Long-Term Effectiveness and Safety of Biologic and Small Molecule Drugs for Moderate to Severe Atopic Dermatitis: A Systematic Review
Abstract
:1. Introduction
2. Material and Method
2.1. Search Strategy and Inclusion Criteria
2.2. Study Selection
2.3. Outcomes
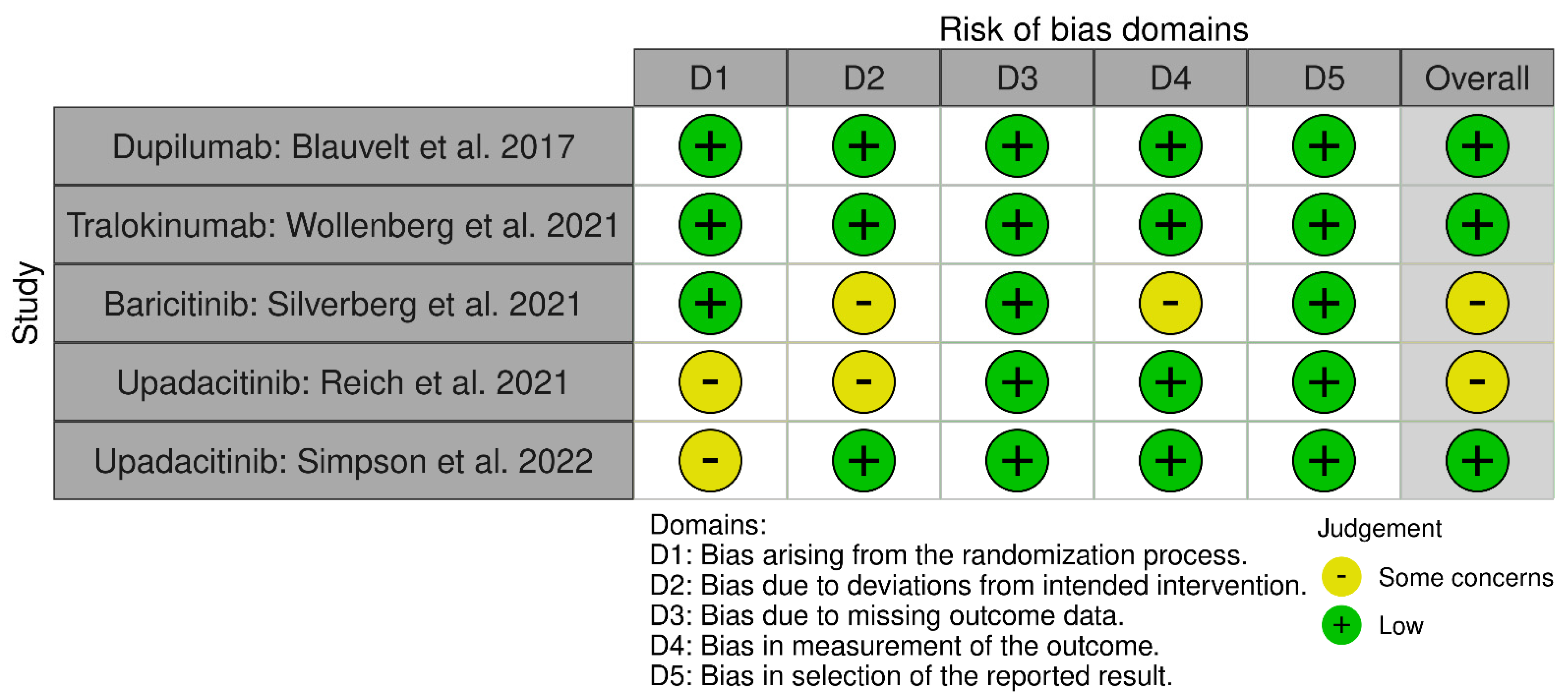
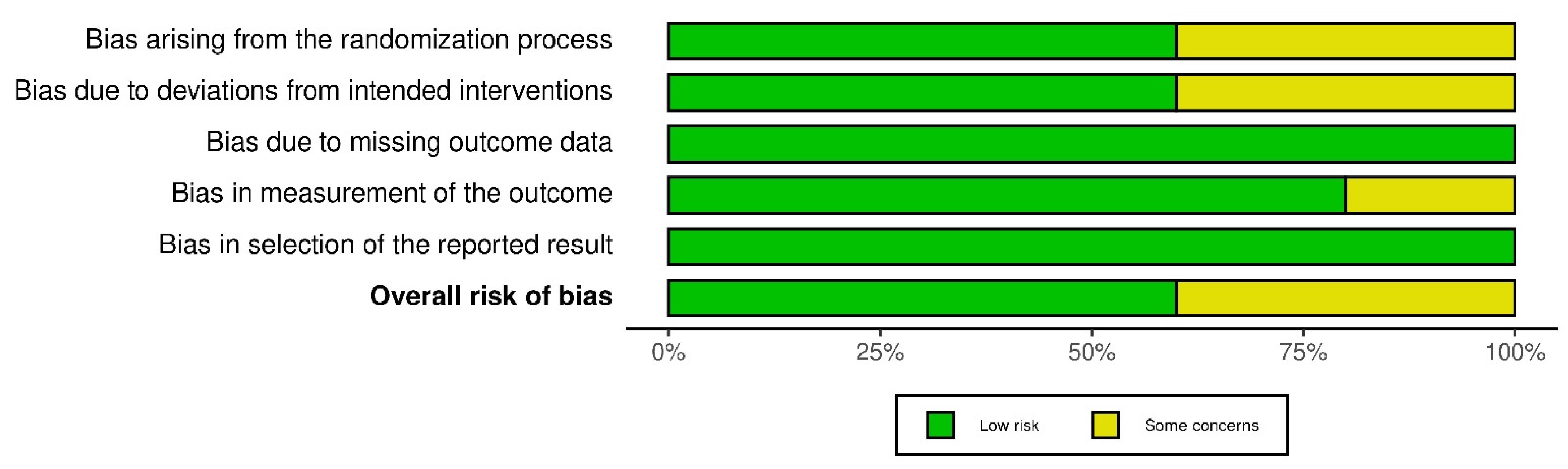
2.4. Assessment of Risk of Bias
2.5. Data Extraction and Quality Assessment
2.6. Strategy for Data Synthesis
3. Results
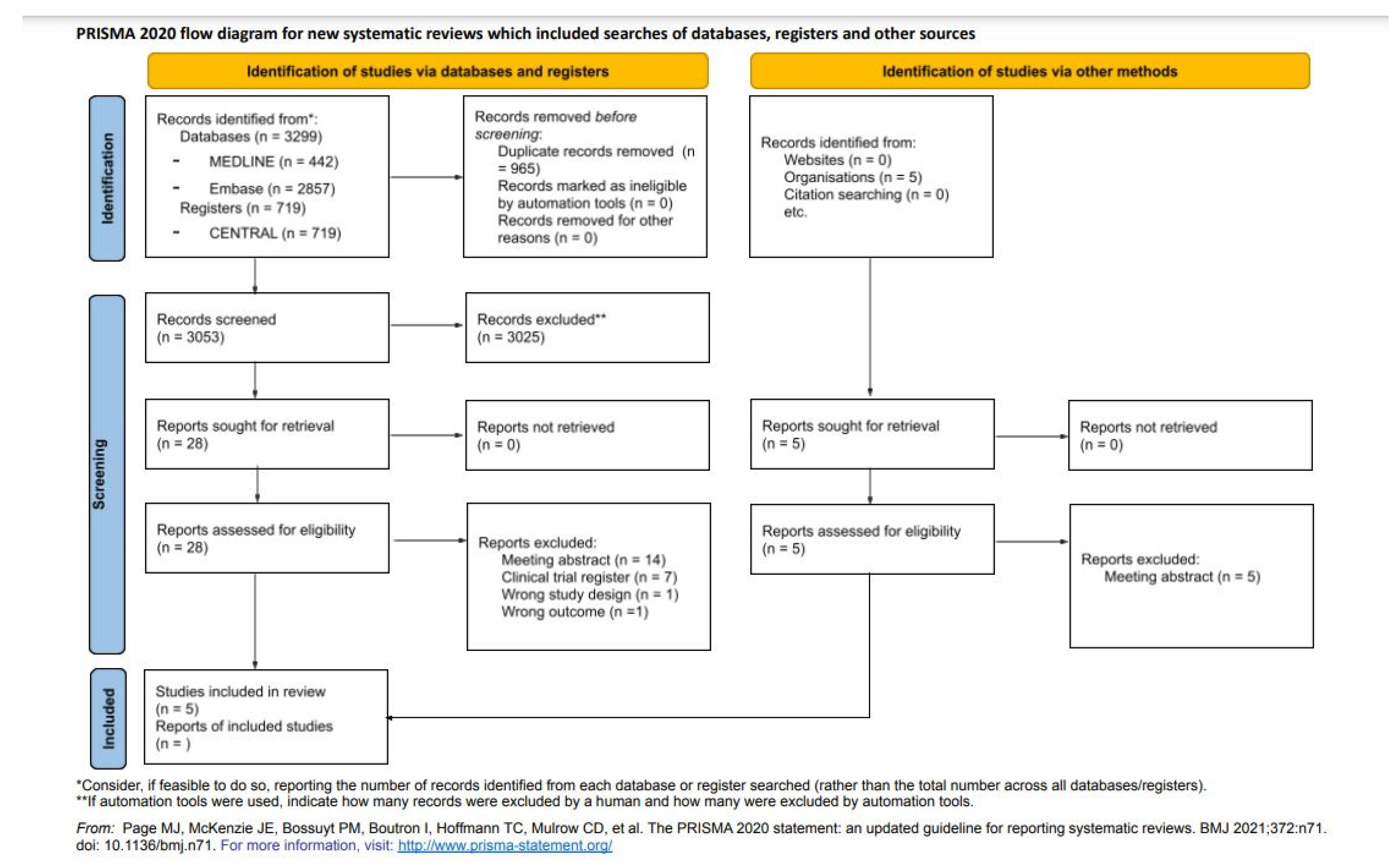
3.1. Study Selection
3.2. Baseline Characteristics
3.3. Efficacy Outcomes
| Publication Data | Study Design | Study Arm Baseline Characteristics | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study ID | Year | Phase | Agent | Dosing, Schedule, Route | n | Males n (%) | Age Mean/Median | Adolescent (12–17 Years) n (%) | Race (White) n (%) | Disease Duration Years Mean/Median | Basal EASI Score Mean/Median | Basal BSA % Mean/Median | Basal SCORAD Score Mean/Median | Weekly WP-NRS Score Mean/Median | vIGA-AD Score = 4 n (%) | DLQI Score Mean/Median |
| LIBERTY AD CHRONOS * [10] | 2017 | 3 | Placebo + TCS | QW sc | 315 | 193 (61.3) | 34.0 (25.0–45.0) | 0 | 208 (66.0) | 26.0 (17.0–38.0) | 29.6 (22.2–40.8) | 55.0 (40.0–75.0) | 64.1 (55.9–76.1) | 7.6 (6.3–8.6) | 147 (46.6) | 14.0 (9.0–20.0) |
| Dupilumab + TCS | 300 mg Q2W sc | 106 | 62 (58.5) | 40.5 (28.0–49.0) | 0 | 74 (69.8) | 28.0 (20.0–44.0) | 30.9 (22.3–41.6) | 58.8 (43.5–78.5) | 69.7 (60.4–79.8) | 7.7 (6.6–8.5) | 53 (50.0) | 13.5 (8.0–20.0) | |||
| Dupilumab + TCS | 300 mg QW sc | 319 | 191 (59.9) | 34.0 (26.0–45.0) | 0 | 208 (65.2) | 26.0 (18.0–39.0) | 29.0 (21.6–40.7) | 52.0 (36.0–71.5) | 65.3 (55.2–76.3) | 7.4 (6.0–8.6) | 147 (46.1) | 14.0 (8.0–20.0) | |||
| ECZTRA-1 * [11] | 2020 | 3 | Placebo | Q2W sc | 199 | 123 (61.8) | 37.0 (26.0–49.0) | 0 | 138 (69.3) | 28.0 (18.0–41.0) | 30.3 (22.0–41.5) | 52.5 (31.0–77.0) | 70.8 (63.8–81.0) | 7.9 (6.9–8.7) | 102 (51.3) | 16.0 (13.0–22.0) |
| Tralokinumab | 300 mg Q2W sc | 603 | 351 (58.2) | 37.0 (27.0–48.0) | 0 | 426 (70.6) | 27.0 (19.0–38.0) | 28.2 (21.3–40.0) | 50.0 (33.0–70.0) | 69.2 (61.5–79.1) | 7.9 (6.7–8.9) | 305 (50.6) | 17.0 (12.0–22.0) | |||
| ECZTRA-2 * [11] | 2020 | 3 | Placebo | Q2W sc | 201 | 114 (56.7) | 30.0 (23.0–46.0) | 0 | 123 (61.2) | 25.0 (18.0–36.0 | 29.6 (20.6–41.4) | 50.0 (31.0–74.0) | 69.9 (61.9–79.1) | 8.1 (7.1–9.0) | 101 (50.2) | 18.0 (12.5–24.0) |
| Tralokinumab | 300 mg Q2W sc | 593 | 359 (60.5) | 34.0 (25.0–48.0) | 0 | 374 (63.1) | 25.5 (17.0–39.0) | 28.2 (19.8–40.8) | 50.0 (31.0–74.0) | 69.5 (60.5–79.1) | 8.0 (7.0–9.0) | 286 (48.2) | 18.0 (13.0–23.0) | |||
| BREEZE-AD3 ** [12] | 2021 | 3 | Baricitinib | 2 mg QD oral | 54 | 28 (51.9) | 32.8 (12.7) | 0 | 45 (83.3) | 19.2 (11.8) | 24.9 (8.7) | NR | 62.2 (12.0) | 6.1 (2.2) | 18 (33.3) | NR |
| Baricitinib | 4 mg QD oral | 70 | 42 (60.0) | 36.7 (15.5) | 0 | 47 (67.1) | 23.2 (16.8) | 28.1 (10.6) | NR | 63.4 (12.3) | 6.5 (2.1) | 22 (31.4) | NR | |||
| AD Up **, *** [13] | 2021 | 3 | Placebo | QD oral | 304 | 178 (58.6) | 34.3 (12–75) | 40 (13.2) | 225 (74.0) | 24.3 (15.2) | 30.3 (13.0) | 48.6 (23.1) | NR | 7.1 (1.6) | 163 (53.6) | 16.3 (7.0) |
| Upadacitinib + TCS | 15 mg QD oral | 300 | 179 (59.7) | 32.5 (13–74) | 39 (13.0 | 204 (68.0) | 22.9 (13.9) | 29.2 (11.8) | 46.7 (21.6) | NR | 7.1 (1.8) | 157 (52.3) | 16.4 (7.2) | |||
| Upadacitinib + TCS | 30 mg QD oral | 297 | 190 (64.0) | 35.5 (12–72) | 37 (12.5) | 218 (73.4) | 23.1 (16.1) | 29.7 (11.8) | 48.5 (23.1) | NR | 7.4 (1.6) | 157 (52.9) | 17.1 (7.0) | |||
| Measure Up 1 **, *** [14] | 2022 | 3 | Placebo | QD oral | 281 [244] | 144 (51.2) | 34.4 (12–75) | 40 (14.2) | 182 (64.8) | 21.3 (15.3) | 28.8 (12.6) | 45.7 (21.6) | 66.1 (12.9) | 7.3 (1.7) | 122 (44.5) | 17.0 (6.8) |
| Upadacitinib + TCS | 15 mg QD oral | 281 | 157 (55.9) | 34.1 (12–74) | 42 (14.9) | 182 (64.8) | 20.5 (15.9) | 30.6 (12.8) | 48.5 (22.2) | 68.2 (12.6) | 7.2 (1.6) | 127 (45.2) | 16.2 (7.0) | |||
| Upadacitinib + TCS | 30 mg QD oral | 285 | 155 (54.4) | 33.6 (12–75) | 42 (14.7) | 191 (67.0) | 20.4 (14.3) | 29.0 (11.1) | 47.0 (22.0) | 67.3 (12.5) | 7.3 (1.5) | 131 (46.0) | 16.4 (7.0) | |||
| Measure Up 2 **, *** [14] | 2022 | 3 | Placebo | QD oral | 278 [241] | 154 (55.4) | 33.4 (13–71) | 36 (12.9) | 195 (70.1) | 21.1 (13.6) | 29.1 (12.1) | 47.6 (22.7) | 67.9 (12.1) | 7.3 (1.6) | 153 (55.0) | 17.1 (7.2) |
| Upadacitinib + TCS | 15 mg QD oral | 276 | 155 (56.2) | 33.3 (12–74) | 33 (12.0) | 184 (66.7) | 18.8 (13.3) | 28.6 (11.7) | 45.1 (22.4) | 66.6 (12.5) | 7.2 (1.6) | 150 (54.3) | 16.9 (7.0) | |||
| Upadacitinib + TCS | 30 mg QD oral | 282 | 162 (57.4) | 34.1 (12–75) | 35 (12.4) | 198 (70.2) | 20.8 (14.3) | 29.7 (12.2) | 47.0 (23.2) | 66.7 (13.0) | 7.3 (1.6) | 156 (55.3) | 16.7 (6.9) | |||
| Publication Data | Study Design | Efficacy (w52) | Safety (w52) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study ID | Year | Phase | Agent | Dosing, Schedule, Route | n | n 16w | EASI 50 | EASI 75 | EASI 90 | EASI 100 | vIGA-AD 0/1 | Mean Reduction DLQI | WP-NRS Improvement ≥4 | At Least One AE | At Least One Serious AE | At Least One Infectious AE | Withdrawal Due to AE |
| LIBERTY AD CHRONOS [10] | 2017 | 3 | Placebo + TCS | QW sc | E = 264 S = 315 | 29.9% | 21.6% | 15.5% | NR | 12.5% | −5.6 (0.36) | 12.9% (32/249) | 266 (84.4) | 16 (5.1) | 182 (57.8) | 24 (7.6) | |
| Dupilumab + TCS | 300 mg Q2W sc | E = 89 S = 110 | 78.7% * | 65.2% * | 50.6% * | NR | 36.0% * | −10.9 (0.59) * | 51.2% (44/86) * | 97 (88.2) | 4 (3.6) | 63 (57.3) | 2 (1.8) | ||||
| Dupilumab + TCS | 300 mg QW sc | E = 270 S = 315 | 70.0% * | 64.1% * | 50.7% * | NR | 40.0% * | −10.7 (0.36) * | 39.0% (97/249) * | 261 (82.9) | 9 (2.9) | 166 (52.7) | 9 (2.9) | ||||
| ECZTRA-1 [11] | 2020 | 3 | Placebo | 199 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Tralokinumab | 300 mg Q2W sc | 603 | Placebo n = 35 | NR | 33.3% | NR | NR | 47.4% | NR | NR | 25 (71.4) | 0 | NR | 0 | |||
| Q2W n = 68 | 59.6% | 51.3% | 54 (79.4) | 1 (1.5) | 1 (1.5) | ||||||||||||
| Q4W n = 76 | 49.1% | 38.9% | 53 (69.7) | 3 (3.9) | 1 (1.3) | ||||||||||||
| ECZTRA-2 [11] | 2020 | 3 | Placebo | 201 | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ||
| Tralokinumab | 300 mg Q2W sc | 593 | Placebo n = 46 | NR | 21.4% | NR | NR | 25.0% | NR | NR | 32 (69.6) | 0 | NR | 0 | |||
| Q2W n = 91 | 55.8% * | 59.3% * | 62 (68.1) | 0 | 2 (2.2) | ||||||||||||
| Q4W n = 89 | 51.4% * | 44.9% | 56 (62.9) | 3 (3.4) | 1 (1.) | ||||||||||||
| BREEZE-AD3 [12] | 2021 | 3 | Baricitinib | 2 mg QD oral | 216 | 54 | NR | 81.5% | NR | NR | 59.3% | −7.9 (7.9) | NR | NR | NR | NR | NR |
| Baricitinib | 4 mg QD oral | 216 | 70 | NR | 55.7% | NR | NR | 47.1% | −7.1 (6.7) | NR | NR | NR | NR | NR | |||
| AD Up [13] | 2021 | 3 | Placebo + TCS Upadacitinib + TCS | 15 mg QD oral | 144 | NR | 79.1% (71.7–86.6) | 60.8% (51.8–69.8) | 27.0% (18.9–35.1) | 56.9% (47.8–66.0) | NR | 61.3% (52.2–70.3) | 338.0 E/100 PY | 8.0 E/100 PY | NR | 20/443 (4.5) | |
| Upadacitinib + TCS | 15 mg QD oral | 300 | 289 | NR | 50.8% (45.1–56.5) | 37.7% (32.1–43.3) | 13.1% (9.2–16.9) | 33.5% (28.1–38.9%) | NR | 45.3% (39.5–51.0) | |||||||
| Placebo + TCS Upadacitinib + TCS | 30 mg QD oral | 139 | NR | 84.7% (77.3–92.1) | 71.8% (62.2–81.5%) | 26.3% (17.3–35.3) | 65.5% (55.7–75.2) | NR | 70.7% (61.3–80.2) | 346.6 E/100 PY | 8.1 E/100 PY | NR | 20/436 (4.6) | ||||
| Upadacitinib + TCS | 30 mg QD oral | 297 | 287 | NR | 69.0% (63.7–74.3) | 55.4% (49.7–61.2) | 23.6% (18.8–28.5) | 45.2% (39.5–50.9) | NR | 57.5% (51.8–63.2) | |||||||
| Measure Up 1 [14] | 2022 | 3 | Placebo + TCS Upadacitinib + TCS (w16) | 15 mg QD oral | 121 | NR | NR | NR | NR | NR | NR | NR | 262.4 E/100 PY | 6.5 E/100 PY | NR | 22 (5.5) | |
| Upadacitinib + TCS | 15 mg QD oral | 281 | NR | 82.0% (77.0–86.9) | 62.7% (56.5–68.9) | 27.9% (22.1–33.7) | 59.2% (52.9–65.5) | NR | 67.3% (61.1–73.4) | ||||||||
| Placebo + TCS Upadacitinib + TCS (w16) | 30 mg QD oral | 123 | NR | NR | NR | NR | NR | NR | NR | 330.9 E/100 PY | 10.0 E/100 PY | NR | 39 (9.6) | ||||
| Upadacitinib + TCS | 30 mg QD oral | 285 | NR | 84.9% (80.3–89.5) | 73.3% (67.6–79.0) | 35.8% (29.6–41.9) | 62.5% (56.3–68.7) | NR | 67.7% (61.6–73.7) | ||||||||
| Measure Up 2 [14] | 2022 | 3 | Placebo + TCS Upadacitinib + TCS (w16) | 15 mg QD oral | 120 | NR | NR | NR | NR | NR | NR | NR | 240.9 E/100 PY | 7.1 E/100 PY | NR | 21 (5.3) | |
| Upadacitinib + TCS | 15 mg QD oral | 276 | NR | 79.1% (73.9–84.4) | 61.3% (55.0–67.6) | 27.8% (22.0–33.6) | 52.6% (46.2–59.1) | NR | 62.4% (56.1–68.7) | ||||||||
| Placebo + TCS Upadacitinib + TCS (w16) | 30 mg QD oral | 121 | NR | NR | NR | NR | NR | NR | NR | 270.9 E/100 PY | 6.9 E/100 PY | NR | 31 (7.7) | ||||
| Upadacitinib + TCS | 30 mg QD oral | 282 | NR | 84.3 (79.6–89.0) | 70.3% (64.4–76.2) | 35.8% (29.6–42.0) | 65.1% (58.9–71.2) | NR | 72.9% (67.1–78.7) | ||||||||
3.4. Safety
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Eichenfield, L.F.; Tom, W.L.; Chamlin, S.L.; Feldman, S.R.; Hanifin, J.M.; Simpson, E.L.; Berger, T.G.; Bergman, J.N.; Cohen, D.E.; Cooper, K.D.; et al. Guidelines of care for the management of atopic dermatitis: Section 1. Diagnosis and assessment of atopic dermatitis Work Group. J. Am. Acad. Dermatol. 2014, 70, 338–351. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.H.; Patel, K.R.; Singam, V.; Rastogi, S.; Silverberg, J.I. A systematic review and meta-analysis of the prevalence and phenotype of adult-onset atopic dermatitis. J. Am. Acad. Dermatol. 2019, 80, 1526–1532.e7. [Google Scholar] [CrossRef]
- Hanifin, J.M.; Reed, M.L.; Drake, L.A.; Koo, J.; Lebwohl, M.G.; Leung, D.Y.M.; McAlister, R.O.; Pariser, D.M.; Weiss, S.T. A population-based survey of eczema prevalence in the United States. Dermatitis 2007, 18, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Sicras-Mainar, A.; Navarro-Artieda, R.; Armario-Hita, J.C. Severe atopic dermatitis in Spain: A real-life observational study. Ther. Clin. Risk Manag. 2019, 15, 1393–1401. [Google Scholar] [CrossRef] [Green Version]
- Beck, L.A.; Thaçi, D.; Hamilton, J.D.; Graham, N.M.; Bieber, T.; Rocklin, R.; Ming, J.E.; Ren, H.; Kao, R.; Simpson, E.; et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N. Engl. J. Med. 2014, 371, 130–139. [Google Scholar] [CrossRef] [Green Version]
- Gómez-García, F.; Epstein, D.; Isla-Tejera, B.; Lorente, A.; Vélez García-Nieto, A.; Ruano, J. Short-term efficacy and safety of new biological agents targeting the interleukin-23–T helper 17 pathway for moderate-to-severe plaque psoriasis: A systematic review and network meta-analysis. Br. J. Dermatol. 2017, 176, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Bafeta, A.; Trinquart, L.; Seror, R.; Ravaud, P. Reporting of results from network meta-analyses: Methodological systematic review. BMJ 2014, 348, g1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blauvelt, A.; de Bruin-Weller, M.; Gooderham, M.; Cather, J.C.; Weisman, J.; Pariser, D.; Simpson, E.L.; Papp, K.A.; Hong, H.C.; Rubel, D.; et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): A 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet 2017, 389, 2287–2303. [Google Scholar] [CrossRef]
- Wollenberg, A.; Blauvelt, A.; Guttman-Yassky, E.; Worm, M.; Lynde, C.; Lacour, J.P.; Spelman, L.; Katoh, N.; Saeki, H.; Poulin, Y.; et al. ECZTRA 1 and ECZTRA 2 study investigators. Tralokinumab for moderate-to-severe atopic dermatitis: Results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br. J. Dermatol. 2021, 184, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; Simpson, E.L.; Wollenberg, A.; Bissonnette, R.; Kabashima, K.; DeLozier, A.M.; Sun, L.; Cardillo, T.; Nunes, F.P.; Reich, K. Long-term Efficacy of Baricitinib in Adults with Moderate to Severe Atopic Dermatitis Who Were Treatment Responders or Partial Responders: An Extension Study of 2 Randomized Clinical Trials. JAMA Dermatol. 2021, 157, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Teixeira, H.D.; de Bruin-Weller, M.; Bieber, T.; Soong, W.; Kabashima, K.; Werfel, T.; Zeng, J.; Huang, X.; Hu, X.; et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD up): Results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2021, 397, 2169–2181, Erratum in Lancet 2021, 397, 2336. [Google Scholar] [CrossRef]
- Simpson, E.L.; Papp, K.A.; Blauvelt, A.; Chu, C.Y.; Hong, H.C.; Katoh, N.; Calimlim, B.M.; Thyssen, J.P.; Chiou, A.S.; Bissonnette, R.; et al. Efficacy and Safety of Upadacitinib in Patients With Moderate to Severe Atopic Dermatitis: Analysis of Follow-up Data From the Measure up 1 and Measure up 2 Randomized Clinical Trials. JAMA Dermatol. 2022, 158, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.A.; Perche, P.O.; Feldman, S.R. Therapeutic Potential of Tralokinumab in the Treatment of Atopic Dermatitis: A Review on the Emerging Clinical Data. Clin. Cosmet. Investig. Dermatol. 2022, 15, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, J.I.; De Bruin-Weller, M.; Bieber, T.; Soong, W.; Kabashima, K.; Costanzo, A.; Rosmarin, D.; Lynde, C.; Liu, J.; Gamelli, A.; et al. Upadacitinib plus topical corticosteroids in atopic dermatitis: Week 52 AD up study results. J. Allergy Clin. Immunol. 2022, 149, 977–987.e14. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayen-Rodríguez, A.; Pereyra-Rodríguez, J.-J.; Navarro-Triviño, F.J.; Alcantara-Luna, S.; Domínguez-Cruz, J.; Galán-Gutiérrez, M.; Vilar-Palomo, S.; Armario-Hita, J.C.; Ruiz-Villaverde, R. Long-Term Effectiveness and Safety of Biologic and Small Molecule Drugs for Moderate to Severe Atopic Dermatitis: A Systematic Review. Life 2022, 12, 1159. https://doi.org/10.3390/life12081159
Ayen-Rodríguez A, Pereyra-Rodríguez J-J, Navarro-Triviño FJ, Alcantara-Luna S, Domínguez-Cruz J, Galán-Gutiérrez M, Vilar-Palomo S, Armario-Hita JC, Ruiz-Villaverde R. Long-Term Effectiveness and Safety of Biologic and Small Molecule Drugs for Moderate to Severe Atopic Dermatitis: A Systematic Review. Life. 2022; 12(8):1159. https://doi.org/10.3390/life12081159
Chicago/Turabian StyleAyen-Rodríguez, Angela, José-Juan Pereyra-Rodríguez, Francisco J. Navarro-Triviño, Sara Alcantara-Luna, Javier Domínguez-Cruz, Manuel Galán-Gutiérrez, Samuel Vilar-Palomo, Jose Carlos Armario-Hita, and Ricardo Ruiz-Villaverde. 2022. "Long-Term Effectiveness and Safety of Biologic and Small Molecule Drugs for Moderate to Severe Atopic Dermatitis: A Systematic Review" Life 12, no. 8: 1159. https://doi.org/10.3390/life12081159
APA StyleAyen-Rodríguez, A., Pereyra-Rodríguez, J.-J., Navarro-Triviño, F. J., Alcantara-Luna, S., Domínguez-Cruz, J., Galán-Gutiérrez, M., Vilar-Palomo, S., Armario-Hita, J. C., & Ruiz-Villaverde, R. (2022). Long-Term Effectiveness and Safety of Biologic and Small Molecule Drugs for Moderate to Severe Atopic Dermatitis: A Systematic Review. Life, 12(8), 1159. https://doi.org/10.3390/life12081159











