Immune-Checkpoint-Inhibitor-Related Lung Toxicity: A Multicentre Real-Life Retrospective Portrait from Six Italian Centres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Pulmonary Function Tests
2.3. High-Resolution Computed Tomography
2.4. Bronchoalveolar Lavage
2.5. Statistical Analysis
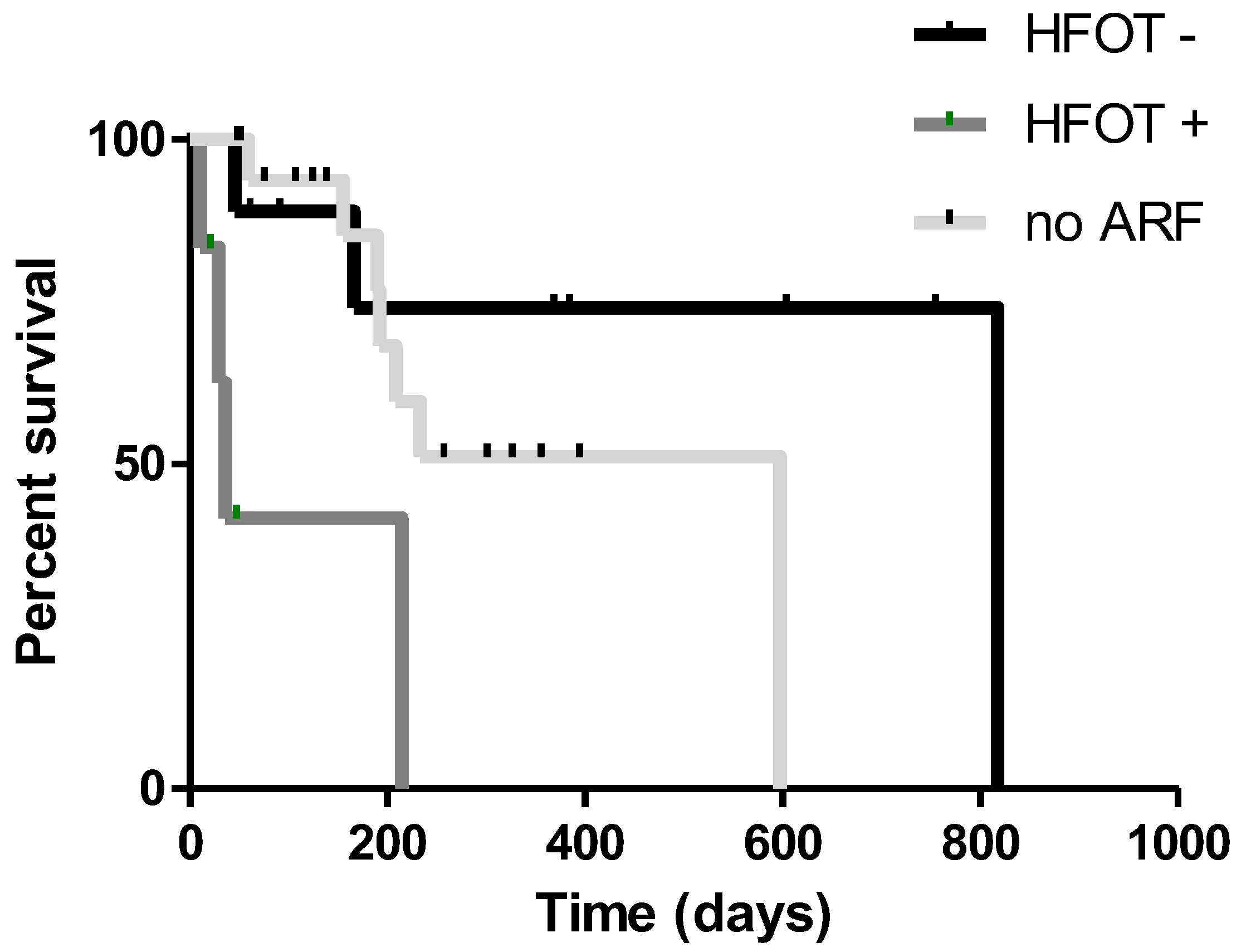
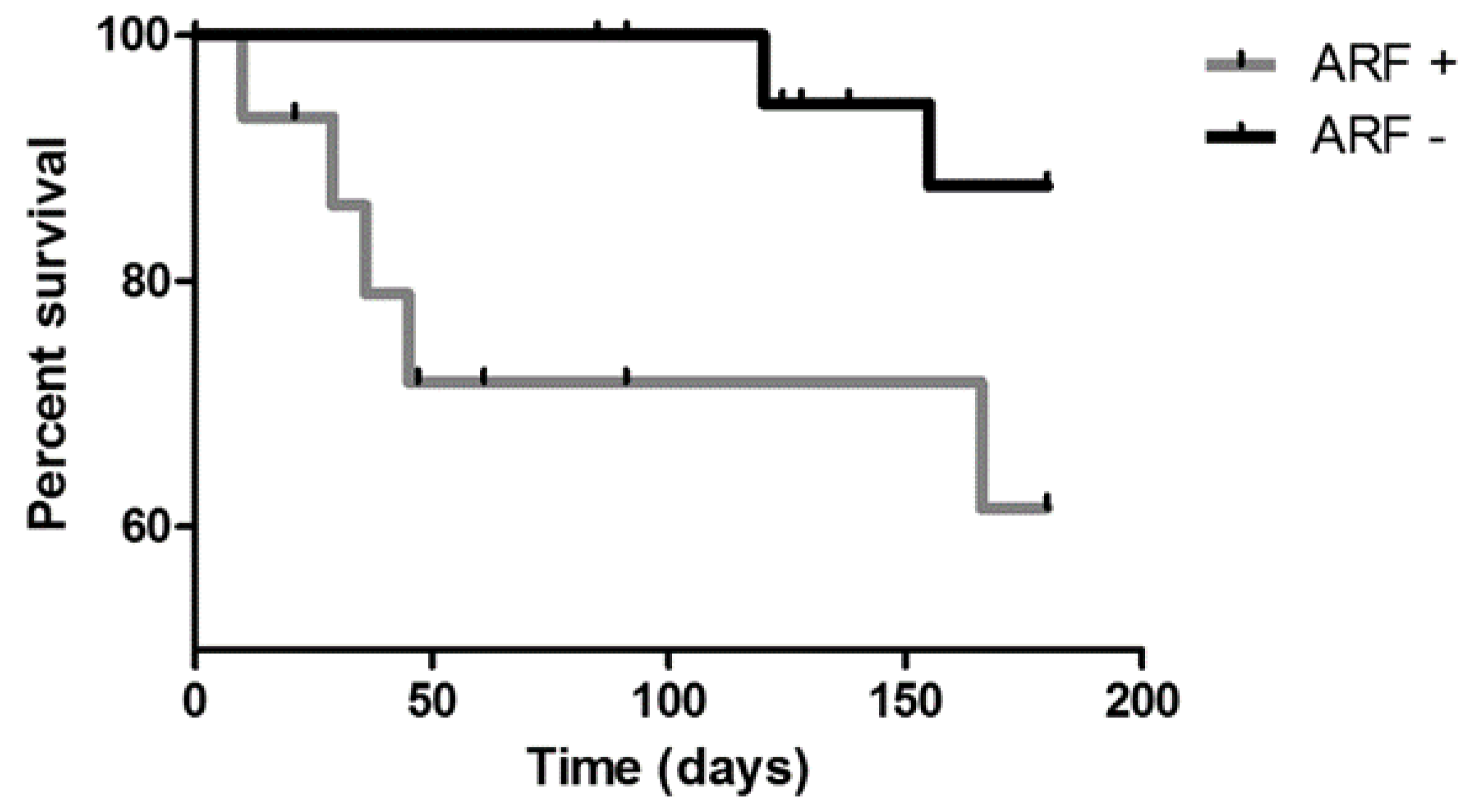
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skeoch, S.; Weatherley, N.; Swift, A.J.; Oldroyd, A.; Johns, C.; Hayton, C.; Giollo, A.; Wild, J.M.; Waterton, J.C.; Buch, M.; et al. Drug-Induced Interstitial Lung Disease: A Systematic Review. J. Clin. Med. 2018, 7, 356. [Google Scholar] [CrossRef] [Green Version]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef]
- Nishino, M.; Brais, L.K.; Brooks, N.V.; Hatabu, H.; Kulke, M.H.; Ramaiya, N.H. Drug-related pneumonitis during mammalian target of rapamycin inhibitor therapy in patients with neuroendocrine tumors: A radiographic pattern-based approach. Eur. J. Cancer Oxf. Engl. 1990, 53, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Qi, W.-X.; Sun, Y.-J.; Shen, Z.; Yao, Y. Risk of interstitial lung disease associated with EGFR-TKIs in advanced non-small-cell lung cancer: A meta-analysis of 24 phase III clinical trials. J. Chemother. Florence Italy 2015, 27, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Tang, J.; Tong, L.; Liu, Z. Risk of interstitial lung disease with gefitinib and erlotinib in advanced non-small cell lung cancer: A systematic review and meta-analysis of clinical trials. Lung Cancer Amst. Neth. 2014, 83, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.; Johnson, D.; Temin, S.; Baker, S.; Brahmer, J.; Ellis, P.M.; Giaccone, G.; Hesketh, P.J.; Jaiyesimi, I.; Leighl, N.B.; et al. Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 3484–3515. [Google Scholar] [CrossRef]
- Naidoo, J.; Page, D.B.; Li, B.T.; Connell, L.C.; Schindler, K.; Lacouture, M.E.; Postow, M.A.; Wolchok, J.D. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 2375–2391. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, J.; Wang, X.; Woo, K.M.; Iyriboz, T.; Halpenny, D.; Cunningham, J.; Jamie, E.C.; Segal, N.H.; Callahan, M.K.; Lesokhin, A.M.; et al. Pneumonitis in Patients Treated With Anti-Programmed Death-1/Programmed Death Ligand 1 Therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 709–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishino, M.; Giobbie-Hurder, A.; Hatabu, H.; Ramaiya, N.H.; Hodi, F.S. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2016, 2, 1607–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margolin, K.; Ernstoff, M.S.; Hamid, O.; Lawrence, D.; McDermott, D.; Puzanov, I.; Wolchok, J.D.; Clark, J.I.; Sznol, M.; Logan, T.F.; et al. Ipilimumab in patients with melanoma and brain metastases: An open-label, phase 2 trial. Lancet Oncol. 2012, 13, 459–465. [Google Scholar] [CrossRef]
- Chuzi, S.; Tavora, F.; Cruz, M.; Costa, R.; Chae, Y.K.; Carneiro, B.A.; Giles, F.J. Clinical features, diagnostic challenges, and management strategies in checkpoint inhibitor-related pneumonitis. Cancer Manag. Res. 2017, 9, 207–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delaunay, M.; Prévot, G.; Collot, S.; Guilleminault, L.; Didier, A.; Mazières, J. Management of pulmonary toxicity associated with immune checkpoint inhibitors. Eur. Respir. Rev. Off. J. Eur. Respir. Soc. 2019, 28, 190012. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, S.; Yang, F.; Qi, X.; Wang, X.; Guan, X.; Shen, C.; Duma, N.; Aguilera, J.V.; Chintakuntlawar, A.; et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Masuda, N.; Nakanishi, Y.; Takahashi, M.; Hida, T.; Sakai, H.; Atagi, S.; Fujita, S.; Tanaka, H.; Takeda, K.; et al. Nivolumab-induced interstitial lung disease analysis of two phase II studies patients with recurrent or advanced non-small-cell lung cancer. Lung Cancer Amst. Neth. 2017, 104, 111–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanai, O.; Kim, Y.H.; Demura, Y.; Kanai, M.; Ito, T.; Fujita, K.; Yoshida, H.; Alkai, M.; Mio, T.; Hirai, T. Efficacy and safety of nivolumab in non-small cell lung cancer with preexisting interstitial lung disease. Thorac. Cancer 2018, 9, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Lacchetti, C.; Schneider, B.J.; Atkins, M.B.; Brassil, K.J.; Caterino, J.M.; Chau, I.; Ernstoff, M.S.; Gardner, J.M.; Ginex, P.; et al. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 1714–1768. [Google Scholar] [CrossRef] [PubMed]
- Savarese, D.M. Common Terminology Criteria for Adverse Events (CTCAE); UpToDate: Waltham, MA, USA, 2013; p. 196. [Google Scholar]
- Haanen, J.B.A.G.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K. ESMO Guidelines Committee. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29 (Suppl. S4), iv264–iv266. [Google Scholar] [CrossRef]
- Miller, M.R.; Crapo, R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Enright, P.; van der Grinten, C.P.M.; Gustafsson, P.; et al. General considerations for lung function testing. Eur. Respir. J. 2005, 26, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; MacIntyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS standards for single-breath carbon monoxide uptake in the lung. Eur. Respir. J. 2017, 49, 1600016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, K.C.; Raghu, G.; Baughman, R.P.; Brown, K.K.; Costabel, U.; du Bois, R.M.; Drent, M.; Haslam, P.L.; Kim, D.S.; Nagai, S.; et al. An official American Thoracic Society clinical practice guideline: The clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am. J. Respir. Crit. Care Med. 2012, 185, 1004–1014. [Google Scholar] [CrossRef]
- Barjaktarevic, I.Z.; Qadir, N.; Suri, A.; Santamauro, J.T.; Stover, D. Organizing pneumonia as a side effect of ipilimumab treatment of melanoma. Chest 2013, 143, 858–861. [Google Scholar] [CrossRef]
- Akella, P.; Loganathan, S.; Jindal, V.; Akhtar, J.; Lal, A. Anti PD-1 immunotherapy related interstitial lung disease presenting as respiratory failure—A review with case series. Respir. Med. Case Rep. 2019, 2, 17–22. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Ramaiya, N.H.; Awad, M.M.; Sholl, L.M.; Maattala, J.A.; Taibi, M.; Hatabu, H.; Ott, P.A.; Armand, P.F.; Hodi, F.S. PD-1 Inhibitor-Related Pneumonitis in Advanced Cancer Patients: Radiographic Patterns and Clinical Course. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 6051–6060. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Ota, T.; Hayashi, M.; Ishikawa, H.; Otsubo, A.; Shoji, S.; Nozaki, K.; Ichikawa, K.; Kondo, R.; Miyabayashi, T.; et al. Prognostic significance of radiologic features of pneumonitis induced by anti-PD-1 therapy. Cancer Med. 2020, 9, 3070–3077. [Google Scholar] [CrossRef] [Green Version]
- Pneumotox » Drug [Internet]. Available online: https://www.pneumotox.com/drug/index/ (accessed on 25 December 2019).
- Johkoh, T.; Lee, K.S.; Nishino, M.; Travis, W.D.; Ryu, J.H.; Lee, H.Y.; Ryerson, C.J.; Franquet, T.; Bankier, A.A.; Brown, K.K.; et al. Chest CT Diagnosis and Clinical Management of Drug-Related Pneumonitis in Patients Receiving Molecular Targeting Agents and Immune Checkpoint Inhibitors: A Position Paper From the Fleischner Society. Chest 2021, 159, 1107–1125. [Google Scholar] [CrossRef]
- Possick, J.D. Pulmonary Toxicities from Checkpoint Immunotherapy for Malignancy. Clin. Chest Med. 2017, 38, 223–232. [Google Scholar] [CrossRef]
- Delaunay, M.; Cadranel, J.; Lusque, A.; Meyer, N.; Gounant, V.; Moro-Sibilot, D.; Michot, J.-M.; Raimbourg, J.; Girard, N.; Guisier, F.; et al. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur. Respir. J. 2017, 50, 1700050. [Google Scholar] [CrossRef]


| Study Population | |
|---|---|
| N° | 41 |
| Male (%) | 25 (60.9) |
| Age (yrs) | 66.8 ± 9.9 |
| BMI (kg/m2) | 26.6 ± 4.6 |
| Smoking history (pack-years) | 31.2 ± 24.6 |
| Current: number (%) | 8 (19.5) |
| Former: number (%) | 26 (63.4) |
| Never: number (%) | 7 (17.1) |
| Clinical onset | |
| Asymptomatic (%) | 13 (31.7) |
| Cough (%) | 8 (19.5) |
| Dyspnea (%) | 22 (53.6) |
| Fever (%) | 4 (9.7) |
| Respiratory failure (%) | 15 (36.5) |
| Grading Lung toxicity | |
| 1 (%) | 14 (34.1) |
| 2 (%) | 12 (29.2) |
| 3 (%) | 9 (21.9) |
| 4 (%) | 6 (14.6) |
| PFTs (N° patients) | 15 |
| FVC l (% predicted value) | 2.2 ± 0.6 (80.5 ± 17.9) |
| FEV1 l (% predicted value) | 1.7 ± 0.6 (75.3 ± 23.9) |
| FEV1/FVC | 71.5 ± 12.6 |
| DLCO (% predicted value) | 52.7 ± 15.4 |
| Therapy status | |
| Nivolumab—1st line (%) | 2 (4.8) |
| Nivolumab—2nd line (%) | 25 (60.9) |
| Nivolumab—3rd line (%) | 5 (12.2) |
| Pembrolizumab—1st line (%) | 5 (12.2) |
| Pembrolizumab—2nd line (%) | 3 (7.3) |
| Nivolumab + ipilimumab—1st line (%) | 1 (2.4) |
| Predominant CT Patterns | |
|---|---|
| Organizing pneumonia (%) | 6 (14.6) |
| Ground glass opacities (%) | 23 (56) |
| HP-like parenchymal features (%) | 8 (19.5) |
| Lung fibrosis (%) | 4 (9.7) |
| Emphysema (%) | 14 (34.1) |
| Pleural effusion (%) | 3 (7.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cameli, P.; Faverio, P.; Ferrari, K.; Bonti, V.; Marsili, S.; Mazzei, M.A.; Mazzoni, F.; Bartolucci, M.; Scotti, V.; Bertolini, F.; et al. Immune-Checkpoint-Inhibitor-Related Lung Toxicity: A Multicentre Real-Life Retrospective Portrait from Six Italian Centres. Life 2022, 12, 1149. https://doi.org/10.3390/life12081149
Cameli P, Faverio P, Ferrari K, Bonti V, Marsili S, Mazzei MA, Mazzoni F, Bartolucci M, Scotti V, Bertolini F, et al. Immune-Checkpoint-Inhibitor-Related Lung Toxicity: A Multicentre Real-Life Retrospective Portrait from Six Italian Centres. Life. 2022; 12(8):1149. https://doi.org/10.3390/life12081149
Chicago/Turabian StyleCameli, Paolo, Paola Faverio, Katia Ferrari, Viola Bonti, Stefania Marsili, Maria Antonietta Mazzei, Francesca Mazzoni, Maurizio Bartolucci, Vieri Scotti, Federica Bertolini, and et al. 2022. "Immune-Checkpoint-Inhibitor-Related Lung Toxicity: A Multicentre Real-Life Retrospective Portrait from Six Italian Centres" Life 12, no. 8: 1149. https://doi.org/10.3390/life12081149
APA StyleCameli, P., Faverio, P., Ferrari, K., Bonti, V., Marsili, S., Mazzei, M. A., Mazzoni, F., Bartolucci, M., Scotti, V., Bertolini, F., Barbieri, F., Baldessari, C., Veronese, C., Boffi, R., Brighenti, M., Cortinovis, D., Dominici, M., Pesci, A., Bargagli, E., & Luppi, F. (2022). Immune-Checkpoint-Inhibitor-Related Lung Toxicity: A Multicentre Real-Life Retrospective Portrait from Six Italian Centres. Life, 12(8), 1149. https://doi.org/10.3390/life12081149










