Artificial Scaffolds in Cardiac Tissue Engineering
Abstract
1. Introduction
1.1. Lack of Self-Regenerative Capabilities of Mammalian Heart Cells
1.2. Cardiovascular Diseases
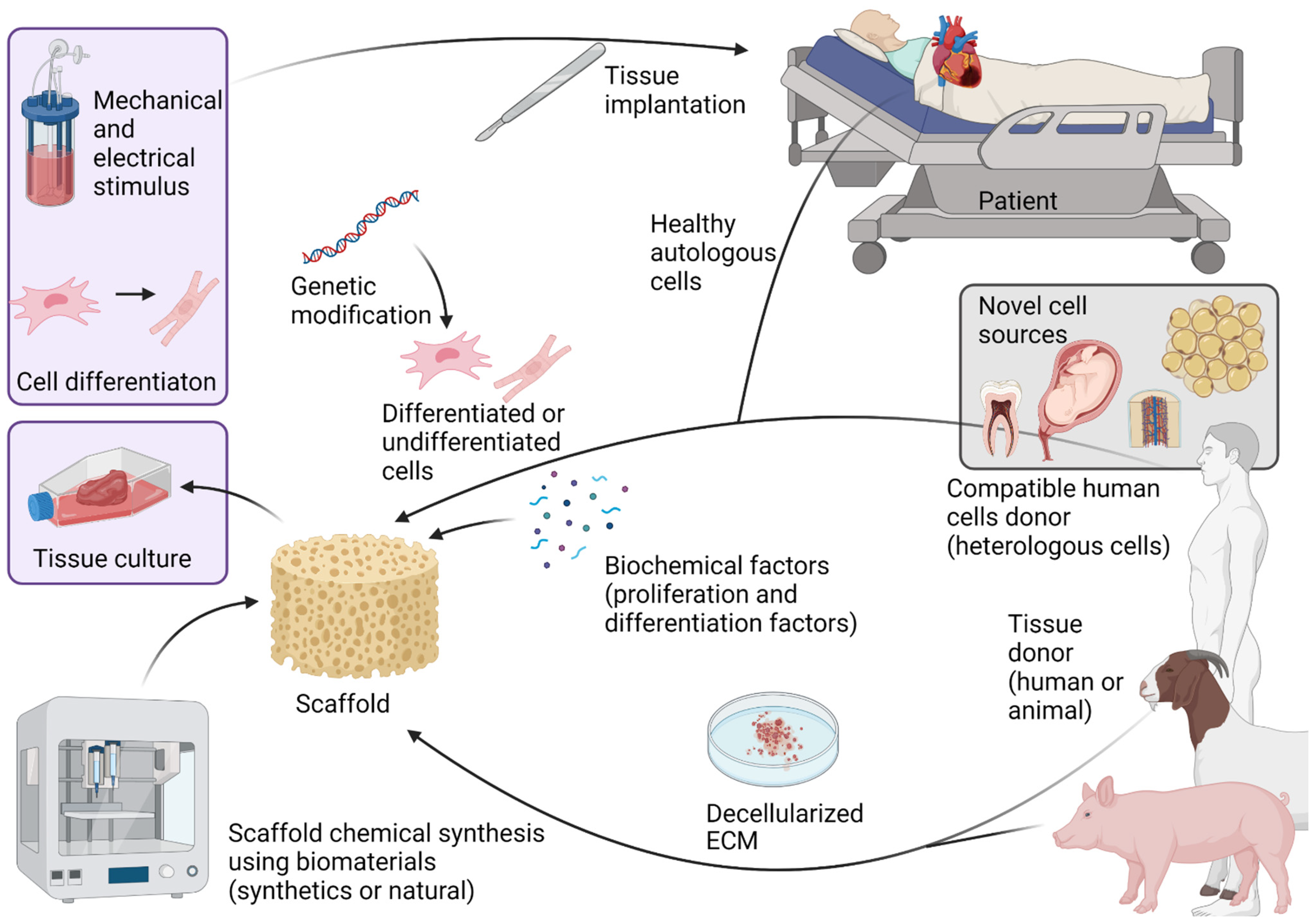
2. Cardiac Tissue Engineering
3. Cells and Growth Factors
3.1. Cells
3.2. Growth Factors
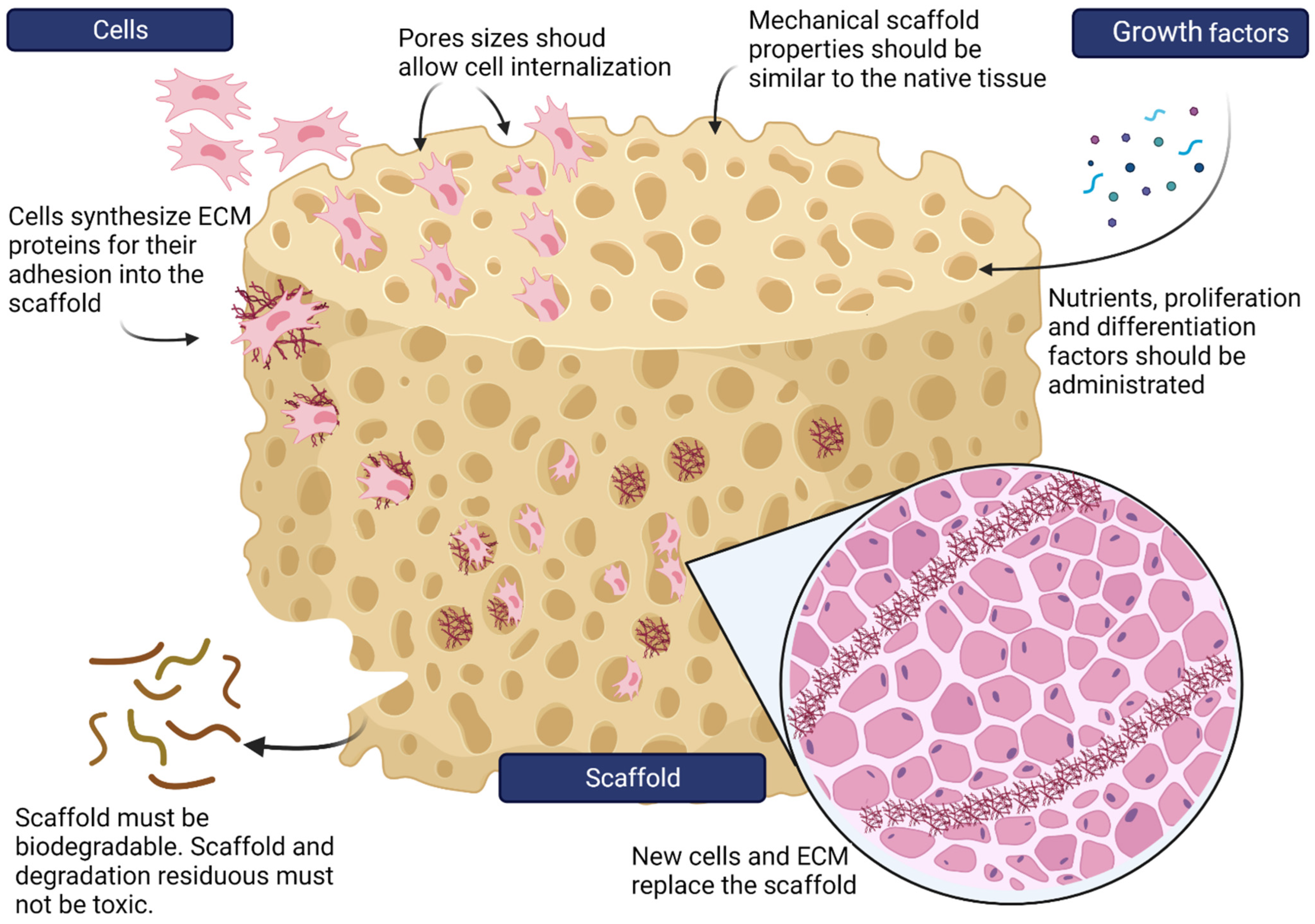
4. Scaffolds
4.1. Porosity
4.2. Topography
4.3. Surface Functionalization
4.4. Mechanical Properties
4.5. Electrical Properties
4.6. Biodegradation
4.7. Bioactivity
4.8. Non-Toxic
5. Decellularized Extracellular Matrices
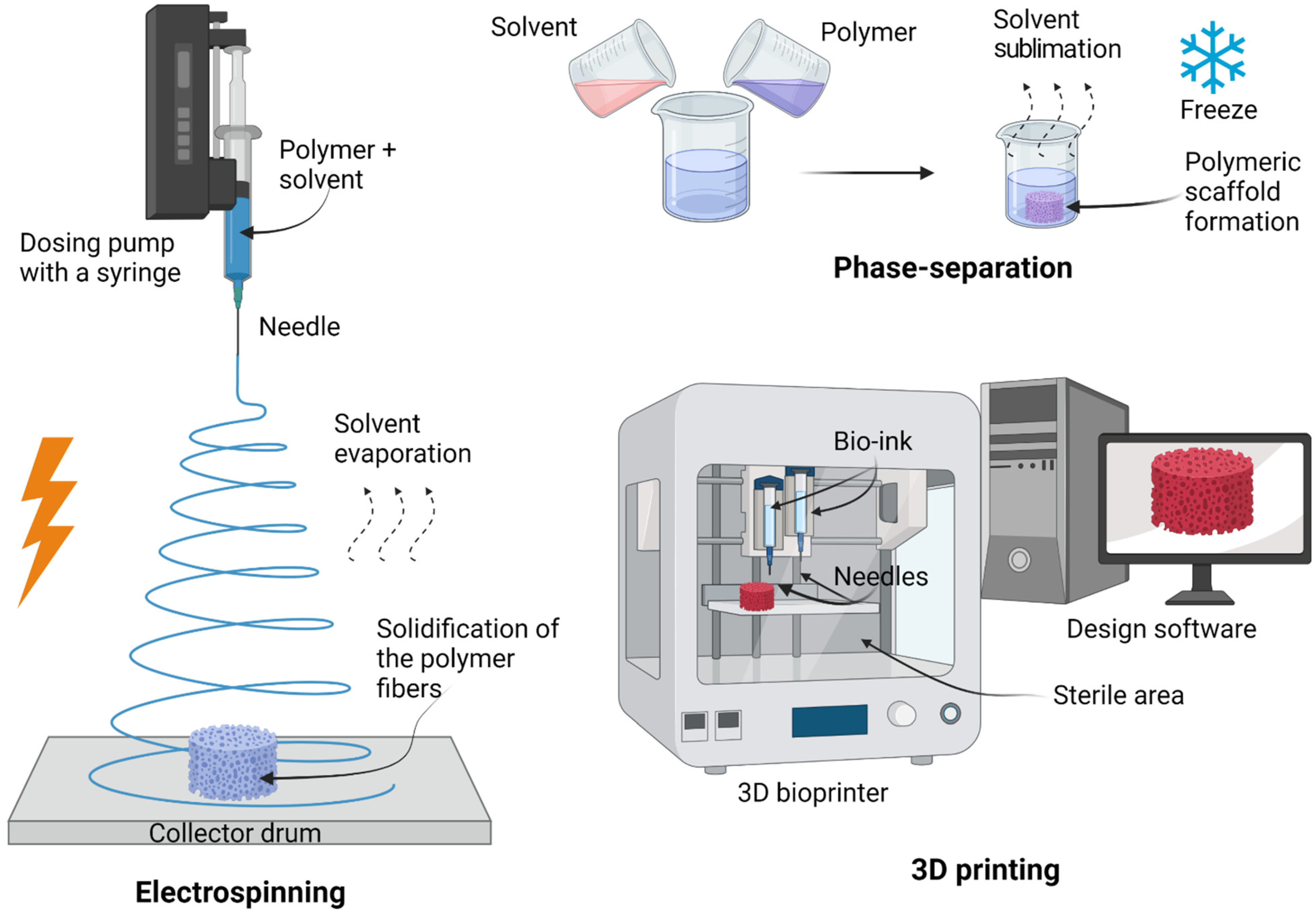
6. Biofabrication Approaches Used to Develop Artificial Scaffolds
6.1. Electrospinning
- Advantages: simple methodology, relatively low preparation cost, sample uniformity, generation of aligned fibers smaller than a micrometer, scaffold incrementation of surface-to-volume ratio (it improves cell attachment, proliferation, and differentiation), tunable porosity interconnectivity over 80%, unique pore shapes, and superior mechanical properties [70,71,75,76];
- Disadvantages: the need for high-voltage appliances and the use of toxic solvents [76].
6.2. Phase-Separation
- Advantages: simplicity of the technique, and preservation of the scaffold structure because the process does not involve high temperatures [76];
- Disadvantages: lengthy process, poor architecture, limited control of size range, irregular porosity, unsuitable mechanical properties for replacing human tissue, and remaining solvents are potentially toxic [76].
6.3. 3D Printing
- Advantages: probably the most attractive technique in terms of micro-architecture, allows the use of a wide range of biomaterials, and excellent controllability over structural properties such as porosity, pore size, and pore interconnectivity [79];
- Disadvantages: initial investment in the instrument, the use of toxic solvents, and mechanical instability [72].
6.4. Solvent Casting and Solvent Casting/Particulate Leaching
6.5. Emulsion Templating
- Emulsion templating is a promising biofabrication approach for scaffolds with up to 99% porosity and high interconnectivity. The technique is based on two steps. The first step is the preparation of an emulsion of two immiscible liquids; one phase is the internal or dispersed phase, and the other one is the external or continuous phase. The second step is the solidification of the continuous phase of the emulsion, while the dispersed phase (which works as a template by its addition drop-by-drop) is removed to obtain a porous scaffold. Emulsion can be given by water-in-oil (w/o) or oil-in-water (o/w) [83].
7. Materials Used for Artificial Scaffold Construction
7.1. Synthetic Materials for Artificial Scaffolds
7.2. Natural Biomaterials for Artificial Scaffolds
7.2.1. Collagen
7.2.2. Fibrinogen
7.2.3. Silk
7.2.4. Alginate
7.2.5. Chitosan
8. Composite Artificial Scaffolds
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CF | Cardiac fibroblast |
| CM | Cardiomyocytes |
| CS | Chitosan |
| CPC | Cardiac progenitor cells |
| EC | Endothelial cells |
| EPC | Endothelial progenitor cells |
| ECM | Extracellular matrix |
| GO | Graphene oxide |
| HSC | Hematopoietic stem cells |
| iPSC | Induced pluripotent stem cells |
| MSC | Mesenchymal stem cells |
| NHSC | Non-hematopoietic stem cells |
| PCL | Poly(ε-caprolactone) |
| PDMS | Polydimethylsiloxane |
| PEG | Polyethylene glycol |
| PGS | Poly(glycerol sebacate) |
| PLA | Polylactic acid |
| PLCL | Poly-l-lactic-co-ε-caprolactone |
| PLGA | Poly(lactic-co-glycolic acid) |
| PLLA | Poly(l-lactide acid) |
| PVA | Polyvinyl alcohol |
| RCSC | Resident cardiac stem cells |
| SMC | Smooth muscle cells |
| VSMC | Vascular smooth muscle cells |
References
- Rossi, G.; Broguiere, N.; Miyamoto, M.; Boni, A.; Guiet, R.; Girgin, M.; Kelly, R.G.; Kwon, C.; Lutolf, M.P. Capturing cardiogenesis in gastruloids. Cell Stem Cell 2021, 28, 230–240.e6. [Google Scholar] [CrossRef]
- Huang, N.F.; Serpooshan, V.; Morris, V.B.; Sayed, N.; Pardon, G.; Abilez, O.J.; Nakayama, K.H.; Pruitt, B.L.; Wu, S.M.; Yoon, Y.S.; et al. Big bottlenecks in cardiovascular tissue engineering. Commun. Biol. 2018, 1, 199. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, O.; Zdunek, S.; Felker, A.; Salehpour, M.; Alkass, K.; Bernard, S.; Sjostrom, S.L.; Szewczykowska, M.; Jackowska, T.; Dos Remedios, C.; et al. Dynamics of cell generation and turnover in the human heart. Cell 2015, 161, 1566–1575. [Google Scholar] [CrossRef]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heider, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for cardiomyocyte renewal in humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef]
- Sawabe, M. Vascular aging: From molecular mechanism to clinical significance. Geriatr. Gerontol. Int. 2010, 10 (Suppl. 1), S213–S220. [Google Scholar] [CrossRef]
- Chiu, J.J.; Chien, S. Effects of disturbed flow on vascular endothelium: Pathophysiological basis and clinical perspectives. Physiol. Rev. 2011, 91, 327–387. [Google Scholar] [CrossRef]
- Severino, P.; D’Amato, A.; Pucci, M.; Infusino, F.; Adamo, F.; Birtolo, L.I.; Netti, L.; Montefusco, G.; Chimenti, C.; Lavalle, C.; et al. Ischemic heart disease pathophysiology paradigms overview: From plaque activation to microvascular dysfunction. Int. J. Mol. Sci. 2020, 21, 8118. [Google Scholar] [CrossRef]
- Riching, A.S.; Song, K. Cardiac regeneration: New insights into the frontier of ischemic heart failure therapy. Front. Bioeng. Biotechnol. 2021, 8, 637538. [Google Scholar] [CrossRef]
- de Bakker, D.E.M.; Bouwman, M.; Dronkers, E.; Simões, F.C.; Riley, P.R.; Goumans, M.J.; Smits, A.M.; Bakkers, J. Prrx1b restricts fibrosis and promotes Nrg1-dependent cardiomyocyte proliferation during zebrafish heart regeneration. Development 2021, 148, dev198937. [Google Scholar] [CrossRef]
- Gemberling, M.; Karra, R.; Dickson, A.L.; Poss, K.D. Nrg1 is an injury-induced cardiomyocyte mitogen for the endogenous heart regeneration program in zebrafish. Elife 2015, 4, e05871. [Google Scholar] [CrossRef]
- Locatelli, P.; Giménez, C.S.; Vega, M.U.; Crottogini, A.; Belaich, M.N. Targeting the cardiomyocyte cell cycle for heart regeneration. Curr. Drug Targets 2019, 20, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Arias, A.G.; Bobadilla-Serrano, M.E.; Dimas-Altamirano, B.; Gómez-Ortega, M.; González-González, G. Enfermedad cardiovascular: Primera causa de morbilidad en un hospital de tercer nivel. Rev. Mex. Cardiol. 2016, 27 (Suppl. 3), 98–102. [Google Scholar]
- Francula-Zaninovic, S.; Nola, I.A. Management of measurable variable cardiovascular disease’ risk factors. Curr. Cardiol. Rev. 2018, 14, 153–163. [Google Scholar] [CrossRef]
- European Association for Cardiovascular Prevention & Rehabilitation; Reiner, Z.; Catapano, A.L.; De Backer, G.; Graham, I.; Taskinen, M.R.; Wiklund, O.; Agewall, S.; Alegria, E.; Chapman, M.J.; et al. ESC Committee for Practice Guidelines (CPG) 2008–2010 and 2010–2012 Committees. ESC/EAS Guidelines for the manage-ment of dyslipidaemias: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur. Heart J. 2011, 32, 1769–1818. [Google Scholar] [CrossRef] [PubMed]
- Costantino, S.; Paneni, F.; Cosentino, F. Ageing, metabolism and cardiovascular disease. J. Physiol. 2016, 594, 2061–2073. [Google Scholar] [CrossRef]
- Shufelt, C.L.; Pacheco, C.; Tweet, M.S.; Miller, V.M. Sex-specific physiology and cardiovascular disease. Adv. Exp. Med. Biol. 2018, 1065, 433–454. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhang, C.; Cheng, M.; Huang, J.; Liu, Q.; Yuan, G.; Lin, K.; Yu, H. Challenges and strategies for in situ endothelialization and long-term lumen patency of vascular grafts. Bioact. Mater. 2020, 6, 1791–1809. [Google Scholar] [CrossRef]
- Müller, P.; Lemcke, H.; David, R. Stem cell therapy in heart diseases—cell types, mechanisms and improvement strategies. Cell Physiol. Biochem. 2018, 48, 2607–2655. [Google Scholar] [CrossRef]
- Miyamoto, M.; Nam, L.; Kannan, S.; Kwon, C. Heart organoids and tissue models for modeling development and disease. Semin. Cell Dev. Biol. 2021, 118, 119–128. [Google Scholar] [CrossRef]
- Davidson, S.M.; Padró, T.; Bollini, S.; Vilahur, G.; Duncker, D.J.; Evans, P.C.; Guzik, T.; Hoefer, I.E.; Waltenberger, J.; Wojta, J.; et al. Progress in cardiac research: From rebooting cardiac regeneration to a complete cell atlas of the heart. Cardiovasc. Res. 2021, 117, 2161–2174. [Google Scholar] [CrossRef]
- Voges, H.K.; Mills, R.J.; Elliott, D.A.; Parton, R.G.; Porrello, E.R.; Hudson, J.E. Development of a human cardiac organoid injury model reveals innate regenerative potential. Development 2017, 144, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, E.; Bellin, M.; Sala, L.; van Meer, B.J.; Tertoolen, L.G.; Orlova, V.V.; Mummery, C.L. Three-dimensional cardiac microtissues composed of cardiomyocytes and endothelial cells co-differentiated from human pluripotent stem cells. Development 2017, 144, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Tavakol, D.N.; Fleischer, S.; Vunjak-Novakovic, G. Harnessing organs-on-a-chip to model tissue regeneration. Cell Stem Cell 2021, 28, 993–1015. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Dan, P.; Hasan, A.; Khalaf, I.M.; Prasad, P.; Ghosal, K.; Gentile, C.; McClements, L.; Maureira, P. Stem cell-based approaches in cardiac tissue engineering: Controlling the microenvironment for autologous cells. Biomed. Pharmacother 2021, 138, 111425. [Google Scholar] [CrossRef]
- Campostrini, G.; Windt, L.M.; van Meer, B.J.; Bellin, M.; Mummery, C.L. Cardiac tissues from stem cells: New routes to maturation and cardiac regeneration. Circ. Res. 2021, 128, 775–801. [Google Scholar] [CrossRef]
- Montero, P.; Flandes-Iparraguirre, M.; Musquiz, S.; Pérez Araluce, M.; Plano, D.; Sanmartín, C.; Orive, G.; Gavira, J.J.; Prosper, F.; Mazo, M.M. Cells, materials, and fabrication processes for cardiac tissue engineering. Front. Bioeng. Biotechnol. 2020, 8, 955. [Google Scholar] [CrossRef]
- Alrefai, M.T.; Murali, D.; Paul, A.; Ridwan, K.M.; Connell, J.M.; Shum-Tim, D. Cardiac tissue engineering and regeneration using cell-based therapy. Stem Cells Cloning 2015, 8, 81–101. [Google Scholar] [CrossRef][Green Version]
- Roura, S.; Pujal, J.M.; Gálvez-Montón, C.; Bayes-Genis, A. Impact of umbilical cord blood-derived mesenchymal stem cells on cardiovascular research. Biomed. Res. Int. 2015, 2015, 975302. [Google Scholar] [CrossRef]
- Sun, Q.; Zhang, Z.; Sun, Z. The potential and challenges of using stem cells for cardiovascular repair and regeneration. Genes Dis. 2014, 1, 113–119. [Google Scholar] [CrossRef]
- Bruun, K.; Schermer, E.; Sivendra, A.; Valaik, E.; Wise, R.B.; Said, R.; Bracht, J.R. Therapeutic applications of adipose-derived stem cells in cardiovascular disease. Am. J. Stem. Cells 2018, 7, 94–103. [Google Scholar]
- Arjmand, B.; Abedi, M.; Arabi, M.; Alavi-Moghadam, S.; Rezaei-Tavirani, M.; Hadavandkhani, M.; Tayanloo-Beik, A.; Kordi, R.; Roudsari, P.P.; Larijani, B. Regenerative medicine for the treatment of ischemic heart disease; status and future perspectives. Front. Cell Dev. Biol. 2021, 9, 704903. [Google Scholar] [CrossRef] [PubMed]
- Chopra, H.; Hung, M.K.; Kwong, D.L.; Zhang, C.F.; Pow, E.H.N. Insights into endothelial progenitor cells: Origin, classification, potentials, and prospects. Stem Cells Int. 2018, 2018, 9847015. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Qi, X.; Han, Z.; Liang, L.; Kong, D.; Han, Z.; Zhao, S.; He, Z.X.; Li, Z. Bone marrow is a reservoir for cardiac resident stem cells. Sci. Rep. 2016, 6, 28739. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Zhang, E.; Joshi, J.; Yang, J.; Zhang, J.; Zhu, W. Utilization of human induced pluripotent stem cells for cardiac repair. Front. Cell Dev. Biol. 2020, 8, 36. [Google Scholar] [CrossRef]
- Chen, W.; Bian, W.; Zhou, Y.; Zhang, J. Cardiac fibroblasts and myocardial regeneration. Front. Bioeng. Biotechnol. 2021, 9, 599928. [Google Scholar] [CrossRef]
- Durrani, S.; Konoplyannikov, M.; Ashraf, M.; Haider, K.H. Skeletal myoblasts for cardiac repair. Regen. Med. 2010, 5, 919–932. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Ieda, M.; Fu, J.D.; Delgado-Olguin, P.; Vedantham, V.; Hayashi, Y.; Bruneau, B.G.; Srivastava, D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 2010, 142, 375–386. [Google Scholar] [CrossRef]
- Burridge, P.W.; Keller, G.; Gold, J.D.; Wu, J.C. Production of de novo cardiomyocytes: Human pluripotent stem cell differentiation and direct reprogramming. Cell Stem Cell 2012, 10, 16–28. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Diaz, A.D.; Benham, A.; Xu, X.; Wijaya, C.S.; Fa’ak, F.; Luo, W.; Soibam, B.; Azares, A.; et al. Mesp1 marked cardiac progenitor cells repair infarcted mouse hearts. Sci. Rep. 2016, 6, 31457. [Google Scholar] [CrossRef]
- Chiapparo, G.; Lin, X.; Lescroart, F.; Chabab, S.; Paulissen, C.; Pitisci, L.; Bondue, A.; Blanpain, C. Mesp1 controls the speed, polarity, and directionality of cardiovascular progenitor migration. J. Cell Biol. 2016, 213, 463–477. [Google Scholar] [CrossRef]
- Song, K.; Nam, Y.J.; Luo, X.; Qi, X.; Tan, W.; Huang, G.N.; Acharya, A.; Smith, C.L.; Tallquist, M.D.; Neilson, E.G.; et al. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature 2012, 485, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.; Hashimoto, H.; Zhou, H.; Morales, M.G.; Chen, B.; Bassel-Duby, R.; Olson, E.N. Notch inhibition enhances cardiac reprogramming by increasing MEF2C transcriptional activity. Stem Cell Rep. 2017, 8, 548–560. [Google Scholar] [CrossRef] [PubMed]
- den Hartogh, S.C.; Wolstencroft, K.; Mummery, C.L.; Passier, R. A comprehensive gene expression analysis at sequential stages of in vitro cardiac differentiation from isolated MESP1-expressing-mesoderm progenitors. Sci. Rep. 2016, 6, 19386. [Google Scholar] [CrossRef]
- Singh, V.P.; Pinnamaneni, J.P.; Pugazenthi, A.; Sanagasetti, D.; Mathison, M.; Wang, K.; Yang, J.; Rosengart, T.K. Enhanced generation of induced cardiomyocytes using a small-molecule cocktail to overcome barriers to cardiac cellular Reprogramming. J. Am. Heart Assoc. 2020, 9, e015686. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, T.M.; Egemnazarov, B.; Finch, E.A.; Zhang, L.; Payne, J.A.; Pandya, K.; Zhang, Z.; Rosenberg, P.; Mirotsou, M.; Dzau, V.J. MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ. Res. 2012, 110, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Raso, A.; Dirkx, E.; Sampaio-Pinto, V.; El Azzouzi, H.; Cubero, R.J.; Sorensen, D.W.; Ottaviani, L.; Olieslagers, S.; Huibers, M.M.; de Weger, R.; et al. A microRNA program regulates the balance between cardiomyocyte hyperplasia and hypertrophy and stimulates cardiac regeneration. Nat. Commun. 2021, 12, 4808. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Yang, Y.; Jackson, L.; Ahmed, R.P. MicroRNAs regulating meis1 expression and inducing cardiomyocyte proliferation. Cardiovasc. Regen. Med. 2016, 3, e1468. [Google Scholar]
- Ellison-Hughes, G.M.; Madeddu, P. Exploring pericyte and cardiac stem cell secretome unveils new tactics for drug discovery. Pharmacol. Ther. 2017, 171, 1–12. [Google Scholar] [CrossRef]
- Rezaei, F.S.; Sharifianjazi, F.; Esmaeilkhanian, A.; Salehi, E. Chitosan films and scaffolds for regenerative medicine applications: A review. Carbohydr. Polym. 2021, 273, 118631. [Google Scholar] [CrossRef]
- Li, H.; Bao, M.; Nie, Y. Extracellular matrix-based biomaterials for cardiac regeneration and repair. Heart Fail. Rev. 2021, 26, 1231–1248. [Google Scholar] [CrossRef] [PubMed]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Curcio, F.; Cassano, R.; Curcio, M.; Cirillo, G.; Iemma, F. Polymeric biomaterials for the treatment of cardiac post-infarction injuries. Pharmaceutics 2021, 13, 1038. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Huang, W.Y.; Chen, L.H.; Liang, N.W.; Wang, H.C.; Lu, J.; Wang, X.; Wang, T.W. Neural tissue engineering: The influence of scaffold surface topography and extracellular matrix microenvironment. J. Mater. Chem. B 2021, 9, 567–584. [Google Scholar] [CrossRef]
- Rana, D.; Ramasamy, K.; Leena, M.; Jiménez, C.; Campos, J.; Ibarra, P.; Haidar, Z.S.; Ramalingam, M. Surface functionalization of nanobiomaterials for application in stem cell culture, tissue engineering, and regenerative medicine. Biotechnol. Prog. 2016, 32, 554–567. [Google Scholar] [CrossRef]
- Abedi, A.; Hasanzadeh, M.; Tayebi, L. Conductive nanofibrous Chitosan/PEDOT:PSS tissue engineering scaffolds. Mater. Chem. Phys. 2019, 237, 121882. [Google Scholar] [CrossRef]
- Patel, B.; Manne, R.; Patel, D.B.; Gorityala, S.; Palaniappan, A.; Kurakula, M. Chitosan as functional biomaterial for designing delivery systems in cardiac therapies. Gels 2021, 7, 253. [Google Scholar] [CrossRef]
- Nguyen-Truong, M.; Li, Y.V.; Wang, Z. Mechanical considerations of electrospun scaffolds for myocardial tissue and regenerative engineering. Bioengineering 2020, 7, 122. [Google Scholar] [CrossRef]
- Martins, A.M.; Eng, G.; Caridade, S.G.; Mano, J.F.; Reis, R.L.; Vunjak-Novakovic, G. Electrically conductive chitosan/carbon scaffolds for cardiac tissue engineering. Biomacromolecules 2014, 15, 635–643. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, D.; Wang, Z.; Zhang, Z.; Xia, Y.; Xue, H.; Liu, Y. Preparation of an electrically conductive graphene oxide/chitosan scaffold for cardiac tissue engineering. Appl. Biochem. Biotechnol. 2019, 188, 952–964. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Parmaksiz, M.; Dogan, A.; Odabas, S.; Elçin, A.E.; Elçin, Y.M. Clinical applications of decellularized extracellular matrices for tissue engineering and regenerative medicine. Biomed. Mater. 2016, 11, 022003. [Google Scholar] [CrossRef] [PubMed]
- Kc, P.; Hong, Y.; Zhang, G. Cardiac tissue-derived extracellular matrix scaffolds for myocardial repair: Advantages and challenges. Regen. Biomater. 2019, 6, 185–199. [Google Scholar] [CrossRef]
- Taylor, D.A.; Parikh, R.B.; Sampaio, L.C. Bioengineering hearts: Simple yet complex. Curr. Stem Cell Rep. 2017, 3, 35–44. [Google Scholar] [CrossRef]
- Tang-Quan, K.R.; Mehta, N.A.; Sampaio, L.C.; Taylor, D.A. Whole cardiac tissue bioscaffolds. Adv. Exp. Med. Biol. 2018, 1098, 85–114. [Google Scholar] [CrossRef]
- Svystonyuk, D.A.; Mewhort, H.E.M.; Hassanabad, A.F.; Heydari, B.; Mikami, Y.; Turnbull, J.D.; Teng, G.; Belke, D.D.; Wagner, K.T.; Tarraf, S.A.; et al. Acellular bioscaffolds redirect cardiac fibroblasts and promote functional tissue repair in rodents and humans with myocardial injury. Sci. Rep. 2020, 10, 9459. [Google Scholar] [CrossRef] [PubMed]
- Prat-Vidal, C.; Rodríguez-Gómez, L.; Aylagas, M.; Nieto-Nicolau, N.; Gastelurrutia, P.; Agustí, E.; Gálvez-Montón, C.; Jorba, I.; Teis, A.; Monguió-Tortajada, M.; et al. First-in-human PeriCord cardiac bioimplant: Scalability and GMP manufacturing of an allogeneic engineered tissue graft. EbioMedicine 2020, 54, 102729. [Google Scholar] [CrossRef] [PubMed]
- Cebotari, S.; Lichtenberg, A.; Tudorache, I.; Hilfiker, A.; Mertsching, H.; Leyh, R.; Breymann, T.; Kallenbach, K.; Maniuc, L.; Batrinac, A.; et al. Clinical application of tissue engineered human heart valves using autologous progenitor cells. Circulation 2006, 114 (Suppl. 1), I132–I137. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Li, Y.; Chen, T. Techniques for fabrication and construction of three-dimensional scaffolds for tissue engineering. Int. J. Nanomed. 2013, 8, 337–350. [Google Scholar] [CrossRef]
- Islam, M.T.; Laing, R.M.; Wilson, C.A.; McConnell, M.; Ali, M.A. Fabrication and characterization of 3-dimensional electrospun poly(vinyl alcohol)/keratin/chitosan nanofibrous scaffold. Carbohydr. Polym. 2022, 275, 118682. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Brann, S.A.; Patiño-Herrera, R.; Navarrete-Damián, J.; Louvier-Hernández, J.F. Electrospinning of chitosan from different acid solutions. AIMS Bioeng. 2021, 8, 112–129. [Google Scholar] [CrossRef]
- Izadifar, Z.; Chen, X.; Kulyk, W. Strategic design and fabrication of engineered scaffolds for articular cartilage repair. J. Funct. Biomater. 2012, 3, 799–838. [Google Scholar] [CrossRef] [PubMed]
- Abedi, A.; Bakhshandeh, B.; Babaie, A.; Mohammadnejad, J.; Vahdat, S.; Mombeiny, R.; Moosavi, S.R.; Amini, J.; Tayebi, L. Concurrent application of conductive biopolymeric chitosan/polyvinyl alcohol/MWCNTs nanofibers, intracellular signaling manipulating molecules and electrical stimulation for more effective cardiac tissue engineering. Mater. Chem. Phys. 2021, 258, 123842. [Google Scholar] [CrossRef]
- Anisiei, A.; Oancea, F.; Marin, L. Electrospinning of chitosan-based nanofibers: From design to prospective applications. Rev. Chem. Eng. 2021, 000010151520210003. [Google Scholar] [CrossRef]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Perez-Puyana, V.; Rubio-Valle, J.F.; Jiménez-Rosado, M.; Guerrero, A.; Romero, A. Alternative processing methods of hybrid porous scaffolds based on gelatin and chitosan. J. Mech. Behav. Biomed. Mater. 2020, 102, 103472. [Google Scholar] [CrossRef]
- Sitab, A.A.; Joya, J.S.; Barman, J.P.; Biswas, S.; Rashid, T.U. Fabrication of chitosan-based biomaterials: Techniques and designs. In Engineering Materials for Stem Cell Regeneration; Sheikh, F.A., Ed.; Springer: Singapore, 2021; pp. 455–518. [Google Scholar] [CrossRef]
- Patel, D.K.; Dutta, S.D.; Hexiu, J.; Ganguly, K.; Lim, K.T. 3D-printable chitosan/silk fibroin/cellulose nanoparticle scaffolds for bone regeneration via M2 macrophage polarization. Carbohydr. Polym. 2022, 281, 119077. [Google Scholar] [CrossRef]
- Rajabi, M.; McConnell, M.; Cabral, J.; Ali, M.A. Chitosan hydrogels in 3D printing for biomedical applications. Carbohydr. Polym. 2021, 260, 117768. [Google Scholar] [CrossRef]
- Camman, M.; Joanne, P.; Agbulut, O.; Hélary, C. 3D models of dilated cardiomyopathy: Shaping the chemical, physical and topographical properties of biomaterials to mimic the cardiac extracellular matrix. Bioact. Mater. 2021, 7, 275–291. [Google Scholar] [CrossRef]
- Roshandel, M.; Dorkoosh, F. Cardiac tissue engineering, biomaterial scaffolds, and their fabrication techniques. Polym. Adv. Technol. 2021, 32, 2290–2305. [Google Scholar] [CrossRef]
- Aldemir-Dikici, B.; Claeyssens, F. Basic principles of emulsion templating and its use as an emerging manufacturing method of tissue engineering scaffolds. Front. Bioeng. Biotechnol. 2020, 8, 875. [Google Scholar] [CrossRef] [PubMed]
- Nii, T.; Katayama, Y. Biomaterial-assisted regenerative medicine. Int. J. Mol. Sci. 2021, 22, 8657. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Park, Y.; Kim, J. Engineering biomaterials to guide heart cells for matured cardiac tissue. Coatings 2020, 10, 925. [Google Scholar] [CrossRef]
- Huyer, L.D.; Montgomery, M.; Zhao, Y.; Xiao, Y.; Conant, G.; Korolj, A.; Radisic, M. Biomaterial based cardiac tissue engineering and its applications. Biomed. Mater. 2015, 10, 034004. [Google Scholar] [CrossRef]
- Thein-Han, W.W.; Saikhun, J.; Pholpramoo, C.; Misra, R.D.; Kitiyanant, Y. Chitosan-gelatin scaffolds for tissue engineering: Physico-chemical properties and biological response of buffalo embryonic stem cells and transfectant of GFP-buffalo embryonic stem cells. Acta Biomater. 2009, 5, 3453–3466. [Google Scholar] [CrossRef] [PubMed]
- Matsubayashi, K.; Fedak, P.W.; Mickle, D.A.; Weisel, R.D.; Ozawa, T.; Li, R.K. Improved left ventricular aneurysm repair with bioengineered vascular smooth muscle grafts. Circulation 2003, 108 (Suppl. 1), II-219–II-225. [Google Scholar] [CrossRef]
- Morgan, K.Y.; Sklaviadis, D.; Tochka, Z.L.; Fischer, K.M.; Hearon, K.; Morgan, T.D.; Langer, R.; Freed, L.E. Multi-material tissue engineering scaffold with hierarchical pore architecture. Adv. Funct. Mater. 2016, 26, 5873–5883. [Google Scholar] [CrossRef]
- Hu, T.; Wu, Y.; Zhao, X.; Wang, L.; Bi, L.; Ma, P.X.; Guo, B. Micropatterned, electroactive, and biodegradable poly(glycerol sebacate)-aniline trimer elastomer for cardiac tissue engineering. Chem. Eng. J. 2019, 366, 208–222. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, B.; Puperi, D.S.; Yonezawa, A.L.; Wu, Y.; Tseng, H.; Cuchiara, M.L.; West, J.L.; Grande-Allen, K.J. Integrating valve-inspired design features into poly(ethylene glycol) hydrogel scaffolds for heart valve tissue engineering. Acta Biomater. 2015, 14, 11–21. [Google Scholar] [CrossRef]
- Sugiura, T.; Matsumura, G.; Miyamoto, S.; Miyachi, H.; Breuer, C.K.; Shinoka, T. Tissue-engineered vascular grafts in children with congenital heart disease: Intermediate term follow-up. Semin. Thorac. Cardiovasc. Surg. 2018, 30, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.A.; Islas, J.F.; Schwartz, R.J.; Birla, R.K. Electrical stimulation of artificial heart muscle: A look into the electrophysiologic and genetic implications. ASAIO J. 2017, 63, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Salazar, B.H.; Reddy, A.K.; Tao, Z.; Madala, S.; Birla, R.K. 32-Channel system to measure the electrophysiological properties of bioengineered cardiac muscle. IEEE Trans. Biomed. Eng. 2015, 62, 1614–1622. [Google Scholar] [CrossRef]
- Gupta, N.; Cruz, M.A.; Nasser, P.; Rosenberg, J.D.; Iatridis, J.C. Fibrin-genipin hydrogel for cartilage tissue engineering in nasal reconstruction. Ann. Otol. Rhinol. Laryngol. 2019, 128, 640–646. [Google Scholar] [CrossRef]
- Setayeshmehr, M.; Esfandiari, E.; Hashemibeni, B.; Tavakoli, A.H.; Rafienia, M.; Samadikuchaksaraei, A.; Moroni, L.; Joghataei, M.T. Chondrogenesis of human adipose-derived mesenchymal stromal cells on the [devitalized costal cartilage matrix/poly(vinyl alcohol)/fibrin] hybrid scaffolds. Eur. Polym. J. 2019, 118, 528–541. [Google Scholar] [CrossRef]
- Saeedi Garakani, S.; Khanmohammadi, M.; Atoufi, Z.; Kamrava, S.K.; Setayeshmehr, M.; Alizadeh, R.; Faghihi, F.; Bagher, Z.; Davachi, S.M.; Abbaspourrad, A. Fabrication of chitosan/agarose scaffolds containing extracellular matrix for tissue engineering applications. Int. J. Biol. Macromol. 2020, 143, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Majid, Q.A.; Fricker, A.T.R.; Gregory, D.A.; Davidenko, N.; Hernandez Cruz, O.; Jabbour, R.J.; Owen, T.J.; Basnett, P.; Lukasiewicz, B.; Stevens, M.; et al. Natural biomaterials for cardiac tissue engineering: A highly biocompatible solution. Front. Cardiovasc. Med. 2020, 7, 554597. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhang, T.; Song, Y.; Sun, W. Assessment of various crosslinking agents on collagen/chitosan scaffolds for myocardial tissue engineering. Biomed. Mater. 2020, 15, 045003. [Google Scholar] [CrossRef]
- Gotenstein, J.R.; Koo, C.C.; Ho, T.W.; Chisholm, A.D. Genetic suppression of basement membrane defects in caenorhabditis elegans by gain of function in extracellular matrix and cell-matrix attachment genes. Genetics 2018, 208, 1499–1512. [Google Scholar] [CrossRef]
- Wu, W.; Peng, S.; Song, Z.; Lin, S. Collagen biomaterial for the treatment of myocardial infarction: An update on cardiac tissue engineering and myocardial regeneration. Drug Deliv. Transl. Res. 2019, 9, 920–934. [Google Scholar] [CrossRef]
- Johnson, T.D.; Christman, K.L. Injectable hydrogel therapies and their delivery strategies for treating myocardial infarction. Expert Opin. Drug Deliv. 2013, 10, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Chachques, J.C.; Trainini, J.C.; Lago, N.; Masoli, O.H.; Barisani, J.L.; Cortes-Morichetti, M.; Schussler, O.; Carpentier, A. Myocardial assistance by grafting a new bioartificial upgraded myocardium (MAGNUM clinical trial): One year follow-up. Cell Transplant. 2007, 16, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M.; Samimi, A.; Khorram, M.; Abdi, M.M.; Golestaneh, S.I. Fabrication and characterization of conductive polypyrrole/chitosan/collagen electrospun nanofiber scaffold for tissue engineering application. Int. J. Biol. Macromol. 2021, 168, 175–186. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, H.; Ahlfeld, T.; Kilian, D.; Liu, Y.; Gelinsky, M.; Hu, Q. Evaluation of different crosslinking methods in altering the properties of extrusion-printed chitosan-based multi-material hydrogel composites. Bio-Des. Manuf. 2022. [Google Scholar] [CrossRef]
- Nezhad-Mokhtari, P.; Akrami-Hasan-Kohal, M.; Ghorbani, M. An injectable chitosan-based hydrogel scaffold containing gold nanoparticles for tissue engineering applications. Int. J. Biol. Macromol. 2020, 154, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Norahan, M.H.; Amroon, M.; Ghahremanzadeh, R.; Mahmoodi, M.; Baheiraei, N. Electroactive graphene oxide-incorporated collagen assisting vascularization for cardiac tissue engineering. J. Biomed. Mater. Res. A 2019, 107, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Raghunathan, S.; Islas, J.F.; Mistretta, B.; Iyer, D.; Shi, L.; Gunaratne, P.H.; Ko, G.; Schwartz, R.J.; McConnell, B.K. Conversion of human cardiac progenitor cells into cardiac pacemaker-like cells. J. Mol. Cell Cardiol. 2020, 138, 12–22. [Google Scholar] [CrossRef]
- Islas, J.F.; Abbasgholizadeh, R.; Dacso, C.; Potaman, V.N.; Navran, S.; Bond, R.A.; Iyer, D.; Birla, R.; Schwartz, R.J. β-Adrenergic stimuli and rotating suspension culture enhance conversion of human adipogenic mesenchymal stem cells into highly conductive cardiac progenitors. J. Tissue Eng. Regen. Med. 2020, 14, 306–318. [Google Scholar] [CrossRef]
- de Melo, B.A.G.; Jodat, Y.A.; Cruz, E.M.; Benincasa, J.C.; Shin, S.R.; Porcionatto, M.A. Strategies to use fibrinogen as bioink for 3D bioprinting fibrin-based soft and hard tissues. Acta Biomater. 2020, 117, 60–76. [Google Scholar] [CrossRef]
- Evans, L.A.; Ferguson, K.H.; Foley, J.P.; Rozanski, T.A.; Morey, A.F. Fibrin sealant for the management of genitourinary injuries, fistulas and surgical complications. J. Urol. 2003, 169, 1360–1362. [Google Scholar] [CrossRef]
- Abbasgholizadeh, R.; Islas, J.F.; Navran, S.; Potaman, V.N.; Schwartz, R.J.; Birla, R.K. A highly conductive 3D cardiac patch fabricated using cardiac myocytes reprogrammed from human adipogenic mesenchymal stem cells. Cardiovasc. Eng. Technol. 2020, 11, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, R.I.; Pieters, M.; de Lange-Loots, Z.; Weisel, J.W. Fibrinogen and fibrin. In Macromolecular Protein Complexes III: Structure and Function; Harris, J.R., Marles-Wright, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 471–501. [Google Scholar]
- Stoppel, W.L.; Hu, D.; Domian, I.J.; Kaplan, D.L.; Black, L.D. Anisotropic silk biomaterials containing cardiac extracellular matrix for cardiac tissue engineering. Biomed. Mater. 2015, 10, 034105. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, H.; Yue, F.; Lv, Q.; Cai, B.; Dong, N.; Wang, Z.; Wang, L. Silk-based biomaterials for cardiac tissue engineering. Adv. Healthc Mater. 2020, 9, 2000735. [Google Scholar] [CrossRef] [PubMed]
- Di Felice, V.; Serradifalco, C.; Rizzuto, L.; De Luca, A.; Rappa, F.; Barone, R.; Di Marco, P.; Cassata, G.; Puleio, R.; Verin, L.; et al. Silk fibroin scaffolds enhance cell commitment of adult rat cardiac progenitor cells. J. Tissue Eng. Regen. Med. 2015, 9, E51–E64. [Google Scholar] [CrossRef]
- Liang, Y.; Mitriashkin, A.; Lim, T.T.; Goh, J.C. Conductive polypyrrole-encapsulated silk fibroin fibers for cardiac tissue engineering. Biomaterials 2021, 276, 121008. [Google Scholar] [CrossRef]
- Rastogi, P.; Kandasubramanian, B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication 2019, 11, 042001. [Google Scholar] [CrossRef]
- Sridharan, D.; Palaniappan, A.; Blackstone, B.N.; Powell, H.M.; Khan, M. Electrospun aligned coaxial nanofibrous scaffold for cardiac repair. Methods Mol. Biol. 2021, 2193, 129–140. [Google Scholar] [CrossRef]
- Tamimi, M.; Rajabi, S.; Pezeshki-Modaress, M. Cardiac ECM/chitosan/alginate ternary scaffolds for cardiac tissue engineering application. Int. J. Biol. Macromol. 2020, 164, 389–402. [Google Scholar] [CrossRef]
- Lu, W.N.; Lü, S.H.; Wang, H.B.; Li, D.X.; Duan, C.M.; Liu, Z.Q.; Hao, T.; He, W.J.; Xu, B.; Fu, Q.; et al. Functional improvement of infarcted heart by co-injection of embryonic stem cells with temperature-responsive chitosan hydrogel. Tissue Eng. Part A 2009, 15, 1437–1447. [Google Scholar] [CrossRef]
- Zadeh, Z.E.; Solouk, A.; Shafieian, M.; Nazarpak, M.H. Electrospun polyurethane/carbon nanotube composites with different amounts of carbon nanotubes and almost the same fiber diameter for biomedical applications. Mater. Sci. Eng. C 2021, 118, 111403. [Google Scholar] [CrossRef]
- Lv, J.; Liu, W.; Shi, G.; Zhu, F.; He, X.; Zhu, Z.; Chen, H. Human cardiac extracellular matrix-chitosan-gelatin composite scaffold and its endothelialization. Exp. Ther. Med. 2020, 19, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Pok, S.; Myers, J.D.; Madihally, S.V.; Jacot, J.G. A multilayered scaffold of a chitosan and gelatin hydrogel supported by a PCL core for cardiac tissue engineering. Acta Biomater. 2013, 9, 5630–5642. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.S.; Niu, J.; Pastor-Cervantes, J.A. Comparison of hemostasis times with a chitosan-based hemostatic pad (Clo-SurPlus Radial™) vs mechanical compression (TR Band®) following transradial access: A pilot study. Cardiovasc. Revasc. Med. 2019, 20, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Pathan, A.Z.; Aijaz, S.; Sheikh, S.; Sattar, S. Randomized trial comparing radial hemostasis techniques; catechol conjugated chitosan pad (InnoSEAL) versus pneumatic compression band. Catheter. Cardiovasc. Interv. 2021, 98, E181–E187. [Google Scholar] [CrossRef]
- Martel-Estrada, S.A.; Martínez-Pérez, C.A.; Chacón-Nava, J.G.; García-Casillas, P.E.; Olivas-Armendáriz, I. Synthesis and thermo-physical properties of chitosan/poly(dl-lactide-co-glycolide) composites prepared by thermally induced phase separation. Carbohydr. Polym. 2010, 81, 775–783. [Google Scholar] [CrossRef]
- Olivas-Armendariz, I.; García-Casillas, P.; Martel Estrada, A.; Martínez-Villafañe, A.; De la Rosa, L.A.A.; Martínez-Pérez, C.A. In vitro evaluation of polyurethane-chitosan scaffolds for tissue engineering. J. Biomater. Nanobiotechnol. 2012, 3, 440–445. [Google Scholar] [CrossRef][Green Version]
- Ahmadi, P.; Nazeri, N.; Derakhshan, M.A.; Ghanbari, H. Preparation and characterization of polyurethane/chitosan/CNT nanofibrous scaffold for cardiac tissue engineering. Int. J. Biol. Macromol. 2021, 180, 590–598. [Google Scholar] [CrossRef]
- De la Paz Orozco, A.; Vega, F.; Martel-Estrada, S.; Aguilar, A.; Mendoza-Duarte, M.; Chavarría-Gaytán, M.; Rodríguez-González, C.; Olivas-Armendáriz, I. Development of chitosan/poly(l-lactide)/multiwalled carbon nanotubes scaffolds for bone tissue engineering. Open J. Regen. Med. 2016, 5, 14–23. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Wang, S.; Zhang, R. Composite poly(lactic acid)/chitosan nanofibrous scaffolds for cardiac tissue engineering. Int. J. Biol. Macromol. 2017, 103, 1130–1137. [Google Scholar] [CrossRef]
- Liu, N.; Chen, J.; Zhuang, J.; Zhu, P. Fabrication of engineered nanoparticles on biological macromolecular (PEGylated chitosan) composite for bio-active hydrogel system in cardiac repair applications. Int. J. Biol. Macromol. 2018, 117, 553–558. [Google Scholar] [CrossRef]
- Wang, H.; Shi, J.; Wang, Y.; Yin, Y.; Wang, L.; Liu, J.; Liu, Z.; Duan, C.; Zhu, P.; Wang, C. Promotion of cardiac differentiation of brown adipose derived stem cells by chitosan hydrogel for repair after myocardial infarction. Biomaterials 2014, 35, 3986–3998. [Google Scholar] [CrossRef]
- Olivas-Armendariz, I.; Martel-Estrada, S.; Mendoza-Duarte, M.; Jiménez-Vega, F.; García-Casillas, P.E.; Martínez-Pérez, C.A. Biodegradable chitosan/multiwalled carbon nanotube composite for bone tissue engineering. J. Biomater. Nanobiotechnol. 2013, 4, 204–211. [Google Scholar] [CrossRef][Green Version]
- Saravanan, S.; Sareen, N.; Abu-El-Rub, E.; Ashour, H.; Sequiera, G.L.; Ammar, H.I.; Gopinath, V.; Shamaa, A.A.; Sayed, S.S.E.; Moudgil, M.; et al. Graphene oxide-gold nanosheets containing chitosan scaffold improves ventricular contractility and function after implantation into infarcted heart. Sci. Rep. 2018, 8, 15069. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, A.; Yin, R.T.; Kireev, D.; Efimov, I.R.; Molokanova, E. Graphene-based scaffolds: Fundamentals and applications for cardiovascular tissue engineering. Front. Bioeng. Biotechnol. 2021, 9, 797340. [Google Scholar] [CrossRef] [PubMed]
| Source | Cells | Definition | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|
| Embryonic | Fetal CM | Fetal heart. | Potential for cardiac integration and regeneration. | Immunogenicity. Malignant potential. Ethical questions. Limited availability. | [26] |
| Human umbilical cord blood-derived cells | Pluripotent stem cells, mesenchymal stem cells (MSC), hematopoietic stem cells (HSC), and non-hematopoietic stem cells (NHSC). Differentiate into different types. | Reduction of infarct after intramyocardial injection. | Immunogenicity. Need to standardize isolation and culture procedures. Senescence and mutational acquisition during in vitro expansion. | [27,28] | |
| Embryonic MSC | Pluripotent stem cell derived from inner cell mass of blastocyst in embryo. | Potential to differentiate into cells from all three embryonic germ layers. | Associated with malignant transformation. Legal issues. | [29] | |
| Adult stem cells | Adipose stem cells | Derived from adipose tissue: heterogeneous mixture of MSC, HSC, and endothelial progenitor cells (EPC). | Multipotent potential. Easily source with minimal effort. Easy harvesting. Low cost. No ethical issues. | Potentially tumorigenic. Limited understanding of the mechanism associated with cardiac repair. | [30] |
| Bone marrow stem cells | Stem cells derived from bone marrow. | Well-known cell precursors. Easy collection. | Extracted in low numbers. Potential contamination during in vitro expansion. | [30,31] | |
| EPC | Originated from different tissues. Have been classified into hematopoietic and nonhematopoietic progenitor cells. | Increase its numbers in response to ischemia/cytokine stimuli. Migrate to injury site and differentiate into myocytes. Participate in repair and maintenance of vascular homeostasis. | Low numbers in peripheral blood and bone marrow makes ex vivo expansion difficult. | [32] | |
| Resident cardiac stem cells (RCSC) | Represent a responsive stem cell reservoir within the adult associated myocardial homeostasis. | Capable of differentiating into multiple cell types such as CM or VSMC. | Limited repair. | [33] | |
| Ault somatic cells | Human-induced pluripotent stem cells (iPSC) | Autologous somatic cells that can be converted into pluripotent cells. | Possibilities of large-scale production. Ability to differentiate into CM, SMC, and vascular EC. | Poor purity. Heterogeneity. Laborious/inefficient techniques of isolation. Can generate teratomas. | [30,34] |
| CF | Source of induced pluripotent cells. Can be reprogrammed directly into CM, EC, and SMC. | Available in large numbers. Phenotypically plastic. Promote the proliferation of endogenous CM by activation of the CM cell cycle. | Primary drivers of fibrosis. Unclear how an in vivo environment with changed ECM compositions influences CF plasticity and integration. | [35] | |
| Skeletal myoblasts | Derived from muscle biopsy. | Ability to engraft, create myotubules, and improve cardiac function after transfer into infarcted myocardium. | Heterogeneous. Associated with arrhythmias, interfering with the propagation of electrical potentials. | [36] |
| Scaffold | Biofabrication Technique | Results | Year | Reference |
|---|---|---|---|---|
| Conductive nanofiber scaffold (polypyrrole hidrogel/chitosan/polyethylene oxide) | Electrospinning | Cell adhesion, growth and proliferation, conductive nanofiber scaffolds appropriate for employing in body parts with electrical signals such as cardiac tissue engineering. | 2021 | [104] |
| Collagen/chitosan composite scaffold | Freezing and lyophilization | High porosity (>65%), excellent mechanical properties, in the physiological range of native myocardium, biocompatibility, CM high expression of cardiac-specific marker protein, and contractile performance. | 2020 | [105] |
| Injectable hidrogel (Collagen/carbon nano tubes/chitosan/gold nanoparticles) | Chemically cross-linking | Non-toxicity, great potential as a new biomaterial for cardiac tissue engineering applications. | 2020 | [106] |
| Collagen/graphene oxide cardiac patch | Freeze-drying method | Interconnected pores with appropriate pore sizes, electrical conductivity suitable for cardiac tissue engineering, no toxic effects on human cells, neonatal cardiomyocyte adhesion, and upregulation of cardiac genes expression. | 2019 | [107] |
| Scaffold | Biofabrication Technique | Results | Year | Reference |
|---|---|---|---|---|
| Polypyrrole scaffold coated with silk fibroin | Electrospinning | Mimic of myocardium fibrils, similar mechanical properties to the native myocardium, sufficient electrical conductivity for cardiomyocytes, support CM contraction. | 2021 | [117] |
| Scaffold | Biofabrication Technique | Results | Year | Reference |
|---|---|---|---|---|
| Alginate scaffolds functionalized with magnetite nanoparticles | Freeze-dry technique | Magnetic alginate scaffolds exposed to an alternating magnetic field create stimulating microenvironments for engineering functional tissues | 2021 | [119] |
| Composite of cardiac ECM with alginate and chitosan | Freezing and lyophilization | Porosity of more than 96%, very high swelling rate, stability in PBS solution, improving of the tensile strength, proliferation of human MSC inside the pores, higher expression of cardiac marker cTnT. | 2020 | [120] |
| Scaffold | Biofabrication Technique | Results | Year | Reference |
|---|---|---|---|---|
| Polyurethane /CS/carbon nanotubes composite | Electrospinning | Biocompatibility, electro conduction, and aligned nanofibers. | 2021 | [122] |
| CS scaffold blending with graphene oxide (GO) | Freezing and lyophilization | Swelling, porosity, and conductive properties. Good cell viability, promotion of cell attachment, intercellular network formation, upregulation of cardiac-specific genes, and protein expression involved in muscle conduction of electrical signals. | 2019 | [62] |
| Cardiac ECM-chitosan-gelatin composite | Freezing and lyophilization | High porosity, biodegradable and biocompatible scaffold, increased cell survival and proliferation, promotion of differentiation process. | 2019 | [123] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roacho-Pérez, J.A.; Garza-Treviño, E.N.; Moncada-Saucedo, N.K.; Carriquiry-Chequer, P.A.; Valencia-Gómez, L.E.; Matthews, E.R.; Gómez-Flores, V.; Simental-Mendía, M.; Delgado-Gonzalez, P.; Delgado-Gallegos, J.L.; et al. Artificial Scaffolds in Cardiac Tissue Engineering. Life 2022, 12, 1117. https://doi.org/10.3390/life12081117
Roacho-Pérez JA, Garza-Treviño EN, Moncada-Saucedo NK, Carriquiry-Chequer PA, Valencia-Gómez LE, Matthews ER, Gómez-Flores V, Simental-Mendía M, Delgado-Gonzalez P, Delgado-Gallegos JL, et al. Artificial Scaffolds in Cardiac Tissue Engineering. Life. 2022; 12(8):1117. https://doi.org/10.3390/life12081117
Chicago/Turabian StyleRoacho-Pérez, Jorge A., Elsa N. Garza-Treviño, Nidia K. Moncada-Saucedo, Pablo A. Carriquiry-Chequer, Laura E. Valencia-Gómez, Elizabeth Renee Matthews, Víctor Gómez-Flores, Mario Simental-Mendía, Paulina Delgado-Gonzalez, Juan Luis Delgado-Gallegos, and et al. 2022. "Artificial Scaffolds in Cardiac Tissue Engineering" Life 12, no. 8: 1117. https://doi.org/10.3390/life12081117
APA StyleRoacho-Pérez, J. A., Garza-Treviño, E. N., Moncada-Saucedo, N. K., Carriquiry-Chequer, P. A., Valencia-Gómez, L. E., Matthews, E. R., Gómez-Flores, V., Simental-Mendía, M., Delgado-Gonzalez, P., Delgado-Gallegos, J. L., Padilla-Rivas, G. R., & Islas, J. F. (2022). Artificial Scaffolds in Cardiac Tissue Engineering. Life, 12(8), 1117. https://doi.org/10.3390/life12081117







