Abstract
Atrial fibrillation is the most common presentation in adult patients with cor triatriatum sinister. The key to successful and safe catheter ablation in these patients is an accurate exploration and thorough understanding of the left atrial anatomy, both before and during the procedure. Catheter manipulation is highly dependable on left atrial anatomy, including the interatrial septum, insertion of pulmonary veins and cor triatriatum membrane. Anatomical variants such as the left common pulmonary trunk may influence the ablation approach and outcome. We report the case of a 52-year-old patient with cor triatriatum sinister and the left common pulmonary vein variant who underwent successful high-power, short-duration catheter ablation for paroxysmal atrial fibrillation.
1. Introduction
Cor triatriatum is a rare congenital cardiac anomaly that accounts for 0.1 to 0.4% of all congenital heart disease [1,2,3,4,5]. The left-sided anomaly cor triatriatum sinister (CTS) is more frequent than the right-sided cor triatriatum dexter, with both conditions being commonly diagnosed in infancy and associated with other congenital heart defects such as atrial septal defect, patent foramen ovale or left superior vena cava [1,2].
In CTS, abnormal fibromuscular septation divides the left atrium into two separate chambers: the pulmonary chamber, usually located posteriorly and superiorly, which most frequently receives the four pulmonary veins, and the true left atrium, located anteriorly and inferiorly, which contains the left atrial appendage and the mitral valve annulus [1,2,3,4,5,6].
CTS is an uncommon diagnosis in adulthood, with the symptoms depending on the degree of hemodynamic obstruction between the two left atrial chambers. As a consequence of this, adult patients with CTS and large non-obstructive fenestrations in the membrane may present as completely asymptomatic, whereas patients with small obstructive fenestrations may mimic the clinical picture of mitral stenosis due to the obstruction through the membrane [1,2,6]. However, although it is still not completely understood why asymptomatic patients with large fenestrations become symptomatic with age, in most of them, atrial fibrillation is the sentinel clinical symptom [2,5,6,7].
AF catheter ablation may be considered in patients with congenital heart defects, as the current practical guidelines recommend [8]. Nevertheless, in patients with CTS, catheter manipulation is highly dependent on left atrial anatomy, including the anatomy of the interatrial septum, membrane and pulmonary veins [4,6,9]. Although the left common pulmonary vein is a common anatomical variant in patients undergoing AF catheter ablation [10,11], there are no reports of this particular anatomy in patients with CTS. We report the case of a patient with CTS and the left common pulmonary vein variant who underwent successful high-power, short-duration catheter ablation for paroxysmal AF.
2. Case Presentation
A 52-year-old Caucasian patient was referred to our arrhythmias department for recurrent paroxysmal AF episodes, which began three months earlier, in order to assess their suitability for catheter ablation. He had no medical history or past interventions and at admission was on antiarrhythmic treatment with Flecainide and Bisoprolol, which had commenced with the onset of symptoms. The patient reported worsening symptoms in the preceding two weeks, with AF episodes becoming more frequent, lasting longer and affecting his everyday activity.
An ECG on admission showed a normal sinus rhythm of 70 bpm. Transthoracic echocardiography revealed normal left ventricular function, mild mitral regurgitation and a mildly dilated left atrium, with a structure that resembled a membrane cutting transversely from left to right at this level. CTS was suspected, which was easily confirmed by transesophageal echocardiography (TEE) (Figure 1). TEE showed no significant gradient at the level of the membrane, suggesting a big fenestration between the superior and the inferior cavities of the left atrium. Multidetector computed tomography angiography further characterized the left atrial anatomy, showing a superoposterior atrial chamber in which a common left pulmonary vein (the superior and inferior left pulmonary veins drain into the LA through a common pulmonary venous ostium) and two separate right pulmonary veins were tributary and an inferoanterior chamber which contained the mitral valve and left atrial appendage. The two chambers were separated by a membrane inserted from the left ridge, in immediate proximity to the left common pulmonary vein, to the interatrial septum in its anterior region (Figure 2).
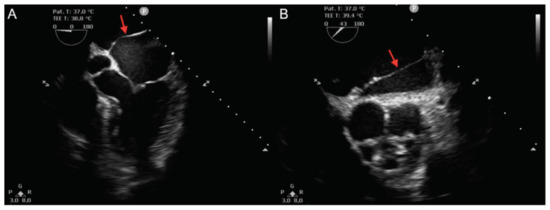
Figure 1.
Transesophageal echocardiography. (A): Mid-esophageal 5-chamber view and (B): Mid-esophageal short-axis view, showing a membrane (red arrows) separating the left atrium in two separate chambers.
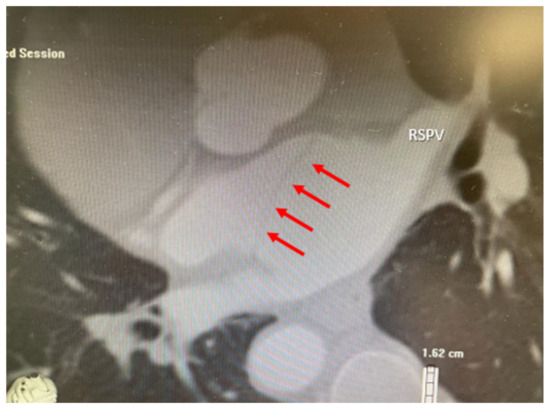
Figure 2.
Multidetector computed tomography angiography confirming the presence of two separate left atrial chambers, the superoposterior atrial chamber which receives the pulmonary veins and the inferoanterior atrial chamber, separated by a membrane (red arrows). RSPV—right superior pulmonary vein.
We decided to perform AF catheter ablation under general anesthesia using live TEE guidance. Transseptal access to the superoposterior part of the left atrium (pulmonary chamber) was achieved. A bidirectional guiding sheath was introduced into the LA in order to gain better catheter stability (CARTO VIZIGO™ Bi-Directional Guiding Sheath; Biosense Webster, Inc., Diamond Bar, CA, USA). Electroanatomical mapping performed using a PentaRay NAV catheter and the CARTO 3 system (Biosense Webster, Inc., Diamond Bar, CA, USA) confirmed the common left pulmonary vein and two distinct pulmonary veins on the right, and no low-voltage areas were detected inside the left atrium, meaning no fibrosis was present (Figure 3). A large fenestration was seen during electroanatomical mapping, localized in the anterior part of the septum, which was not very clear until this moment (Figure 4).
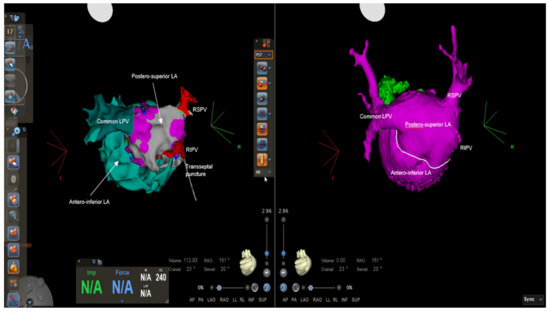
Figure 3.
Electroanatomical mapping using CARTO 3 system and CT reconstruction showing the anatomy of the pulmonary veins: two separate pulmonary veins on the right and a common left pulmonary vein draining into the posterosuperior left-atrial chamber, where we can also notice the transseptal puncture. LA = left atrium, LPV = left pulmonary vein, RIPV = right inferior pulmonary vein, RSPV = right superior pulmonary vein.
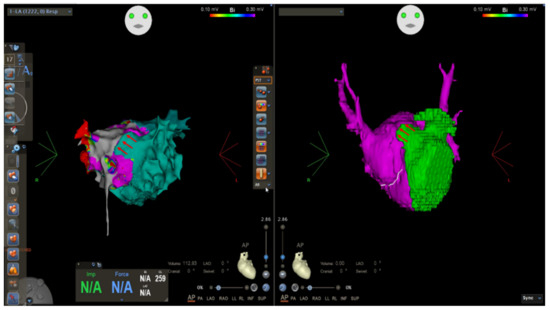
Figure 4.
Electroanatomical mapping using CARTO 3 system and CT reconstruction showing anterior fenestration in the CTS membrane (red arrows).
We performed circumferential point-by-point high-power, short-duration (50 W, ablation index 450 on the anterior wall and 320 on the posterior wall) radiofrequency isolation of the left common pulmonary vein and right pulmonary veins using a SmartTouch SF force-sensing catheter (Biosense Webster, Inc., Diamond Bar, CA, USA). Catheter stability was lower on the left ridge, in the proximity of the CTS membrane, and there was an unusual catheter curve to the left ridge, but good contact force was finally achieved (>10 g) and the vein was successfully isolated. No extra ablation points were applied inside the left atrium. The isolation of the pulmonary veins was verified at the end of the procedure by both entrance- and exit-block. No complications appeared during and after the procedure and the patient was symptom-free at six months post-ablation, with 24 h ECG monitoring showing no AF recurrence.
3. Discussion
Cor triatriatum sinister is a rare congenital cardiac anomaly, most often diagnosed during childhood or infancy [1,2,3,4,5,6]. When it is diagnosed in adults, the most common presentation is for new onset atrial fibrillation [2,5,6,7]. Taking into account that most of these patients are in their young adulthood, catheter ablation can be considered as first line therapy [8].
CTS is a rare finding in the electrophysiology laboratory that could create difficulties during atrial fibrillation catheter ablation even for the most experienced electrophysiologists. A thorough imaging evaluation before performing the ablation for the purpose of an assessment of the exact anatomy of the left atrium and pulmonary veins is crucial in order to achieve good outcomes in terms of safety and efficacy [4,6,9,12,13]. Furthermore, electroanatomical mapping further defines the anatomy of important left atrial structures in patients with CTS during catheter ablation for atrial fibrillation [6].
The first case of atrial fibrillation ablation in a patient with CTS was reported by Yamada et al. in 2008 [14]. Since then, 14 other clinical cases were published, including one re-do by Yamada, focusing on the importance of anatomy in order to achieve a safe left atrial access through transseptal puncture to the posterosuperior atrial chamber [4,6,9,12,13,14,15,16,17,18,19,20]. However, neither of these reports focused on the impact of the anatomy of pulmonary veins on catheter manipulation and procedure outcome.
In this case, the left common pulmonary vein posed a challenge for catheter manipulation, as the CTS membrane inserted directly in its proximity, on the left ridge. While the left common pulmonary vein is a common anatomical variant in patients undergoing AF catheter ablation [10,11], there are no reports of this particular anatomy in patients with CTS. Moreover, the left ridge is recognized as a region where the catheter has reduced contact with the tissue [21], with the presence of both the CTS membrane and left common pulmonary vein posing further challenges for successful isolation of pulmonary veins. Despite the fact that, in our patient, catheter stability was lower on the left ridge, given the unusual curve of the ablation catheter passing through the interatrial septum and directed towards the narrow ridge between the ostium of the left common pulmonary vein and the insertion of the CTS membrane, we achieved a good contact force (>10 g) in this region and performed the successful isolation of all pulmonary veins, including the left common pulmonary vein. However, it is noteworthy that the left atrial anatomy in such patients, including that of pulmonary veins and CTS membrane insertion, may pose a challenge in the context of obtaining good contact force and catheter stability.
Thirteen out of the 15 previous reported cases of AF ablation in patients with CTS do not describe the used power-duration approach [4,6,9,13,14,15,16,17,18,19,20]. The remaining two case reports include one patient for whom low-power, long-duration settings (25–30 W, 30 s) were used [12] and one cryoballoon ablation [6]. Considering that high-power, short-duration is a relatively new approach for AF catheter ablation [22,23,24] and only 2 out of these 13 cases were published after 2018 [4,9], the present case may be the first report of high-power, short-duration AF ablation in a CTS patient.
In studied populations, results on arrhythmia recurrence after atrial fibrillation catheter ablation in patients with the left common pulmonary vein variant are controversial [10,11,21,25]. Although there are studies that show that atrial fibrillation recurrence is higher in patients with the left common pulmonary vein variant [11], most studies show good outcomes in these patients [21,25]. In our case, the patient was free of atrial fibrillation 6 months after ablation.
As the majority of the literature reports recommend, transseptal puncture was performed to the posterosuperior atrial chamber, which gave us direct access to all pulmonary veins, including the left pulmonary common vein. However, in some of the published cases [4,14,17], the transseptal approach was performed into the anteroinferior atrial chamber, with the authors reporting challenges in terms of obtaining access to the pulmonary veins’ antra due to the passing of the catheters through the fenestration. Still, Yamada et al. reported that the ablation of the right inferior pulmonary vein was more challenging when left atrial cannulation was performed to the posterosuperior compared to anteroinferior chamber in the same re-do patient [14,15]. Nevertheless, access to the posterosuperior atrial chamber should be performed first in all patients [6], including patients with the left common pulmonary vein variant.
Tokuda et al. reported a case of atrial fibrillation catheter ablation in a CTS patient in which all pulmonary veins, including left and right veins, drained into the posterosuperior atrium through a total common trunk [16]. However, the anatomy of the pulmonary veins was different to that of to our patient, with ablation being performed by isolating the single total pulmonary trunk.
In our patient, there was no need for extra ablation sites inside the left atrium, as electroanatomical mapping showed no low-voltage areas and no electrical potentials on or adjacent to the membrane. Other studies reported electrical potentials on or around the membrane [9,16], and depending on the type of atrial fibrillation, the need of secondary sites of ablation [9,16,17,18,20], including left atrial roof line, box or mitral isthmus ablation.
Finally, although intracardiac echocardiography was used for live guidance during the procedure in most of the reported cases, for the purpose of transseptal puncture [4,6,9,12,13,16,18,20], transesophageal echocardiography was also used in several reports [17,19], including ours, without any disadvantages reported. Intraoperative imaging by ICE or TEE, together with electroanatomical mapping and aided by preoperative advanced imaging techniques such as multidetector computed tomography, is essential for describing the exact anatomy of the left atrium and pulmonary veins in CTS patients undergoing AF catheter ablation. All together, these imaging techniques enable electrophysiologists to plan and perform procedures more safely and efficiently.
4. Conclusions
AF is the most common presentation in adult patients with CTS. A thorough understanding of the anatomy of the left atrium, including that of the pulmonary veins, CTS membrane and fenestration, and interatrial septum allows electrophysiologists to perform AF catheter ablation safely and with good efficacy. The presence of a left common pulmonary vein in patients with CTS may pose challenges with respect to catheter manipulation, particularly on the left ridge where the membrane is inserted. Good contact force (>10 g) using a high-power, short-duration approach can result in a good procedural outcome in patients with CTS and the left common pulmonary vein variant.
Author Contributions
Conceptualization, I.-A.M. and G.C.; validation and formal analysis, M.P. and R.T.; resources and investigation, D.A. (Daniela Anghelina) and A.G.; writing—original draft preparation, I.-A.M. and R.T.; writing—review and editing, G.C., M.P. and R.R.; visualization, D.A. (Denis Amet) and M.A.; supervision, D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for these case reports after the information was deidentified, resulting in this report being exempt from review by the Institutional Review Board.
Informed Consent Statement
Written informed consent was obtained from the patient involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest regarding this case report.
References
- Jha, A.K.; Makhija, N. Cor Triatriatum: A Review. Semin. Cardiothorac. Vasc. Anesth. 2017, 21, 178–185. [Google Scholar] [CrossRef]
- Rudienė, V.; Hjortshøj, C.M.S.; Glaveckaitė, S.; Zakarkaitė, D.; Petrulionienė, Ž.; Gumbienė, L.; Aidietis, A.; Sondergaard, L. Cor triatriatum sinistrum diagnosed in the adulthood: A systematic review. Heart 2019, 105, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Ullah, W.; Sattar, Y.; Rauf, H.; Roomi, S.; Shah, M. A Systematic Review of a Long-forgotten Cause of Atrial Fibrillation and Stroke: Cor Triatriatum. Cureus 2019, 11, e6371. [Google Scholar] [CrossRef] [PubMed]
- Morishima, I.; Kanzaki, Y.; Furui, K.; Yamauchi, R.; Morita, Y.; Tsuboi, H. Three-dimensional visualization of the left atrium by intracardiac echocardiography facilitates trans-septal catheterization and atrial fibrillation catheter ablation in cor triatriatum sinister: A case report and literature review. J. Cardiol. Cases 2020, 22, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Zepeda, I.A.; Morcos, P.; Castellanos, L.R. Cor Triatriatum Sinister Identified after New Onset Atrial Fibrillation in an Elderly Man. Case Rep. Med. 2014, 2014, 674018. [Google Scholar] [CrossRef]
- Karimianpour, A.; Cai, A.W.; Cuoco, F.A.; Sturdivant, J.L.; Litwin, S.E.; Wharton, J.M. Catheter ablation of atrial fibrillation in patients with cor triatriatum sinister; case series and review of literature. Pacing Clin. Electrophysiol. 2021, 44, 2084–2091. [Google Scholar] [CrossRef]
- Hayes, C.; Liu, S.; Tam, J.W.; Kass, M. Cor Triatriatum Sinister: An Unusual Cause of Atrial Fibrillation in Adults. Case Rep. Cardiol. 2018, 2018, 9242519. [Google Scholar] [CrossRef]
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Okada, A.; Kato, K.; Shoda, M.; Tabata, H.; Yoshie, K.; Kuwahara, K. Successful catheter ablation of atrial tachycardia in cor triatriatum sinister: A figure-of-8 reentry in the left atrial membrane. HeartRhythm Case Rep. 2020, 7, 109–111. [Google Scholar] [CrossRef]
- Istratoaie, S.; Roșu, R.; Cismaru, G.; Vesa, C.; Puiu, M.; Zdrenghea, D.; Pop, D.; Buzoianu, A.D. The Impact of Pulmonary Vein Anatomy on the Outcomes of Catheter Ablation for Atrial Fibrillation. Medicina 2019, 55, 727. [Google Scholar] [CrossRef]
- Xu, B.; Xing, Y.; Xu, C.; Peng, F.; Sun, Y.; Wang, S.; Guo, H. A left common pulmonary vein: Anatomical variant predicting good outcomes of repeat catheter ablation for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2019, 30, 717–726. [Google Scholar] [CrossRef]
- Bhatia, N.L.; Humphries, J.; Chandrasekaran, K.; Srivathsan, K. Atrial fibrillation ablation in cor triatriatum: Value of intracardiac echocardiography. J. Interv. Card. Electrophysiol. 2009, 28, 153–155. [Google Scholar] [CrossRef]
- Fukumoto, K.; Takatsuki, S.; Miyoshi, S.; Tanimoto, K.; Nishiyama, N.; Aizawa, Y.; Kimura, T.; Fukuda, Y.; Sato, T.; Fukuda, K. Cor triatriatum sinister. Herz 2011, 37, 217–218. [Google Scholar] [CrossRef]
- Yamada, T.; Tabereaux, P.B.; McElderry, H.T.; Kay, G.N. Successful catheter ablation of atrial fibrillation in a patient with cor triatriatum sinister. Heart Rhythm 2008, 5, 903–904. [Google Scholar] [CrossRef]
- Yamada, T.; Tabereaux, P.B.; McElderry, H.T.; Doppalapudi, H.; Kay, G.N. Transseptal catheterization in the catheter ablation of atrial fibrillation in a patient with cor triatriatum sinister. J. Interv. Card. Electrophysiol. 2009, 25, 79–82. [Google Scholar] [CrossRef]
- Tokuda, M.; Yamane, T.; Tokutake, K.; Yokoyama, K.; Hioki, M.; Narui, R.; Tanigawa, S.-I.; Yamashita, S.; Inada, K.; Matsuo, S.; et al. Catheter ablation of persistent atrial fibrillation in a patient with cor triatriatum sinister demonstrating a total common trunk of the pulmonary vein. Heart Vessel. 2014, 31, 261–264. [Google Scholar] [CrossRef]
- Gavin, A.; Singleton, C.B.; McGavigan, A.D. Successful Multi-chamber Catheter Ablation of Persistent Atrial Fibrillation in Cor Triatriatum Sinister. Indian Pacing Electrophysiol. J. 2011, 11, 50–55. [Google Scholar]
- Borne, R.T.; Gonzalez, J.; Khanna, A.; Sauer, W.H.; Nguyen, D.T. Getting to the right left atrium: Catheter ablation of atrial fibrillation and mitral annular flutter in cor triatriatum. HeartRhythm Case Rep. 2016, 2, 502–505. [Google Scholar] [CrossRef]
- Paelinck, B.P.; Van Herck, P.L.; Vandaele, L.; Sarkozy, A. Echocardiographic guidance of pulmonary vein isolation catheter ablation procedure for recurrent atrial fibrillation in partial cor triatriatum. Kardiol. Pol. 2018, 76, 1575. [Google Scholar] [CrossRef]
- Shah, S.R.; Mohanty, G.P.; Gilligan, D.M.; Newton, C.M. Long-standing persistent atrial fibrillation ablation without use of fluoroscopy in a patient with cor triatriatum. HeartRhythm Case Rep. 2018, 5, 88–92. [Google Scholar] [CrossRef][Green Version]
- Ronsoni, R.M.; Silvestrini, T.L.; Essebag, V.; Lopes, R.D.; Saffi, M.A.L.; Leiria, T.L.L. Association of the left common ostium with clinical outcome after pulmonary vein isolation in atrial fibrillation. Indian Pacing Electrophysiol. J. 2020, 21, 95–100. [Google Scholar] [CrossRef]
- Winkle, R.A. HPSD ablation for AF high-power short-duration RF ablation for atrial fibrillation: A review. J. Cardiovasc. Electrophysiol. 2020, 32, 2813–2823. [Google Scholar] [CrossRef]
- Leo, M.; Pedersen, M.; Rajappan, K.; Ginks, M.R.; Hunter, R.J.; Bowers, R.; Kalla, M.; Bashir, Y.; Betts, T.R. Power, Lesion Size Index and Oesophageal Temperature Alerts during Atrial Fibrillation Ablation. Circ. Arrhythm. Electrophysiol. 2020, 13, 1102–1112. [Google Scholar] [CrossRef]
- Shin, D.G.; Ahn, J.; Han, S.-J.; Lim, H.E. Efficacy of high-power and short-duration ablation in patients with atrial fibrillation: A prospective randomized controlled trial. EP Eur. 2020, 22, 1495–1501. [Google Scholar] [CrossRef]
- McLellan, A.J.; Ling, L.-H.; Ruggiero, D.; Wong, M.C.; Walters, T.E.; Nisbet, A.; Shetty, A.K.; Azzopardi, S.; Taylor, A.J.; Morton, J.B.; et al. Pulmonary vein isolation: The impact of pulmonary venous anatomy on long-term outcome of catheter ablation for paroxysmal atrial fibrillation. Heart Rhythm 2014, 11, 549–556. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).