Immediate Evaluation of the Effect of Infrared LED Photobiomodulation on Childhood Sleep Bruxism: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
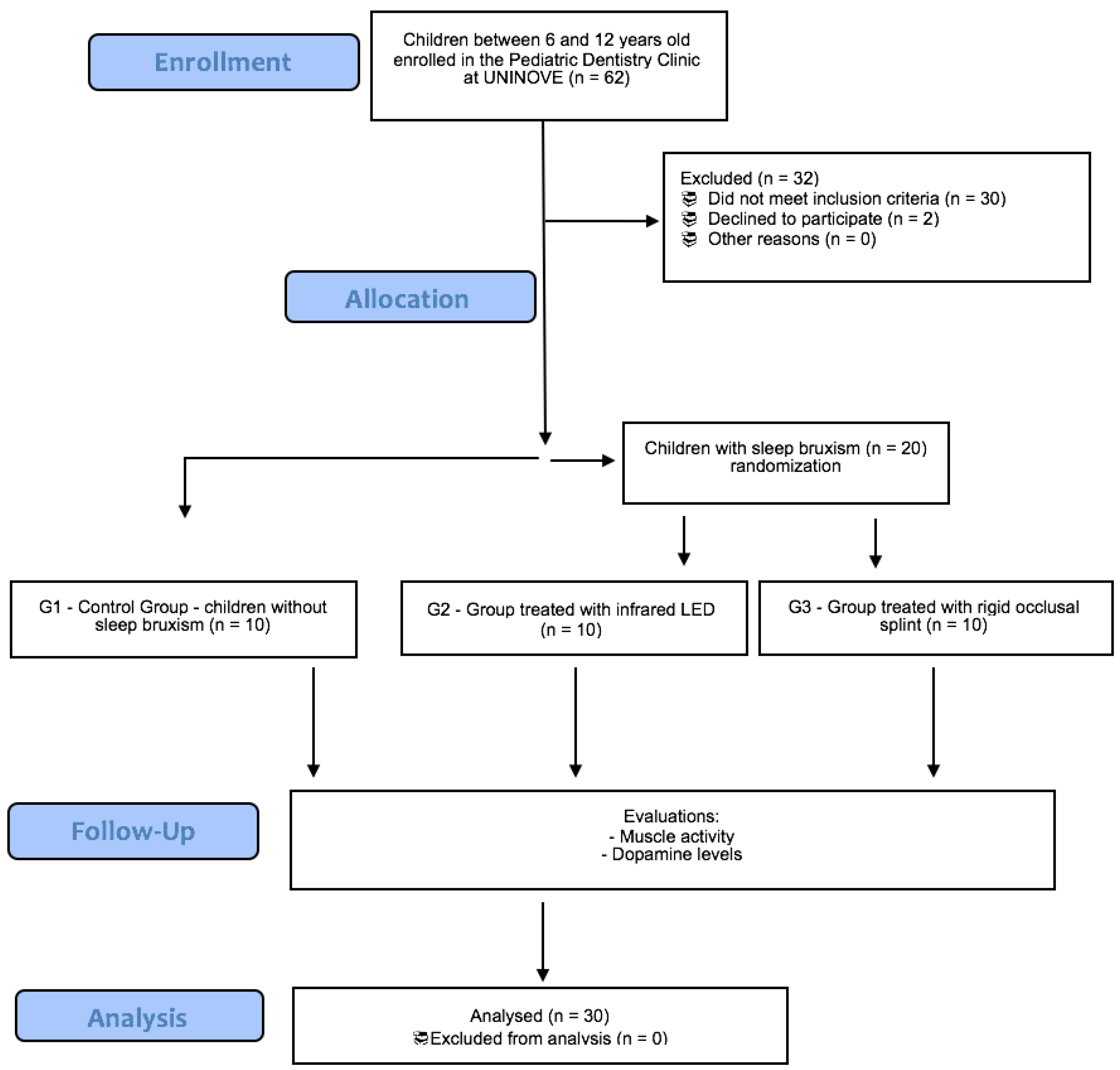
2.3. Allocation Procedure
2.4. Groups
- -
- Group 1 (n = 10): control—absence of SB (4 girls; mean age 7.0 ± 0.9 years);
- -
- Group 2 (n = 10): children with SB treated with infrared LED (5 girls; mean age 7.1 ± 1.1 years);
- -
- Group 3 (n = 10): children with SB treated with occlusal splint (7 girls; mean age 7.6 ± 1.3 years).
2.5. Clinical Evaluation
2.6. Photobiomodulation Treatment
2.7. Occlusal Splints Treatment
2.8. Electromyographic Assessment
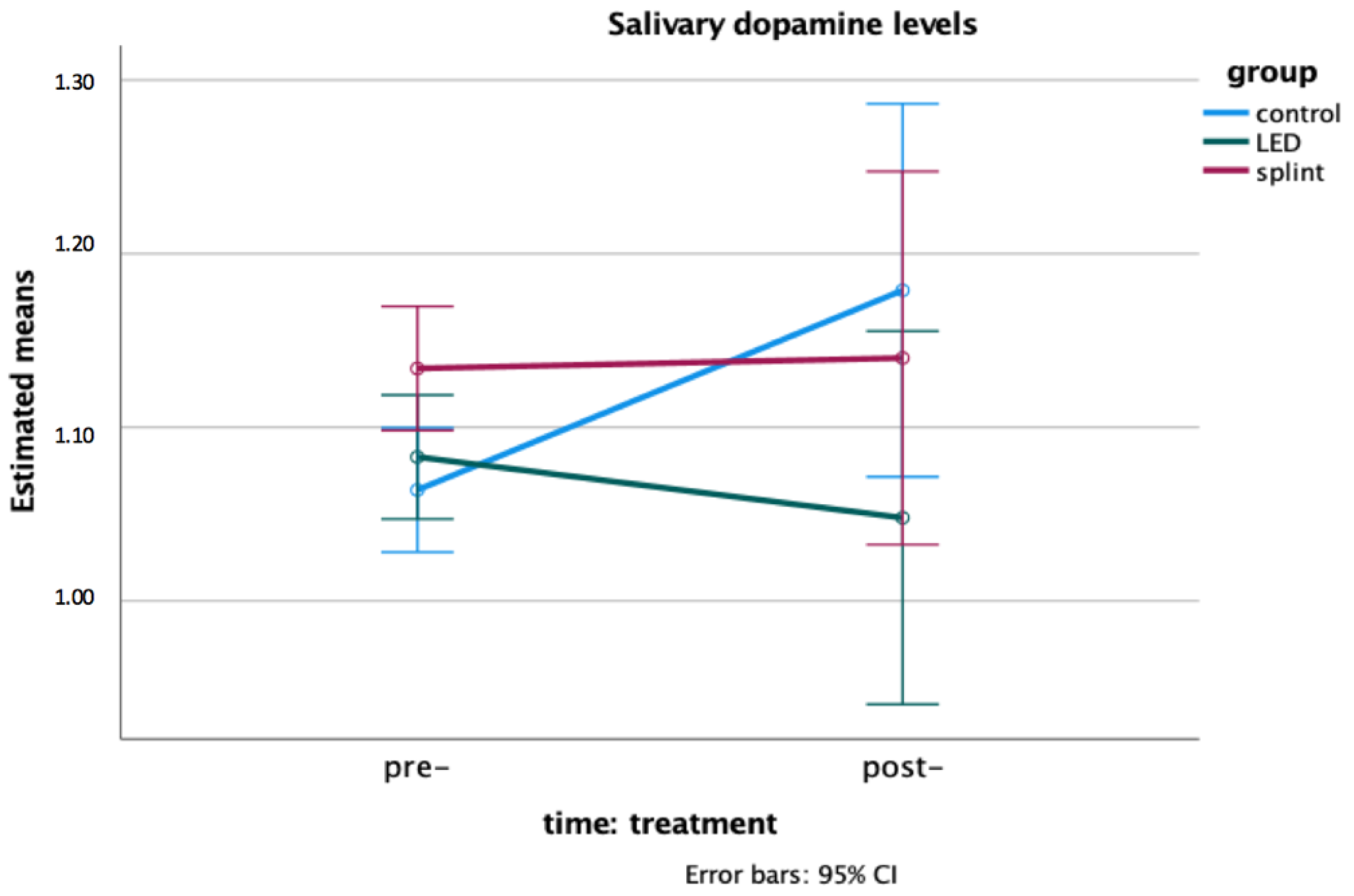
2.9. Salivary Dopamine Levels
2.10. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lobbezoo, F.; Ahlberg, J.; Raphael, K.G.; Wetselaar, P.; Glaros, A.G.; Kato, T.; Santiago, V.; Winocur, E.; De Laat, A.; De Leeuw, R.; et al. International consensus on the assessment of bruxism: Report of a work in progress. J. Oral Rehabil. 2018, 45, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Salgueiro, M.D.C.C.; Bortoletto, C.C.; Horliana, A.C.R.; Mota, A.C.C.; Motta, L.J.; Motta, P.d.B.; MesquitaFerrari, R.A.; Fernandes, K.P.S.; Bussadori, S.K. Evaluation of muscle activity, bite force and salivary cortisol in children with bruxism before and after low level laser applied to acupoints: Study protocol for a randomised controlled trial. BMC Complement. Altern. Med. 2017, 17, 391. [Google Scholar] [CrossRef] [PubMed]
- Lucas, B.d.L.; Barbosa, T.d.S.; Pereira, L.J.; Gavião, M.B.; Castelo, P.M. Electromyographic evaluation of masticatory muscles at rest and maximal intercuspal positions of the mandible in children with sleep bruxism. Eur. Arch. Paediatr. Dent. 2014, 15, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Giannasi, L.C.; Freitas Batista, S.R.; Matsui, M.Y.; Hardt, C.T.; Gomes, C.P.; Oliveira Amorim, J.B.; Oliveira, C.S.; de Oliveira, L.V.; Gomes, M.F. Effect of a hyperbolide mastication apparatus for the treatment of severe sleep bruxism in a child with cerebral palsy: Long-term follow-up. J. Bodyw. Mov. Ther. 2014, 18, 62–67. [Google Scholar] [CrossRef]
- Ferrari-Piloni, C.; Barros, L.A.N.; Evangelista, K.; Serra-Negra, J.M.; Silva, M.A.G.; Valladares-Neto, J. Prevalence of Bruxism in Brazilian Children: A Systematic Review and Meta-Analysis. Pediatr. Dent. 2022, 44, 8–20. [Google Scholar]
- Wieckiewicz, M.; Paradowska-Stolarz, A.; Wieckiewicz, W. Psychosocial aspects of bruxism: The most paramount factor influencing teeth grinding. BioMed Res. Int. 2014, 2014, 469187. [Google Scholar] [CrossRef]
- Takemura, T.; Takahashi, T.; Fukuda, M.; Ohnuki, T.; Asunuma, T.; Masuda, Y.; Kondoh, H.; Kanbayashi, T.; Shimizu, T. A psychological study on patients with masticatory muscle disorder and sleep bruxism. Cranio 2006, 24, 191–196. [Google Scholar] [CrossRef]
- Rodríguez Cerdeira, C.; Sánchez-Blanco, E.; Sánchez-Blanco, B.; González-Cespón, J.L.; Working Group of IISGS. Protein biomarkers of mood disorders. Int. J. Immunopathol. Pharmacol. 2017, 30, 7–12. [Google Scholar] [CrossRef]
- Kang, W.Y.; Yang, Q.; Jiang, X.F.; Chen, W.; Zhang, L.Y.; Wang, X.Y.; Zhang, L.N.; Quinn, T.J.; Liu, J.; Chen, S.D. Salivary DJ-1 could be an indicator of Parkinson’s disease progression. Front. Aging Neurosci. 2014, 6, 102. [Google Scholar] [CrossRef]
- Oporto, G.H., 5th; Bornhardt, T.; Iturriaga, V.; Salazar, L.A. Single nucleotide polymorphisms in genes of dopaminergic pathways are associated with bruxism. Clin. Oral Investig. 2018, 22, 331–337. [Google Scholar] [CrossRef]
- Yang, J.; Qi, M.; Guan, L.; Hou, Y.; Yang, Y. The time course of psychological stress as revealed by event-related potentials. Neurosci. Lett. 2012, 530, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Boone, J.L.; Anthony, J.P. Evaluating the impact of stress on systemic disease: The MOST protocol in primary care. J. Am. Osteopath. Assoc. 2003, 103, 239–246. [Google Scholar] [PubMed]
- Marker, R.J.; Stephenson, J.L.; Kluger, B.M.; Curran-Everett, D.; Maluf, K.S. Modulation of intracortical inhibition in response to acute psychosocial stress is impaired among individuals with chronic neck pain. J. Psychosom. Res. 2014, 76, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.V.; Rangarajan, P.; Mounissamy, A. Bruxism: Conceptual discussion and review. J. Pharm. Bioallied Sci. 2015, 7 (Suppl. 1), S265–S270. [Google Scholar] [CrossRef]
- Huang, Y.F.; Lin, J.C.; Yang, H.W.; Lee, Y.H.; Yu, C.H. Clinical effectiveness of laser acupuncture in the treatment of temporomandibular joint disorder. J. Formos. Med. Assoc. 2014, 113, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.M.; Albertini, R.; Leal-Junior, E.C.; de Tarso Camillo de Carvalho, P.; Silva, J.A., Jr.; Bussadori, S.K.; de Oliveira, L.V.; Casarin, C.A.; Andrade, E.L.; Bocalini, D.S.; et al. Effects of exercise training and photobiomodulation therapy (EXTRAPHOTO) on pain in women with fibromyalgia and temporomandibular disorder: Study protocol for a randomized controlled trial. Trials 2015, 16, 252. [Google Scholar] [CrossRef]
- Restrepo, C.C.; Medina, I.; Patiño, I. Effect of occlusal splints on the temporomandibular disorders, dental wear and anxiety of bruxist children. Eur. J. Dent. 2011, 5, 441–450. [Google Scholar] [CrossRef]
- Kobayashi, F.Y.; Castelo, P.M.; Gonçalves, M.L.L.; Motta, L.J.; Mota, A.C.D.C.; Altavista, O.M.; Pinto, M.M.; Salgueiro, M.C.; Ferreira, K.P.S.; Bussadori, S.K. Evaluation of the effectiveness of infrared light-emitting diode photobiomodulation in children with sleep bruxism: Study protocol for randomized clinical trial. Medicine 2019, 98, e17193. [Google Scholar] [CrossRef]
- Tartaglia, G.M.; Lodetti, G.; Paiva, G.D.; Felicio, C.M.; Sforza, C. Surface eletromyography assessment of patients with long lasting temporomandibular joint disorder pain. J. Electromyogr. Kinesiol. 2011, 21, 659–664. [Google Scholar] [CrossRef]
- Miller, C.S.; Dembo, J.B.; Falace, D.A.; Kaplan, A.L. Salivary cortisol response to dental treatment of varying stress. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1995, 79, 436–441. [Google Scholar] [CrossRef]
- Crowther, J.R. The Elisa Guidebook; Humana Press: New York, NY, USA, 2001. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Sağlam, E.; Akça, Ö.F. Treatment of Sleep Bruxism with a Single Daily Dose of Buspirone in a 7-Year-Old Boy. Clin. Neuropharmacol. 2019, 42, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Akbaş, B.; Bilgiç, A. Fluoxetine-Induced Sleep Bruxism Rapidly Treated with Once-Nightly Dosing of Buspirone in a 6-Year-Old Girl. Clin. Neuropharmacol. 2018, 41, 197–198. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi, S.N.; Jafari, A.; Hoseini, S.G.; Khademian, M.; Kelishadi, R. The efficacy of low and moderate dosage of diazepam on sleep bruxism in children: A randomized placebo-controlled clinical trial. J. Res. Med. Sci. 2019, 24, 8. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, B.P.; Souza, G.A.D.S.; Filho, A.A.D.N.; Pinto, A.P.; Guimarães, C.L.; Pereira, A.P.C.; Neves, M.F.D.; Martins, P.S.L.L.; Lima, F.P.S.; Lopes-Martins, R.A.B.; et al. Analysis of the effects of low-level laser therapy on muscle fatigue of the biceps brachii muscle of healthy individuals and spastic individuals: Study protocol for a single-center, randomized, double-blind, and controlled clinical trial. Medicine 2019, 98, e17166. [Google Scholar] [CrossRef]
- Shahimoridi, D.; Shafiei, S.A.; Yousefian, B. The Effectiveness of the Polarized Low-Level Laser in the Treatment of Patients with Myofascial Trigger Points in the Trapezius Muscles. J. Lasers Med. Sci. 2020, 11, 14–19. [Google Scholar] [CrossRef]
- Kuo, J.R.; Lin, S.S.; Liu, J.; Chen, S.H.; Chio, C.C.; Wang, J.J.; Liu, J.M. Deep brain light stimulation effects on glutamate and dopamine concentration. Biomed. Opt. Express. 2014, 6, 23–31. [Google Scholar] [CrossRef][Green Version]
- Isabella, A.P.J.; Silva, J.T.C.; da Silva, T.; Rodrigues, M.F.S.D.; Horliana, A.C.R.T.; Motta, L.J.; Bussadori, S.K.; Pavani, C.; Silva, D.F.T.D. Effect of irradiation with intravascular laser on the hemodynamic variables of hypertensive patients: Study protocol for prospective blinded randomized clinical trial. Medicine 2019, 98, e15111. [Google Scholar] [CrossRef]
- Huang, S.F.; Tsai, Y.A.; Wu, S.B.; Wei, Y.H.; Tsai, P.Y.; Chuang, T.Y. Effects of intravascular laser irradiation of blood in mitochondria dysfunction and oxidative stress in adults with chronic spinal cord injury. Photomed. Laser Surg. 2012, 30, 579–586. [Google Scholar] [CrossRef]
- Burduli, N.M.; Aksenova, I.Z. The effects of intravenous laser irradiation of blood on the system hemodynamics of patients with chronic obstructive bronchitis exacerbation. Klin. Med. 2006, 84, 37–39. [Google Scholar]
- Herpich, C.M.; Leal-Junior, E.C.P.; Gomes, C.A.F.P.; Gloria, I.P.D.S.; Amaral, A.P.; Amaral, M.F.R.S.; Politti, F.; Biasotto-Gonzalez, D.A. Immediate and short-term effects of phototherapy on pain, muscle activity, and joint mobility in women with temporomandibular disorder: A randomized, double-blind, placebo-controlled, clinical trial. Disabil. Rehabil. 2018, 40, 2318–2324. [Google Scholar] [CrossRef]
- Akat, B.; Görür, S.A.; Bayrak, A.; Eren, H.; Eres, N.; Erkcan, Y.; Kılıçarslan, M.A.; Orhan, K. Ultrasonographic and electromyographic evaluation of three types of occlusal splints on masticatory muscle activity, thickness, and length in patients with bruxism. Cranio 2020, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lukic, N.; Saxer, T.; Hou, M.Y.; Zumbrunn Wojczyńska, A.; Gallo, L.M.; Colombo, V. Short-Term effects of NTI-tss and Michigan splint on nocturnal jaw muscle activity: A pilot study. Clin. Exp. Dent. Res. 2021, 7, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Palinkas, M.; Bataglion, C.; de Luca Canto, G.; Machado Camolezi, N.; Theodoro, G.T.; Siéssere, S.; Semprini, M.; Regalo, S.C. Impact of sleep bruxism on masseter and temporalis muscles and bite force. Cranio 2016, 34, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Lavigne, G.J.; Kato, T.; Kolta, A.; Sessle, B.J. Neurobiological mechanisms involved in sleep bruxism. Crit. Rev. Oral. Biol. Med. 2003, 14, 30–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Lu, Y.C.; Lui, C.C.; Liu, J.S. A proposed mechanism for diurnal/nocturnal bruxism: Hypersensitivity of presynaptic dopamine receptors in the frontal lobe. J. Clin. Neurosci. 2005, 12, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Alencar, M.J.S.; Martins, B.M.C.; Vieira, B.N. Relation between bruxism and dopamine. Rev. Bras. Odontol. 2014, 71, 62–66. [Google Scholar] [CrossRef][Green Version]

| Control Group | LED Group | Occlusal Splint Group | Two-Way Mixed Model ANOVA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | p-Value | eta Partial Squared | F | |
| Rest position | |||||||||
| Right temporalis (%MVC) | 9.6 (6.3) | 7.4 (5.6) | 4.9 (4.3) | 7.7 (6.4) | 6.5 A (6.3) | 13.8 B (10.5) | interaction effect: p = 0.019 | 0.314 | 4.807 |
| Right masseter (%MVC) | 8.1 A (4.8) | 8.6 B (5.0) | 5.1 A (3.6) | 8.2 B (4.9) | 8.6 A (6.2) | 12.8 B (8.6) | time effect: p = 0.044 | 0.180 | 4.598 |
| Left temporalis (%MVC) | 9.4 (5.2) | 7.3 (4.8) | 5.3 (3.8) | 8.8 (4.8) | 6.0 A (4.8) | 12.5 B (6.6) | interaction effect: p = 0.021 | 0.308 | 4.669 |
| Left masseter (%MVC) | 7.2 (3.0) | 5.8 (3.4) | 5.5 (2.8) | 6.5 (3.9) | 6.0 A (2.9) | 9.6 B (5.2) | interaction effect: p = 0.037 | 0.270 | 3.888 |
| Chewing | |||||||||
| Right temporalis (%MVC) | 32.0 (13.5) | 27.7 (14.0) | 28.9 (12.5) | 34.8 (25.9) | 28.7 (17.8) | 28.7 (15.9) | NS | - | |
| Right masseter (%MVC) | 25.3 (18.9) | 23.9 (13.0) | 22.4 (12.8) | 33.4 (34.4) | 27.3 (12.5) | 30.1 (12.6) | NS | - | |
| Left temporalis (%MVC) | 34.3 (11.5) | 28.9 (9.4) | 35.5 (12.1) | 37.6 (16.5) | 27.9 (15.6) | 31.4 (13.5) | NS | - | |
| Left masseter (%MVC) | 23.9 (10.2) | 23.3 (5.4) | 22.6 (10.5) | 26.9 (14.7) | 23.9 (13.0) | 25.8 (10.1) | NS | - | |
| Arm elevation | |||||||||
| Upper trapezius (%MVC) | 36.2 (10.4) | 36.5 (8.8) | 35.0 (10.5) | 28.1 (12.6) | 38.4 (15.6) | 32.6 (11.6) | NS | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, F.Y.; Castelo, P.M.; Politti, F.; Rocha, M.M.; Beltramin, R.Z.; Salgueiro, M.D.C.C.; Gonçalves, M.L.L.; Nammour, S.; Brugnera Júnior, A.; Sfalcin, R.A.; et al. Immediate Evaluation of the Effect of Infrared LED Photobiomodulation on Childhood Sleep Bruxism: A Randomized Clinical Trial. Life 2022, 12, 964. https://doi.org/10.3390/life12070964
Kobayashi FY, Castelo PM, Politti F, Rocha MM, Beltramin RZ, Salgueiro MDCC, Gonçalves MLL, Nammour S, Brugnera Júnior A, Sfalcin RA, et al. Immediate Evaluation of the Effect of Infrared LED Photobiomodulation on Childhood Sleep Bruxism: A Randomized Clinical Trial. Life. 2022; 12(7):964. https://doi.org/10.3390/life12070964
Chicago/Turabian StyleKobayashi, Fernanda Yukie, Paula Midori Castelo, Fabiano Politti, Monise Mendes Rocha, Rafael Zaratin Beltramin, Mônica Da Consolação Canuto Salgueiro, Marcela Leticia Leal Gonçalves, Samir Nammour, Aldo Brugnera Júnior, Ravana Angelini Sfalcin, and et al. 2022. "Immediate Evaluation of the Effect of Infrared LED Photobiomodulation on Childhood Sleep Bruxism: A Randomized Clinical Trial" Life 12, no. 7: 964. https://doi.org/10.3390/life12070964
APA StyleKobayashi, F. Y., Castelo, P. M., Politti, F., Rocha, M. M., Beltramin, R. Z., Salgueiro, M. D. C. C., Gonçalves, M. L. L., Nammour, S., Brugnera Júnior, A., Sfalcin, R. A., & Bussadori, S. K. (2022). Immediate Evaluation of the Effect of Infrared LED Photobiomodulation on Childhood Sleep Bruxism: A Randomized Clinical Trial. Life, 12(7), 964. https://doi.org/10.3390/life12070964








