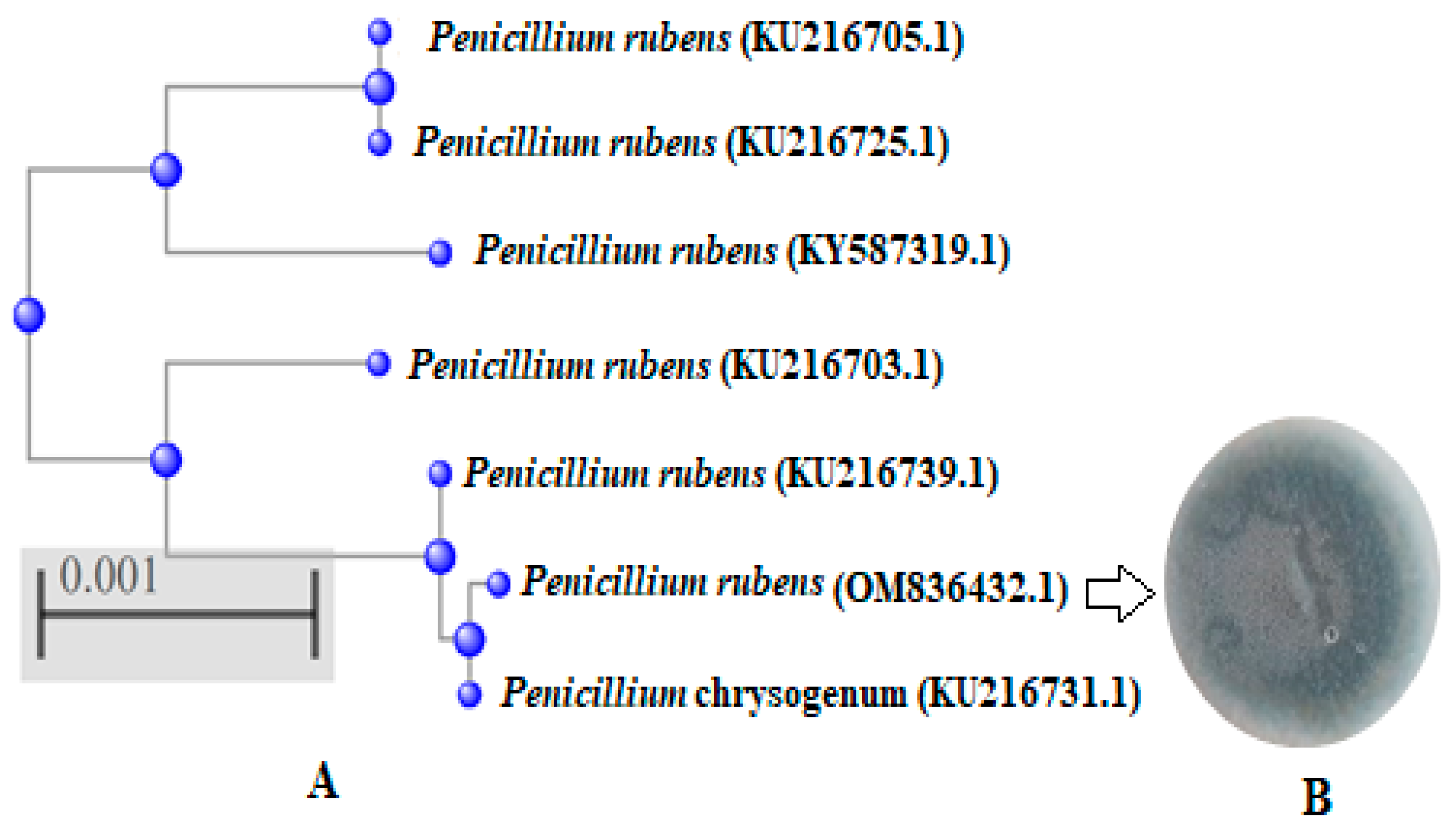
2.3. Identification of Fungal Isolates
2.4. Preparation of Marine Fungal Extract
2.5. GC-MS Analysis of Fungal Filtrate Extract
2.6. Disk Diffusion Method and MIC Detection for Antimicrobial Activity of Fungal Extract
2.7. Cytotoxicity Assay against Cancer Cells
2.8. Cytotoxicity Assay against Normal Cells
2.9. Molecular Docking Studies with MOE
2.10. Statistical Analysis
3. Results and Discussion
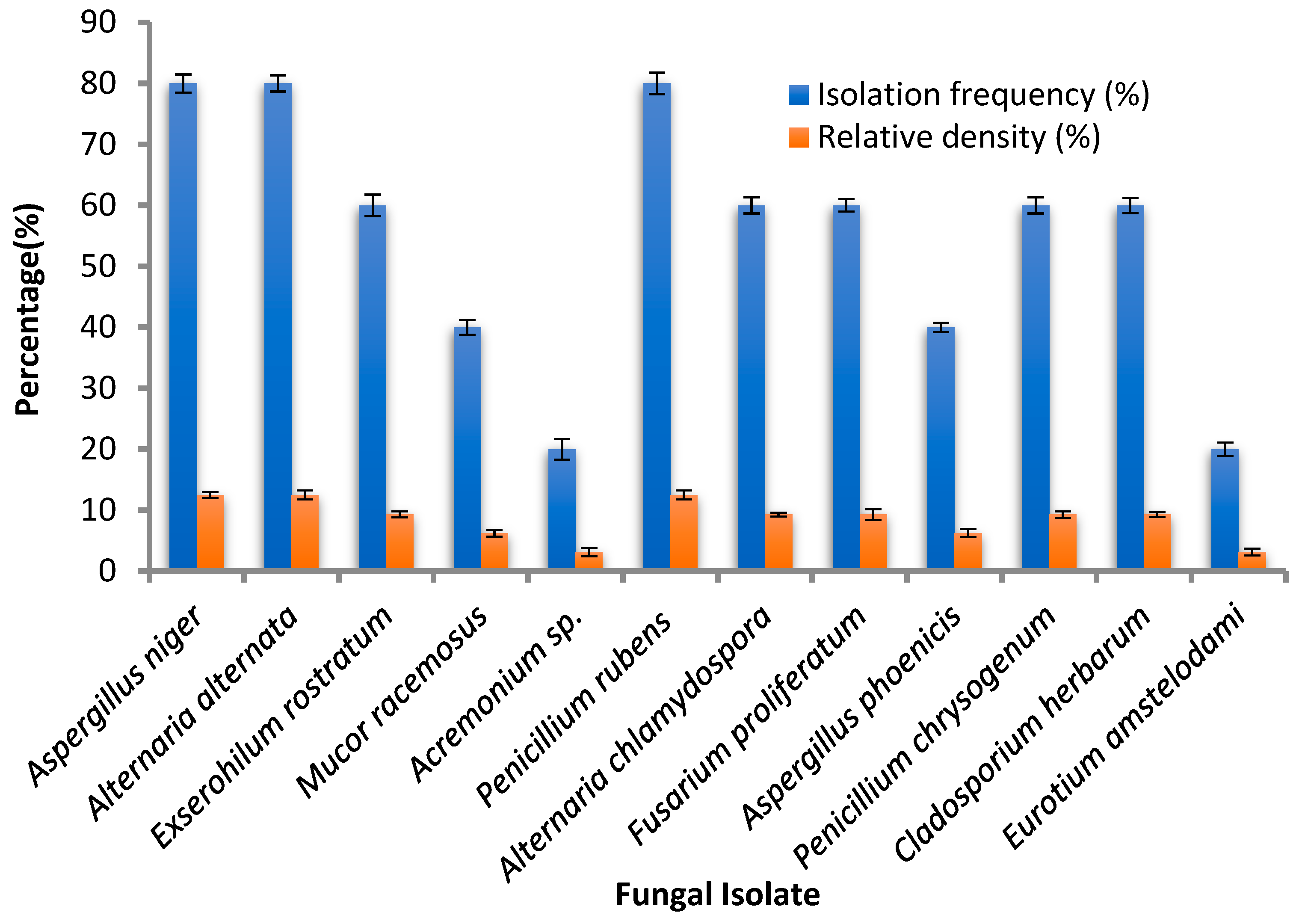
3.1. Endophytic Fungi Isolates
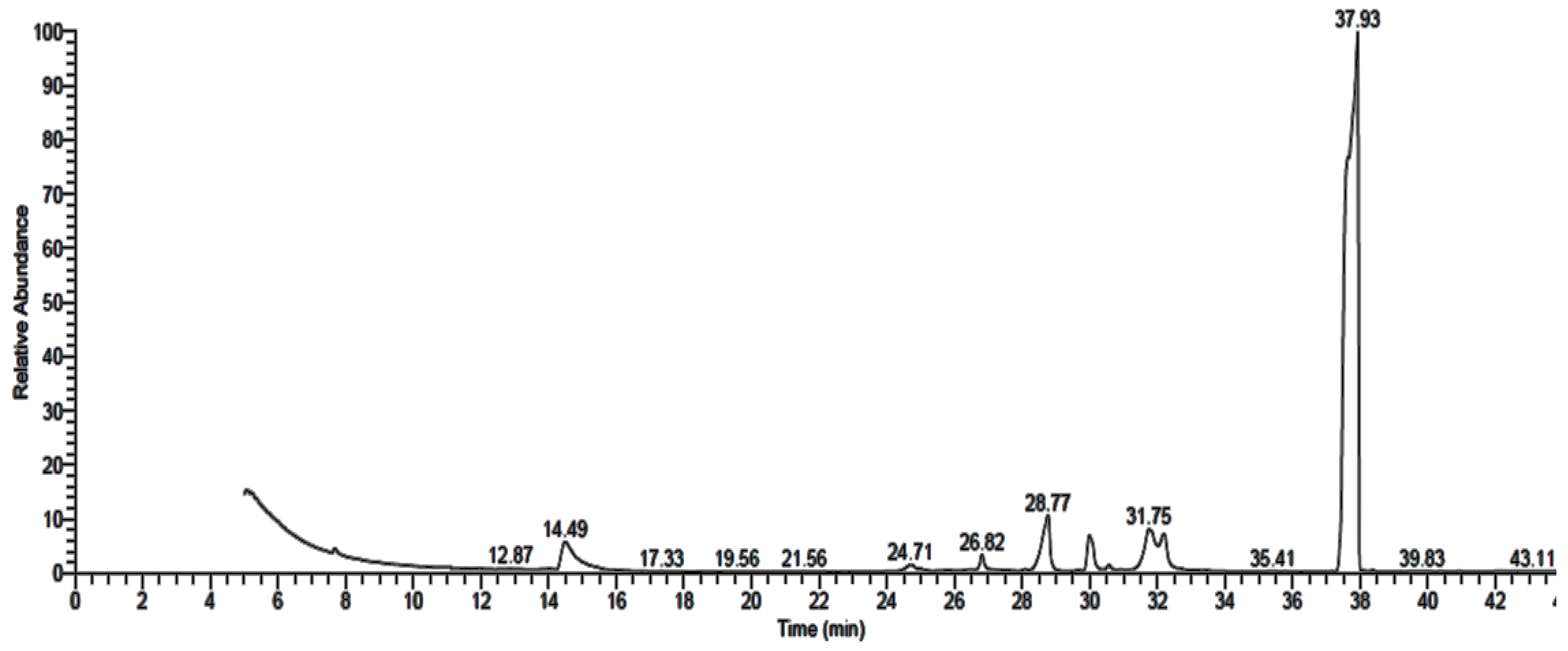
3.2. GC-MS Analysis of Fungal Extract
3.3. Antimicrobial Activity of Fungal Extract
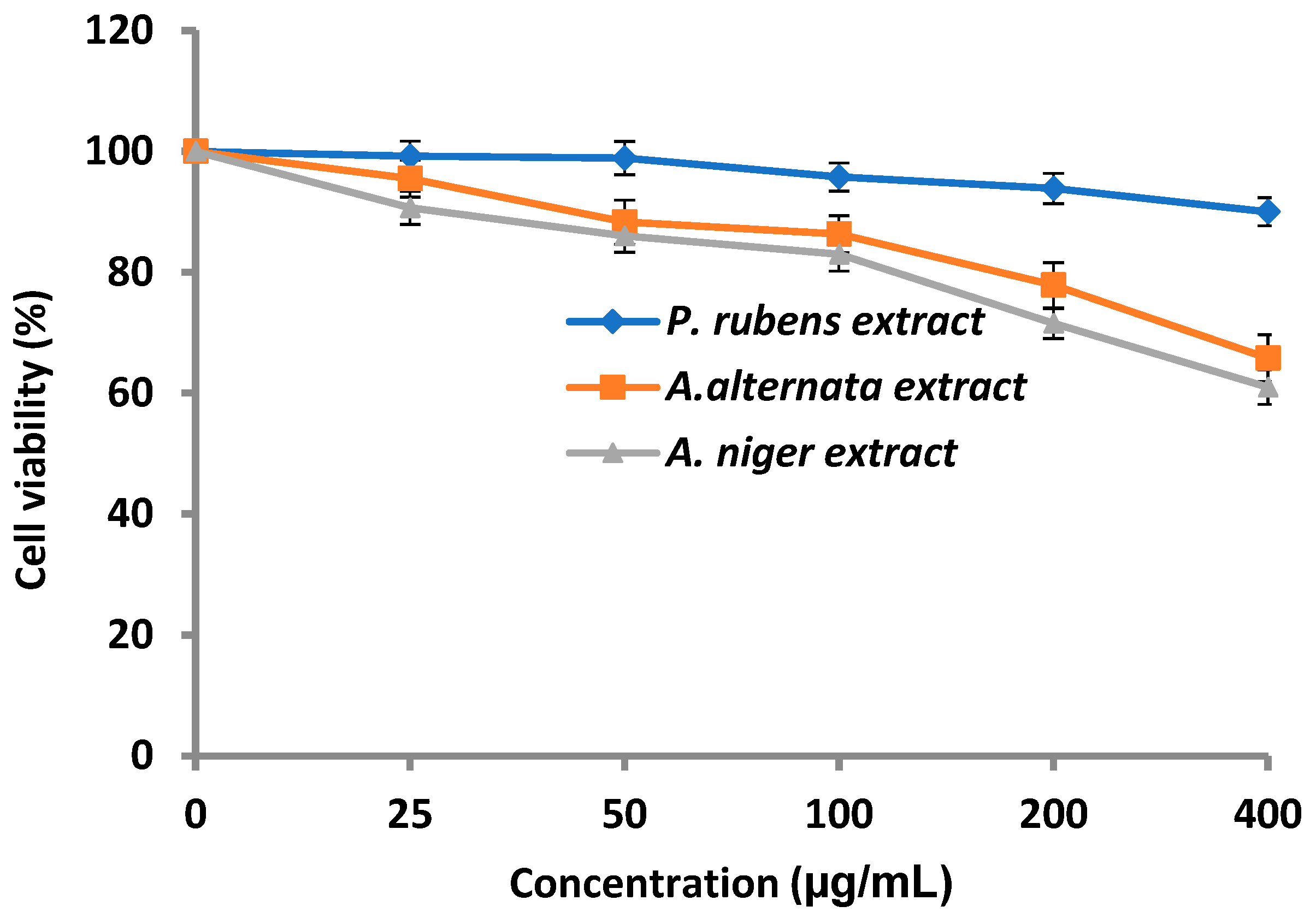
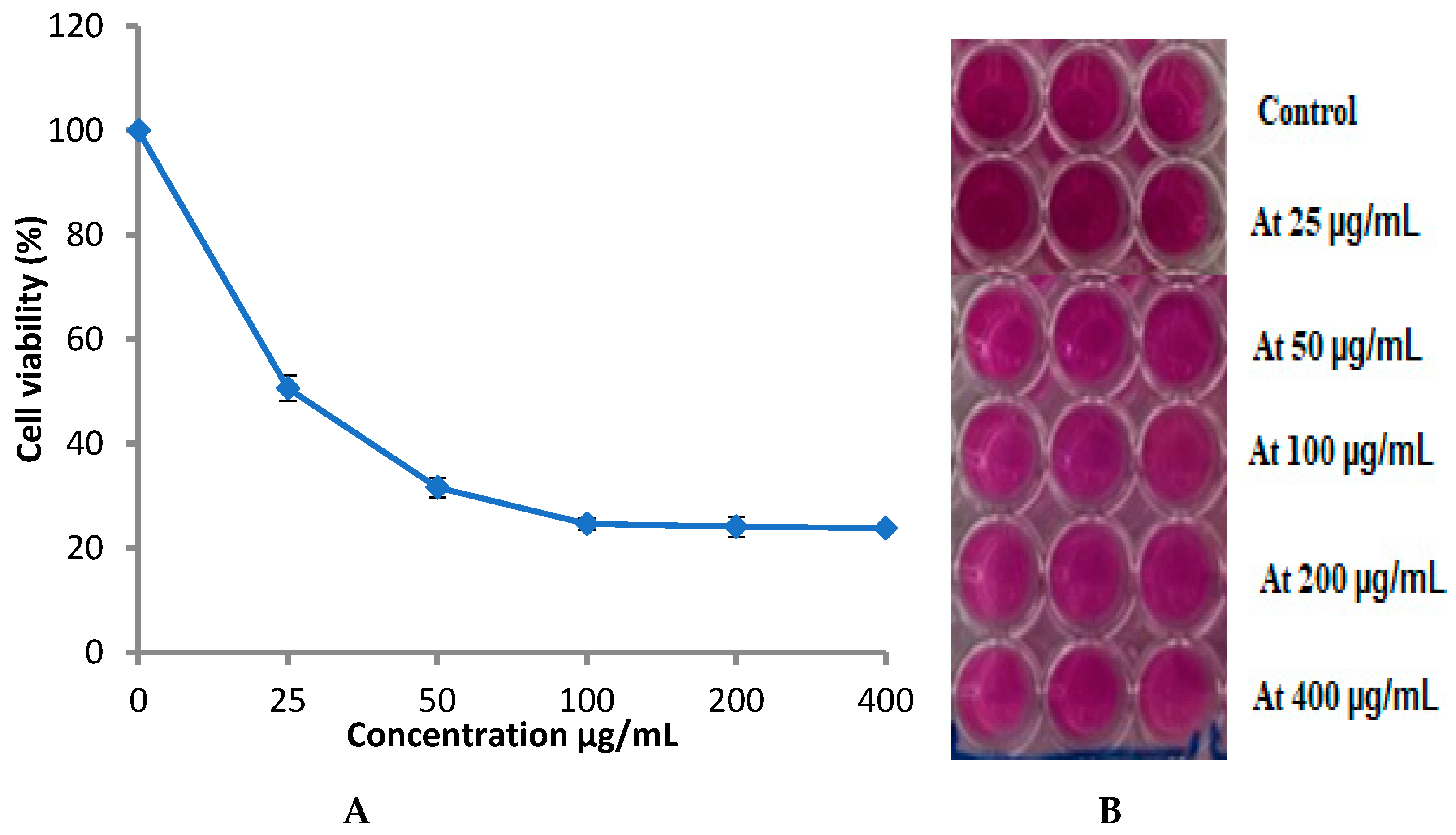
3.4. Cytotoxicity of Fungal Extract
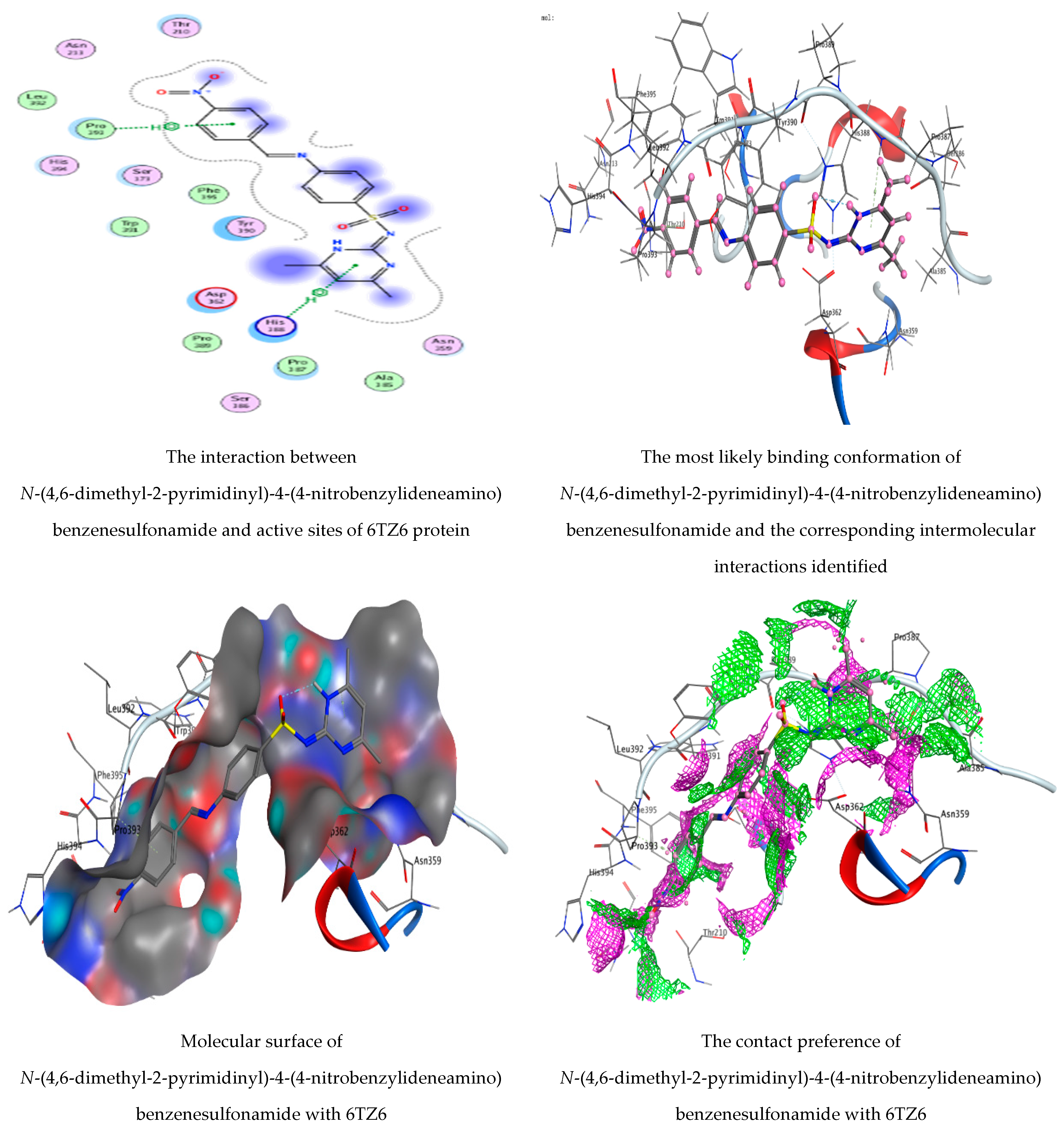
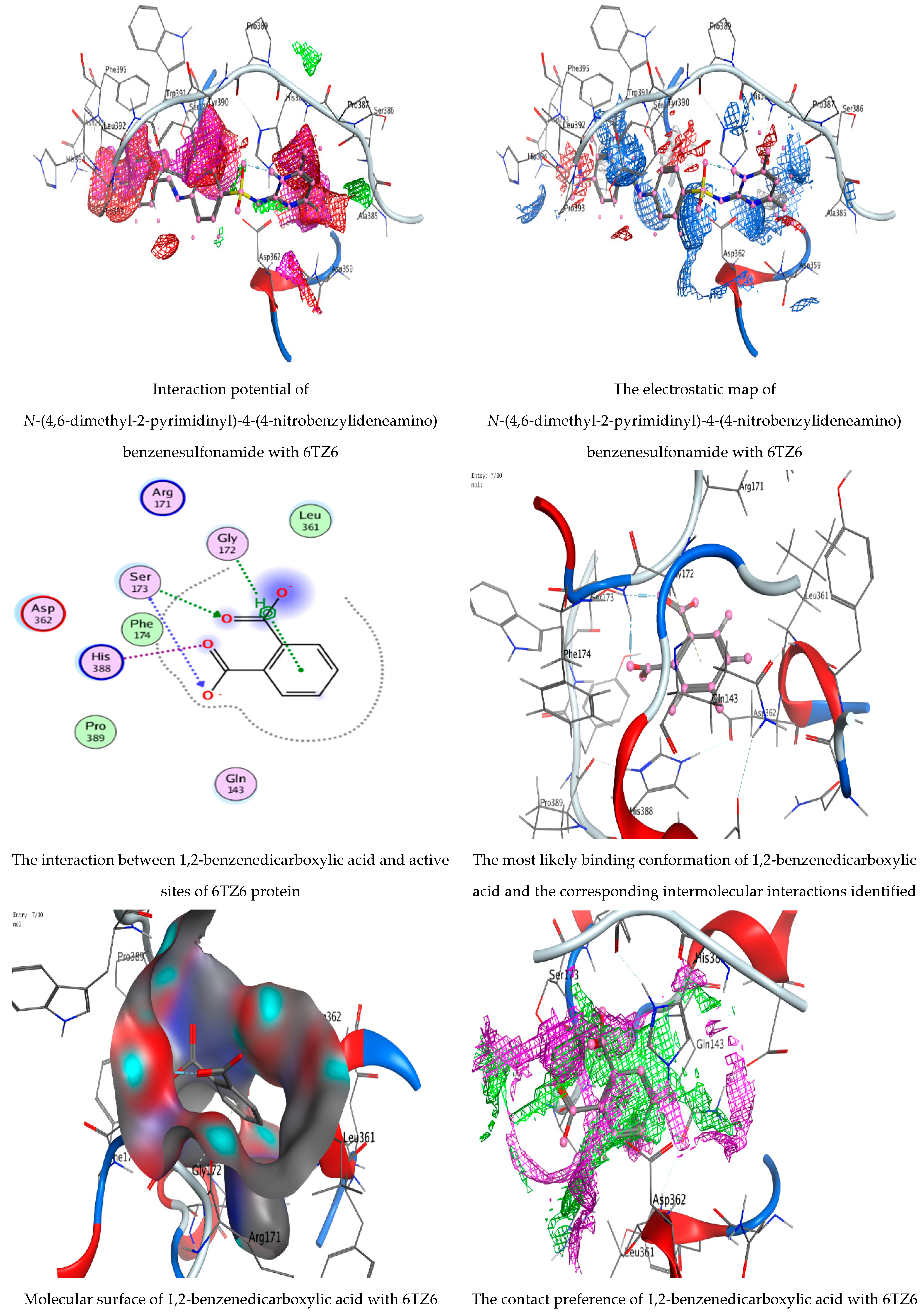
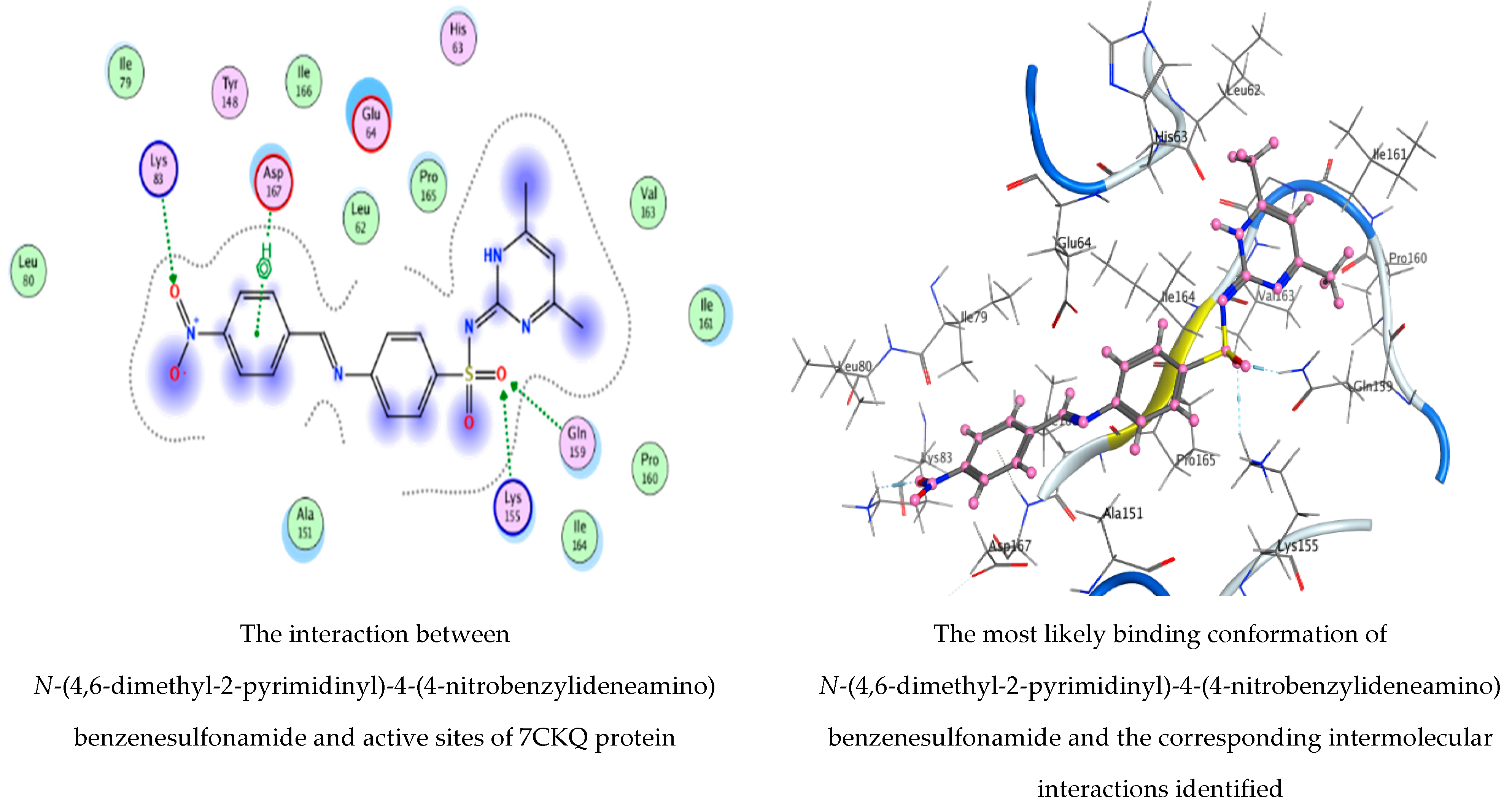
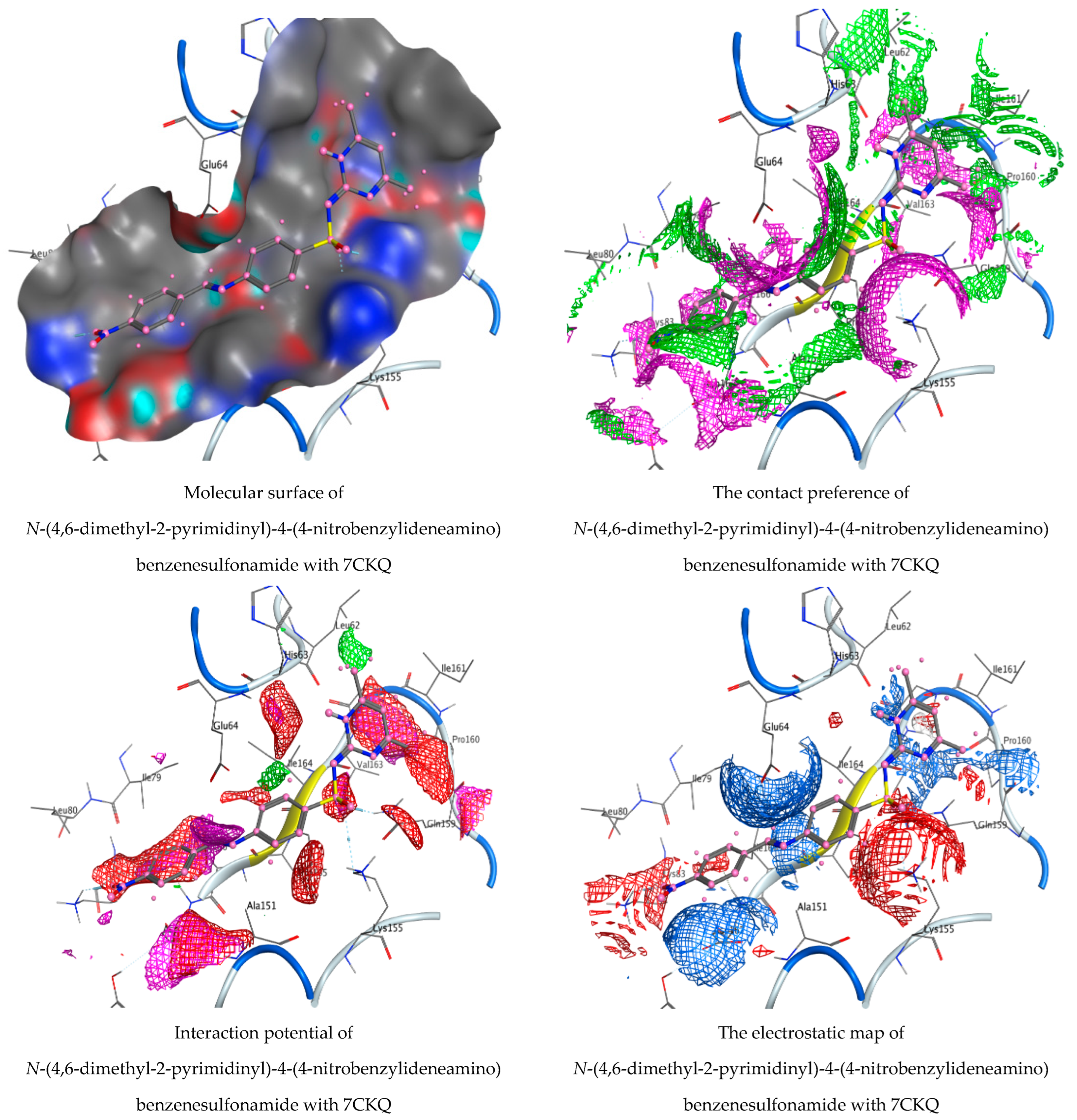
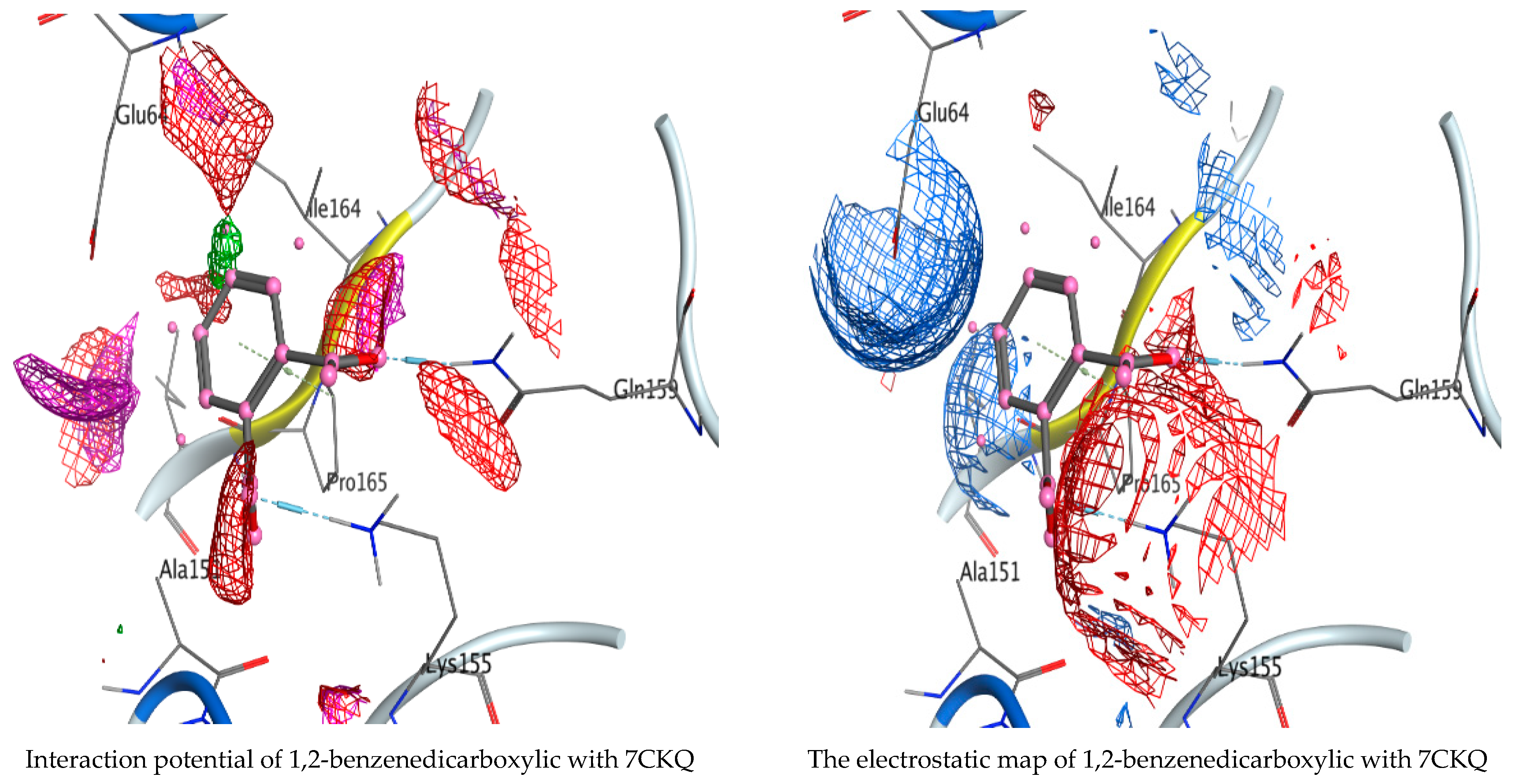
3.5. Molecular Docking Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GC-MS | Gas chromatography–mass spectrometry |
| RT | Retention time |
| MIC | Minimum inhibitory concentration |
| SD | Standard deviation |
| PC3 | Prostate cancer cell |
| MOE | Molecular Operating Environment |
| RMSD | Root mean square deviation |
| GBVI/WSA | Generalized Born Volume Integral/Weighted Surface Area |
References
- Amer, M.S.; Abd Ellatif, H.H.; Hassan, S.W.; Aboelela, G.M.; Gad, A.M. Characterization of some fungal strains isolated from the Eastern coast of Alexandria, Egypt, and some applications of Penicillium crustosum. Egypt. J. Aquat. Res. 2019, 45, 211–217. [Google Scholar] [CrossRef]
- Qader, M.M.; Hamed, A.A.; Soldatou, S.; Abdelraof, M.; Elawady, M.E.; Hassane, A.S.I.; Belbahri, L.; Ebel, R.; Rateb, M.E. Antimicrobial and Antibiofilm Activities of the Fungal Metabolites Isolated from the Marine Endophytes Epicoccum nigrum M13 and Alternaria alternata 13A. Mar. Drugs 2021, 19, 232. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Liu, Y.; Li, J.; Wang, X.; He, S.; Yan, X.; Shi, Y.; Zhang, W.; Ding, L. Marine natural products and their synthetic analogs as promising antibiofilm agents for antibiotics discovery and development. Eur. J. Med. Chem. 2022, 239, 114513. [Google Scholar] [CrossRef] [PubMed]
- Al Abboud, M.A.; Al-Rajhi, A.M.; Shater AR, M.; Alawlaqi, M.M.; Mashraqi, A.; Selim, S.; Al Jaouni, S.K.; Ghany, T.M.A. Halostability and Thermostability of Chitinase Produced by Fungi Isolated from Salt Marsh Soil in Subtropical Region of Saudi Arabia. BioResources 2022, 17, 4763–4780. [Google Scholar]
- Pinheiro, E.A.; Carvalho, J.M.; dos Santos, D.C.; Feitosa Ade, O.; Marinho, P.S.; Guilhon, G.M.; de Souza, A.D.; da Silva, F.M.; Marinho, A.M. Antibacterial activity of alkaloids produced by endophytic fungus Aspergillus sp. EJC08 isolated from medical plant Bauhinia guianensis. Nat. Prod. Res. 2013, 27, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, J.D.; Nascimento, C.C.; Barbosa, R.; da Silva, D.C.; Svedese, V.M.; Silva-Nogueira, E.B.; Gomes, B.S.; Paiva, L.M.; Souza-Motta, C.M. Endophytic fungi from medicinal plant Bauhinia forficata: Diversity and biotechnological potential. Braz. J. Microbiol. 2015, 46, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, M.; Feng, Q.; Lin, Y.; Zhao, H. A study on biological activity of marine fungi from different habitats in coastal regions. SpringerPlus 2016, 5, 1966. [Google Scholar] [CrossRef]
- Hamzah, T.N.T.; Lee, S.Y.; Hidayat, A.; Terhem, R.; Faridah-Hanum, I.; Mohamed, R. Diversity and Characterization of Endophytic Fungi Isolated from the Tropical Mangrove Species, Rhizophora mucronata, and Identification of Potential Antagonists Against the Soil-Borne Fungus, Fusarium solani. Front. Microbiol. 2018, 9, 1707. [Google Scholar] [CrossRef]
- Petit, P.; Lucas, E.M.F.; Abreu, L.M.; Pfenning, L.H.; Takahashi, J.A. Novel antimicrobial secondary metabolites from a Penicillium sp. isolated from Brazilian cerrado soil. Electron. J. Biotechnol. 2009, 12, 1–9. [Google Scholar] [CrossRef]
- Gharaei-Fathabad, E.; Tajick-Ghanbary, M.A.; Shahrokhi, N. Antimicrobial Properties of Penicillium Species Isolated from Agricultural Soils of Northern Iran. Res. J. Toxin 2014, 6, 1–7. [Google Scholar] [CrossRef][Green Version]
- Chen, M.; Shen, Y.; Lin, L.; Wei, W.; Wei, D. Mn2+ modulates the production of mycophenolic acid in Penicillium brevicompactum NRRL864 via reactive oxygen species signaling and the investigation of pb-pho. Fungal Biol. 2022, 126, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Lopes, F.C.; Tichota, D.M.; Sauter, I.P.; Meira, S.M.M.; Segalin, J.; Rott, M.B.; Rios, A.O.; Brandelli, A. Active metabolites produced by Penicillium chrysogenum IFL1 growing on agro-industrial residues. Ann. Microbiol. 2013, 63, 771–778. [Google Scholar] [CrossRef]
- Omeike, S.O.; Kareem, S.O.; Lasisi, A.A. Potential antibiotic-producing fungal strains isolated from pharmaceutical waste sludge. Beni-Suef Univ. J. Basic Appl. Sci. 2019, 8, 18. [Google Scholar] [CrossRef]
- Visamsetti, A.; Ramachandran, S.S.; Kandasamy, D. Penicillium chrysogenum DSOA associated with marine sponge (Tedania anhelans) exhibit antimycobacterial activity. Microbiol. Res. 2016, 185, 55–60. [Google Scholar] [CrossRef]
- Nakashima, T.; Mayuzumi, S.; Inaba, S.; Park, J.Y.; Anzai, K.; Suzuki, R.; Kuwahara, N.; Utsumi, N.; Yokoyama, F.; Sato, H.; et al. Production of bioactive compounds based on phylogeny in the genus Penicillium preserved at NBRC. Biosci. Biotechnol. Biochem. 2008, 72, 3051–3054. [Google Scholar] [CrossRef][Green Version]
- Leitao, A.L. Potential of Penicillium species in bioremediation field. Int. J. Environ. Res. Public Health 2009, 6, 1393–1417. [Google Scholar] [CrossRef] [PubMed]
- Monika, G.; Punam, G.; Sarbjot, S.; Gupta, G. An overview on molecular docking. Int. J. Drug Dev. Res. 2010, 2, 219–231. [Google Scholar]
- Dar, A.; Mir, S. Molecular Docking: Approaches, Types, Applications and Basic Challenges. J. Anal. Bioanal. Tech. 2017, 8, 356. [Google Scholar] [CrossRef]
- Guedes, I.A.; de Magalhães, C.S.; Dardenne, L.E. Receptor–ligand molecular docking. Biophys. Rev. 2014, 6, 75–87. [Google Scholar] [CrossRef]
- Kjer, J.; Debbab, A.; Aly, A.H.; Proksch, P. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nat. Protoc. 2010, 5, 479–490. [Google Scholar] [CrossRef]
- Ellis, M.B. Dematiaceous Hyphomycetes; CMI: Kew, UK, 1971. [Google Scholar]
- Raper, K.B.; Fennel, D.I. The Genus Aspergillus; Robert, E., Ed.; Krieger Publishing Company: Huntington, NY, USA, 1973. [Google Scholar]
- Domsch, K.H.; Gams, W.; Anderson, T. Compendium of Soil Fungi; Academic Press Ltd.: London, UK, 1980. [Google Scholar]
- Rotem, J. The Genus Alternaria: Biology, Epidemiology and Pathogenicity; American Phytopathological Society: Saint Paul, MN, USA, 1994. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium, Laboratory Manual, 1st ed.; Blackwell Publishing Professional: Hoboken, NJ, USA, 2006; 274p. [Google Scholar]
- White, T.J.; Bruns, T.; Lee, S.; Tayler, J. Amplification and direct sequencing of fungal ribosomal RNA genes for polygenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Abdel Ghany, T.M.; Ganash, M.; Bakri, M.M.; Al-Rajhi, A.M.H. Molecular characterization of Trichoderma asperellum and lignocellulolytic activity on barley straw treated with silver nanoparticles. BioResources 2018, 13, 1729–1744. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Yahya, R.; Bakri, M.M.; Ganash, M.; Amin, B.H.; Qanash, H. Effect of Thevetia peruviana seeds extract for microbial pathogens and cancer control. Int. J. Pharmacol. 2021, 17, 643–655. [Google Scholar] [CrossRef]
- Bakri, M.M.; Al-Rajhi AM, H.; Abada, E.; Salem OM, A.; Shater, A.-R.; Mahmoud, M.S.; Abdel Ghany, T.M. Mycostimulator of chitinolytic activity: Thermodynamic studies and its activity against human and food-borne microbial pathogens. BioResources 2022, 17, 4378–4394. [Google Scholar] [CrossRef]
- Al-Rajhi AM, H.; Yahya, R.; Abdelghany, T.M.; Fareid, M.A.; Mohamed, A.M.; Amin, B.H.; Masrahi, A.S. Anticancer, anticoagulant, antioxidant and antimicrobial activities of Thevetia peruviana latex with molecular docking of antimicrobial and anticancer activities. Molecules 2022, 27, 3165. [Google Scholar] [CrossRef] [PubMed]
- Qanash, H.; Yahya, R.; Bakri, M.M.; Bazaid, A.S.; Qanash, S.; Shater, A.F.; Abdelghany, T.M. Anticancer, antioxidant, antiviral and antimicrobial activities of Kei Apple (Dovyalis caffra) fruit. Sci. Rep. 2022, 12, 5914. [Google Scholar] [CrossRef]
- Hamed, A.A.; Soldatou, S.; Qader, M.M.; Arjunan, S.; Miranda, K.J.; Casolari, F.; Pavesi, C.; Diyaolu, O.A.; Thissera, B.; Eshelli, M.; et al. Screening Fungal Endophytes Derived from Under-Explored Egyptian Marine Habitats for Antimicrobial and Antioxidant Properties in Factionalised Textiles. Microorganisms 2020, 8, 1617. [Google Scholar] [CrossRef]
- Ashok, G.; Senthilkumar, G.; Panneerselvam, A. Diversity and seasonal variation of soil Fungi isolated from coastal area of Tuticorin Dt., Tamil Nadu, India. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 161–178. [Google Scholar]
- Yadav, M.; Yadav, A.; Yadav, J.P. In vitro antioxidant activity and total phenolic content of endophytic fungi isolated from Eugenia jambolana Lam. Asian Pac. J. Trop. Med. 2014, 7, S256–S261. [Google Scholar] [CrossRef]
- Atiphasaworn, P.; Monggoot, S.; Gentekaki, E.; Brooks, S.; Pripdeevech, P. Antibacterial and Antioxidant Constituents of Extracts of Endophytic Fungi Isolated from Ocimum basilicum var. thyrsiflora Leaves. Curr. Microbiol. 2017, 74, 1185–1193. [Google Scholar] [CrossRef]
- Danagoudar, A.; Joshi, C.; Ravi, S.; Rohit Kumar, H.; Ramesh, B. Antioxidant and cytotoxic potential of endophytic fungi isolated from medicinal plant Tragia involucrata L. Pharmacogn. Res. 2018, 10, 188–194. [Google Scholar]
- Kalpana, D.; Shanmugasundaram, R.; Mohan, V. GC–MS analysis of ethanol extract of Entada pursaetha DC seed. Biosci. Discov. 2012, 3, 30–33. [Google Scholar]
- Pinto, M.E.A.; Araújo, S.G.; Morais, M.I.; Sá, N.P.; Lima, C.M.; Rosa, C.A.; Siqueira, E.P.; Johann, S.; Lima, L.A.R.S. Antifungal and antioxidant activity of fatty acid methyl esters from vegetable oils. An. Acad. Bras. Ciências 2017, 89, 1671–1681. [Google Scholar] [CrossRef] [PubMed]
- Davoodbasha, M.; Edachery, B.; Nooruddin, T.; Lee, S.; Kim, J. An evidence of C16 fatty acid methyl esters extracted from microalga for effective antimicrobial and antioxidant property. Microb. Pathog. 2018, 115, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.D.; Iqbal, M.O. Antioxidant activity, phytochemical analysis and total polyphenolics content of essential oil, methanol extract and methanol fractions from Commelinanudiflora. Int. J. Pharm. Pharm. Sci. 2018, 10, 36–43. [Google Scholar] [CrossRef]
- Basheer, M.A.; Mekawey, A.A.; El-Kafrawy, S.B.; Abouzeid, M.A. Antimicrobial Activities of Endophytic Fungi of Red Sea Aquatic Plant Avicennia marina. Egypt. J. Microbiol. 2018, 53, 231–240. [Google Scholar] [CrossRef]
- Heydari, H.; Koc, A.; Simsek, D.; Gozcelioglu, B.; Altanlar, N.; Konuklugil, B. Isolation, identification and bioactivity screening of turkish marine-derived fungi. Farmacia 2019, 67, 780–788. [Google Scholar] [CrossRef]
- Bladt, T.T.; Frisvad, J.C.; Knudsen, P.B.; Larsen, T.O. Anticancer and antifungal compounds from Aspergillus, Penicillium and other filamentous fungi. Molecules 2013, 18, 11338–11376. [Google Scholar] [CrossRef]
- Yahya, R.; Al-Rajhi, A.M.H.; Alzaid, S.Z.; Al Abboud, M.A.; Almuhayawi, M.S.; Al Jaouni, S.K.; Selim, S.; Ismail, K.S.; Abdelghany, T.M. Molecular Docking and Efficacy of Aloe vera Gel Based on Chitosan Nanoparticles against Helicobacter pylori and Its Antioxidant and Anti-Inflammatory Activities. Polymers 2022, 14, 2994. [Google Scholar] [CrossRef] [PubMed]
- Al-Rajhi, A.M.H.; Qanash, H.; Almuhayawi, M.S.; Al Jaouni, S.K.; Bakri, M.M.; Ganash, M.; Salama, H.M.; Selim, S.; Abdelghany, T.M. Molecular Interaction Studies and Phytochemical Characterization of Mentha pulegium L. Constituents with Multiple Biological Utilities as Antioxidant, Antimicrobial, Anticancer and Anti-Hemolytic Agents. Molecules 2022, 27, 4824. [Google Scholar] [CrossRef]


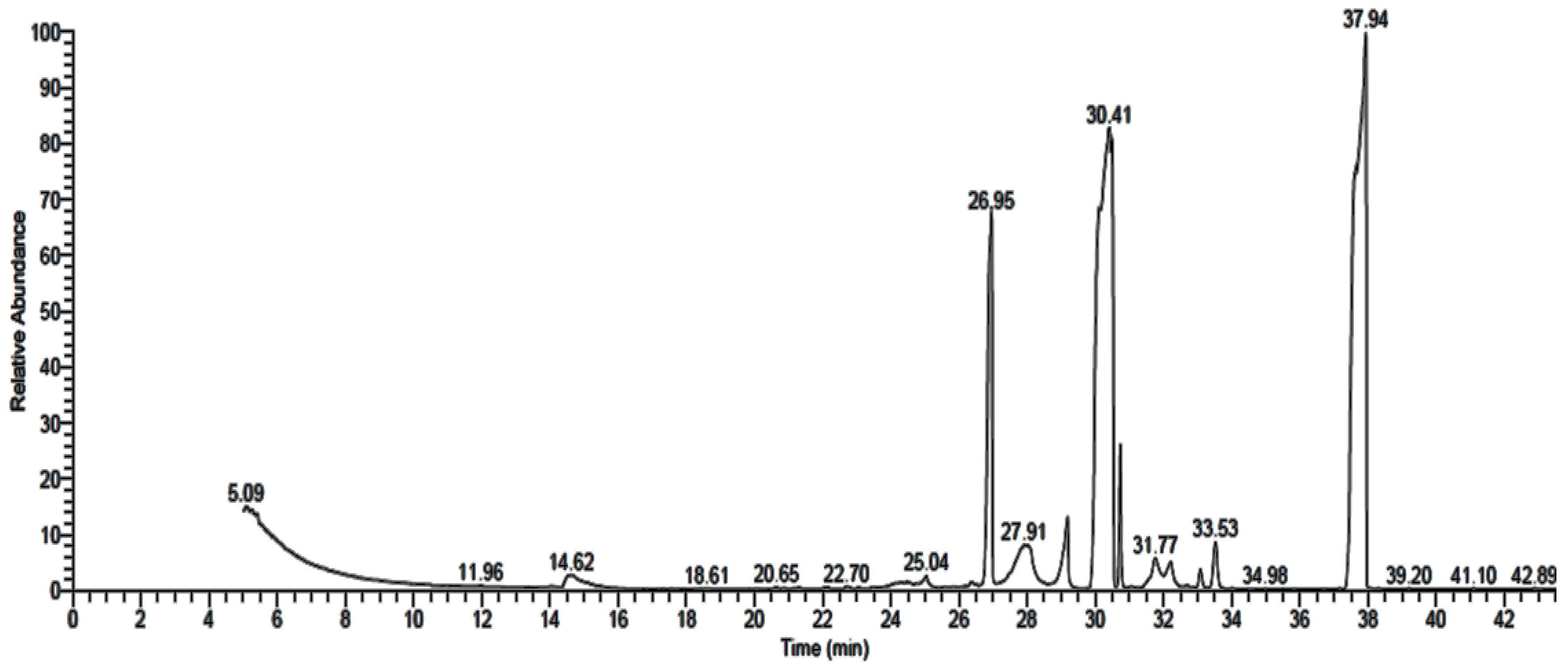
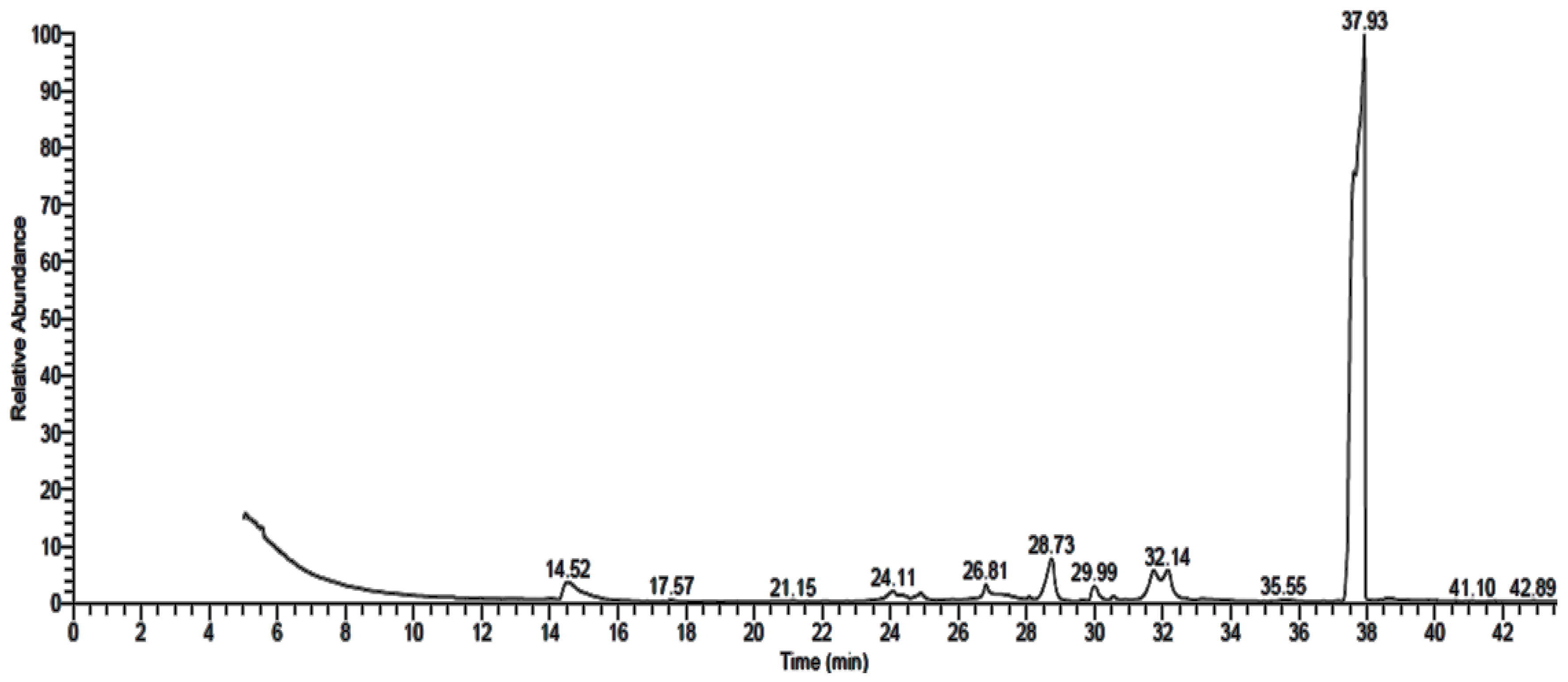



| Detected Constituent | RT * | Area (%) | MF * | MW * |
|---|---|---|---|---|
| Acetic acid ethyl ester | 5.43 | 0.27 | C4H8O2 | 88 |
| N-(4,6-Dimethyl-2-pyrimidinyl)-4-(4-nitrobenzylideneamino)benzenesulfonamide | 14.55 | 0.87 | C19H17N5O4S | 411 |
| Tetradecanoic acid | 25.04 | 0.27 | C14H28O2 | 228 |
| Hexadecanoic acid, methyl ester | 26.95 | 16.41 | C17H34O2 | 270 |
| Propiolic acid, 3-(1-hydroxy-2-isopropyl-5-methylc yclohexyl)-, ethyl ester | 27.91 | 2.57 | C15H24O3 | 252 |
| (1,5,5,8-Tetramethyl-bicyclo [4.2.1]non-9-yl)-acetic acid | 28.04 | 0.94 | C15H26O2 | 238 |
| Hexadecanoic acid | 29.19 | 2.45 | C16H32O2 | 256 |
| 9,12-Octadecadienoic acid (Z,Z)-, methyl ester | 30.05 | 8.10 | C19H34O2 | 294 |
| 9-Octadecenoic acid (Z)-, methyl ester | 30.49 | 29.10 | C19H36O2 | 296 |
| Methyl stearate | 30.74 | 3.34 | C19H38O2 | 298 |
| 9,12-Octadecadienoic acid (Z,Z)- | 31.76 | 0.86 | C18H32O2 | 280 |
| Octadecanoic acid | 32.21 | 0.73 | C18H36O2 | 284 |
| Tributyl acetylcitrate | 33.09 | 0.65 | C20H34O8 | 402 |
| 8,11-Eicosadienoic acid, methyl ester | 33.53 | 1.81 | C21H38O2 | 322 |
| Bis(2-ethylhexyl) phthalate | 37.57 | 9.85 | C24H38O4 | 390 |
| 1,2-Benzenedicarboxylic Acid | 37.94 | 21.77 | C24H38O4 | 390 |
| Detected Constituent | RT * | Area (%) | MF * | MW * |
|---|---|---|---|---|
| Acetic acid ethyl ester | 5.57 | 0.59 | C4H8O2 | 88 |
| N-(4,6-Dimethyl-2-pyrimidinyl)-4-(4-nitrobenzylideneamino)benzenesulfonamide | 14.46 | 3.25 | C19H17N5O4S | 411 |
| Oxiraneundecanoic acid, 3-pentyl-, methyl ester, cis | 26.80 | 0.97 | C19H36O3 | 312 |
| Hexadecanoic acid | 28.74 | 4.07 | C16H32O2 | 256 |
| 9,12-Octadecadienoyl chloride, (Z,Z) | 29.98 | 1.82 | C18H31ClO | 298 |
| 9-Octadecenoic acid (Z)- | 31.73 | 1.94 | C18H34O2 | 282 |
| Octadecanoic acid | 32.17 | 1.80 | C18H36O2 | 284 |
| Phthalic acid, di(2-propylpentyl) ester | 37.56 | 22.30 | C24H38O4 | 390 |
| 1,2-Benzenedicarboxylic acid | 37.93 | 63.26 | C24H38O4 | 390 |
| Detected Constituent | RT * | Area (%) | MF * | MW * |
|---|---|---|---|---|
| Acetic acid ethyl ester | 5.08 | 0.71 | C4H8O2 | 88 |
| N-(4,6-Dimethyl-2-pyrimidinyl)-4-(4-nitrobenzylideneamino) benzenesulfonamide | 14.47 | 4.61 | C19H17N5O4S | 411 |
| Pentadecanoic acid, 14-methyl-, methyl ester | 26.82 | 1.42 | C17H34O2 | 270 |
| Hexadecanoic acid | 28.78 | 7.42 | C16H32O2 | 256 |
| 9,12-Octadecadienoic acid, methyl ester, (E,E)- | 29.99 | 5.21 | C19H34O2 | 294 |
| 9-Octadecenoic acid (Z)- | 31.73 | 3.64 | C18H34O2 | 282 |
| Octadecanoic acid | 32.23 | 2.49 | C18H36O2 | 284 |
| Phthalic acid, di(2-propylpentyl) ester | 37.56 | 22.49 | C24H38O4 | 390 |
| Diisooctyl phthalate | 37.72 | 0.15 | C24H38O4 | 390 |
| 1,2-Benzenedicarboxylic acid | 37.93 | 51.87 | C24H38O4 | 390 |
| Test Organism | Inhibition Zone (mm) | MIC of P. rubens Filtrate Extract µg/mL | |||
|---|---|---|---|---|---|
| P. rubens | A. alternata | A. niger | Control * | ||
| B. subtilis | 27.20 | 17.75 | 20.15 | 22.33 | 19.50 |
| S. aureus | 22.21 | 14.20 | 17.10 | 20.20 | 22.30 |
| E. coli | 26.26 | 21.05 | 21.50 | 24.43 | 21.62 |
| P. aeruginosa | 27.33 | 22.20 | 16.25 | 24.42 | 19.70 |
| C. albicans | 28.25 | 21.15 | 19.33 | 24.50 | 31.2 |
| A. fumigatus | 8.50 | 0.00 | 0.00 | 6.20 | 39.50 |
| Mol | mseq | S | rmsd_Refine | E_Conf | E_Place | E_Score1 | E_Refine | E_Score2 |
|---|---|---|---|---|---|---|---|---|
| N-(4,6-Dimethyl-2-pyrimidinyl)-4-(4-nitrobenzylideneamino) benzenesulfonamide | 1 | −6.04905 | 0.6673 | −69.0445 | −50.0656 | −9.10488 | −25.0128 | −6.04905 |
| 1 | −5.91481 | 1.6653 | −67.9808 | −66.4094 | −7.9697 | −30.5244 | −5.91481 | |
| 1 | −5.82162 | 1.5377 | −78.9276 | −75.9068 | −8.11409 | −30.4167 | −5.82162 | |
| 1 | −5.81604 | 2.2649 | −68.0293 | −45.1237 | −7.79368 | −27.426 | −5.81604 | |
| 1 | −5.73762 | 1.5874 | −77.267 | −44.5125 | −8.87414 | −31.9882 | −5.73762 | |
| 1,2-Benzenedicarboxylic acid | 2 | −4.74927 | 0.6303 | −126.93 | −39.1911 | −8.92265 | −13.603 | −4.74927 |
| 2 | −4.45324 | 0.4808 | −130.454 | −60.5226 | −8.77861 | −16.4348 | −4.45324 | |
| 2 | −4.40914 | 0.6678 | −126.281 | −48.3632 | −9.36907 | −11.4361 | −4.40914 | |
| 2 | −4.35465 | 1.1219 | −127.282 | −37.8587 | −8.83715 | −16.659 | −4.35465 | |
| 2 | −4.30812 | 0.992 | −129.415 | −40.7101 | −8.7569 | −16.2546 | −4.30812 |
| Mol | mseq | S | rmsd_Refine | E_Conf | E_Place | E_Score1 | E_Refine | E_Score2 |
|---|---|---|---|---|---|---|---|---|
| N-(4,6-Dimethyl-2-pyrimidinyl)-4-(4-nitrobenzylideneamino) benzenesulfonamide | 1 | −6.59009 | 1.1327 | −77.8904 | −60.5493 | −10.7919 | −34.7198 | −6.59009 |
| 1 | −6.53716 | 1.2057 | −68.1997 | −72.8364 | −10.2792 | −33.0921 | −6.53716 | |
| 1 | −6.51062 | 4.7166 | −74.721 | −74.2806 | −9.3112 | −35.0797 | −6.51062 | |
| 1 | −6.17644 | 2.6662 | −66.5713 | −69.706 | −9.98946 | −32.9953 | −6.17644 | |
| 1 | −6.06362 | 1.9137 | −80.531 | −63.459 | −9.48588 | −31.6404 | −6.06362 | |
| 1,2-Benzenedicarboxylic acid | 2 | −3.97767 | 0.9546 | −127.43 | −45.723 | −9.74105 | −14.5658 | −3.97767 |
| 2 | −3.94882 | 3.0570 | −130.836 | −39.1438 | −8.63553 | −15.1371 | −3.94882 | |
| 2 | −3.85854 | 1.5905 | −130.089 | −49.7431 | −9.35678 | −13.6882 | −3.85854 | |
| 2 | −3.83116 | 1.5718 | −130.57 | −45.1682 | −8.80624 | −13.9746 | −3.83116 | |
| 2 | −3.67637 | 1.1142 | −131.241 | −36.3385 | −8.59382 | −14.4134 | −3.67637 |
| Mol | Ligand | Receptor | Interaction | Distance | E (kcal/mol) |
|---|---|---|---|---|---|
| N-(4,6-Dimethyl-2-pyrimidinyl)-4-(4-nitrobenzylideneamino) benzenesulfonamide | 6-ring | N HIS 388 (A) | Pi-H | 4.57 | −0.9 |
| 6-ring | CA PRO 393 (A) | Pi-H | 3.55 | −0.6 | |
| 1,2-Benzenedicarboxylic acid | O 14 | N SER 173 (A) | H-acceptor | 2.91 | −3.5 |
| O 16 | OG SER 173 (A) | H-acceptor | 2.81 | −3.1 | |
| 6-ring | CA GLY 172 (A) | Pi-H | 3.89 | −0.5 |
| Mol | Ligand | Receptor | Interaction | Distance | E (kcal/mol) |
|---|---|---|---|---|---|
| N-(4,6-Dimethyl-2-pyrimidinyl)-4-(4-nitrobenzylideneamino) benzenesulfonamide | O 36 | NZ LYS 83 (A) | H-acceptor | 3.11 | −3.6 |
| O 45 | CE LYS 155 (A) | H-acceptor | 3.39 | 0.9 | |
| O 45 | NE2 GLN 159 (A) | H-acceptor | 2.95 | −3.2 | |
| 6-ring | N ASP 167 (A) | Pi-H | 4.05 | −0.5 | |
| 1,2-Benzenedicarboxylic acid | O 15 | NE2 GLN 159 (A) | H-acceptor | 3.08 | −3.5 |
| O 16 | NZ LYS 155 (A) | H-acceptor | 2.98 | −4.3 | |
| 6-ring | CD PRO 165 (A) | Pi-H | 3.77 | −1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Rajhi, A.M.H.; Mashraqi, A.; Al Abboud, M.A.; Shater, A.-R.M.; Al Jaouni, S.K.; Selim, S.; Abdelghany, T.M. Screening of Bioactive Compounds from Endophytic Marine-Derived Fungi in Saudi Arabia: Antimicrobial and Anticancer Potential. Life 2022, 12, 1182. https://doi.org/10.3390/life12081182
Al-Rajhi AMH, Mashraqi A, Al Abboud MA, Shater A-RM, Al Jaouni SK, Selim S, Abdelghany TM. Screening of Bioactive Compounds from Endophytic Marine-Derived Fungi in Saudi Arabia: Antimicrobial and Anticancer Potential. Life. 2022; 12(8):1182. https://doi.org/10.3390/life12081182
Chicago/Turabian StyleAl-Rajhi, Aisha M. H., Abdullah Mashraqi, Mohamed A. Al Abboud, Abdel-Rahman M. Shater, Soad K. Al Jaouni, Samy Selim, and Tarek M. Abdelghany. 2022. "Screening of Bioactive Compounds from Endophytic Marine-Derived Fungi in Saudi Arabia: Antimicrobial and Anticancer Potential" Life 12, no. 8: 1182. https://doi.org/10.3390/life12081182
APA StyleAl-Rajhi, A. M. H., Mashraqi, A., Al Abboud, M. A., Shater, A.-R. M., Al Jaouni, S. K., Selim, S., & Abdelghany, T. M. (2022). Screening of Bioactive Compounds from Endophytic Marine-Derived Fungi in Saudi Arabia: Antimicrobial and Anticancer Potential. Life, 12(8), 1182. https://doi.org/10.3390/life12081182







