Demyelination Lesions Do Not Correlate with Clinical Manifestations by Bordetella pertussis Toxin Concentrations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. EAE Induction
2.4. Clinical Signs of EAE
2.5. Euthanasia
2.6. Histopathological Analysis
2.7. Statistical Analysis
3. Results
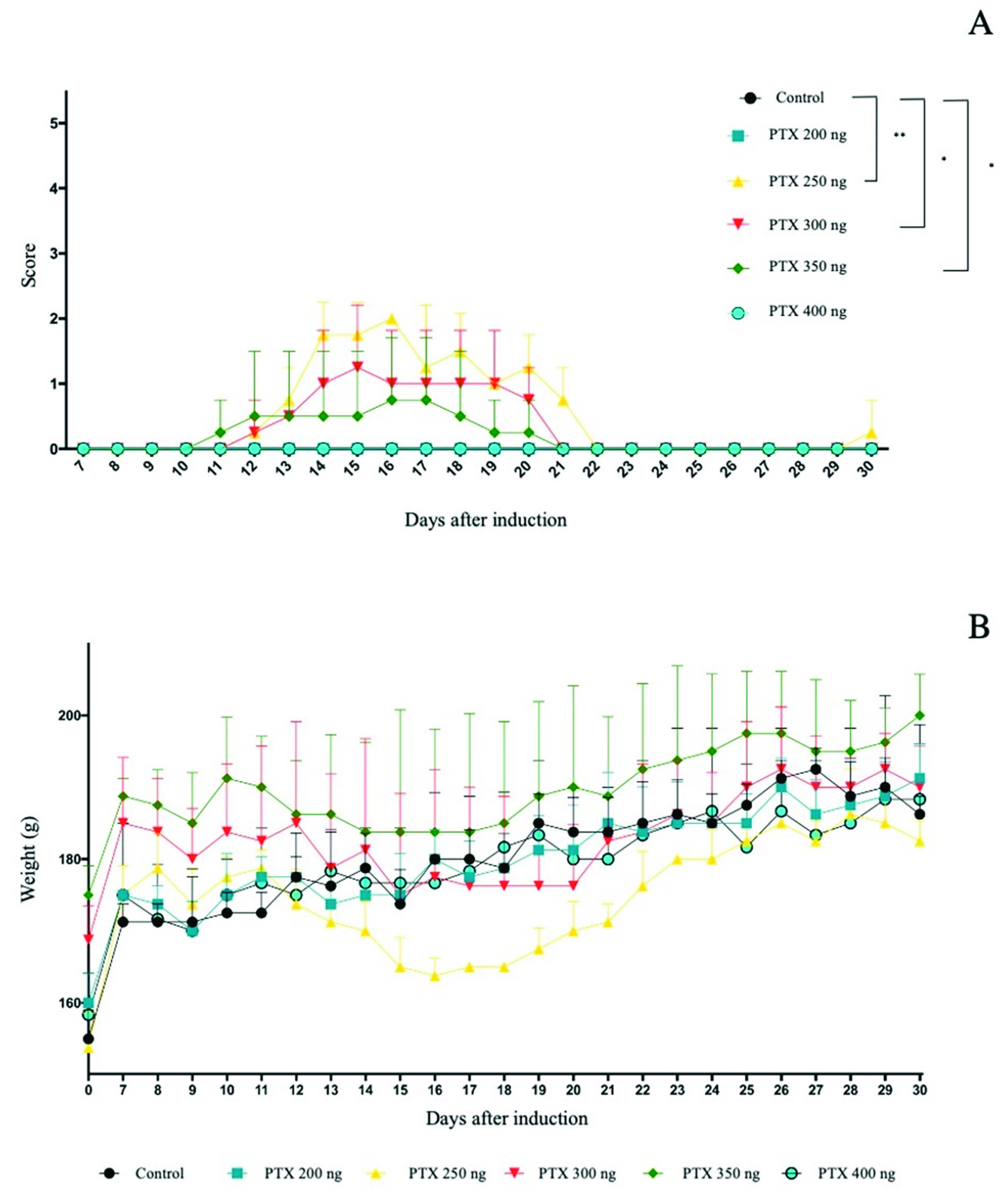
3.1. Clinical Signs
3.2. Weight as a Clinical Sign
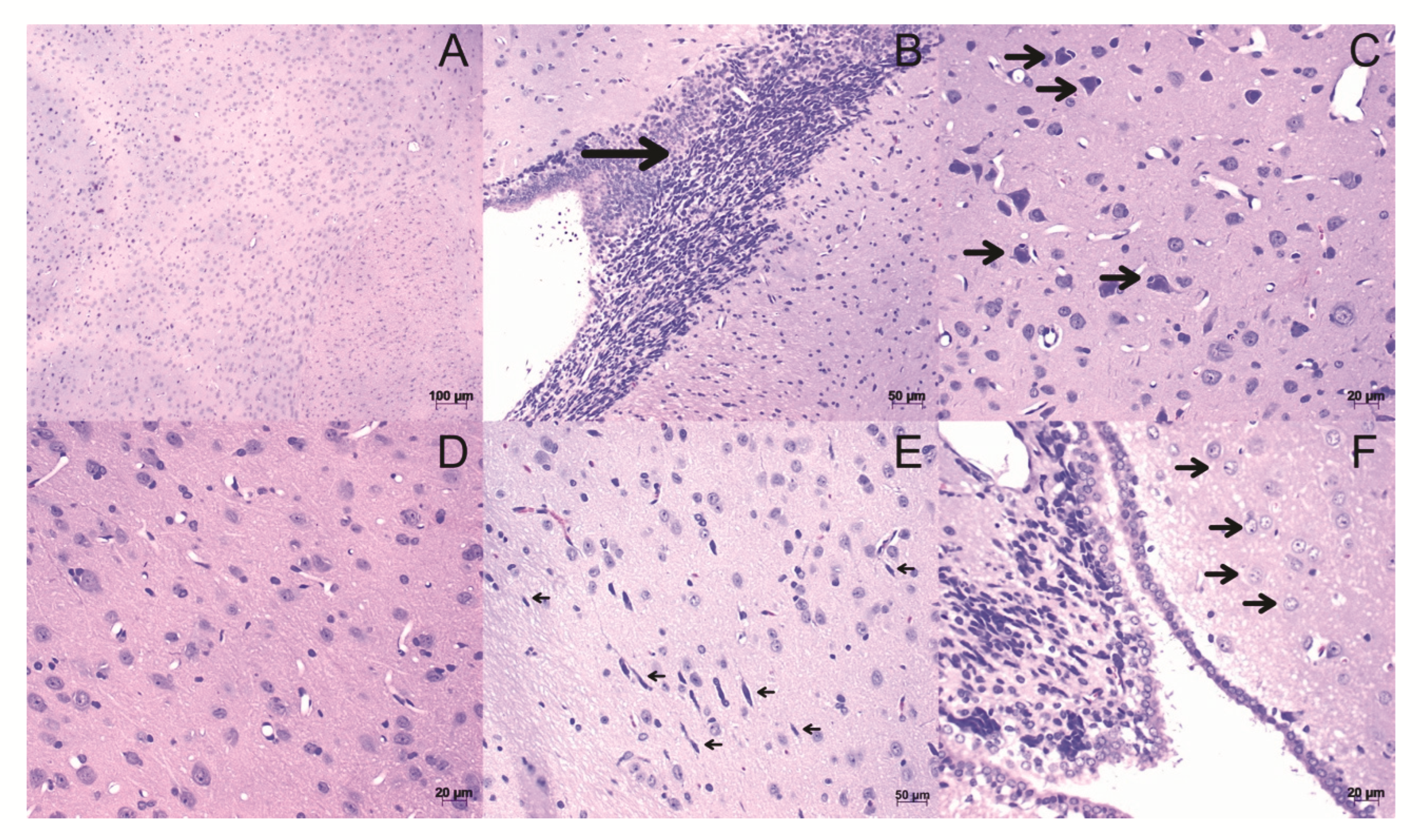
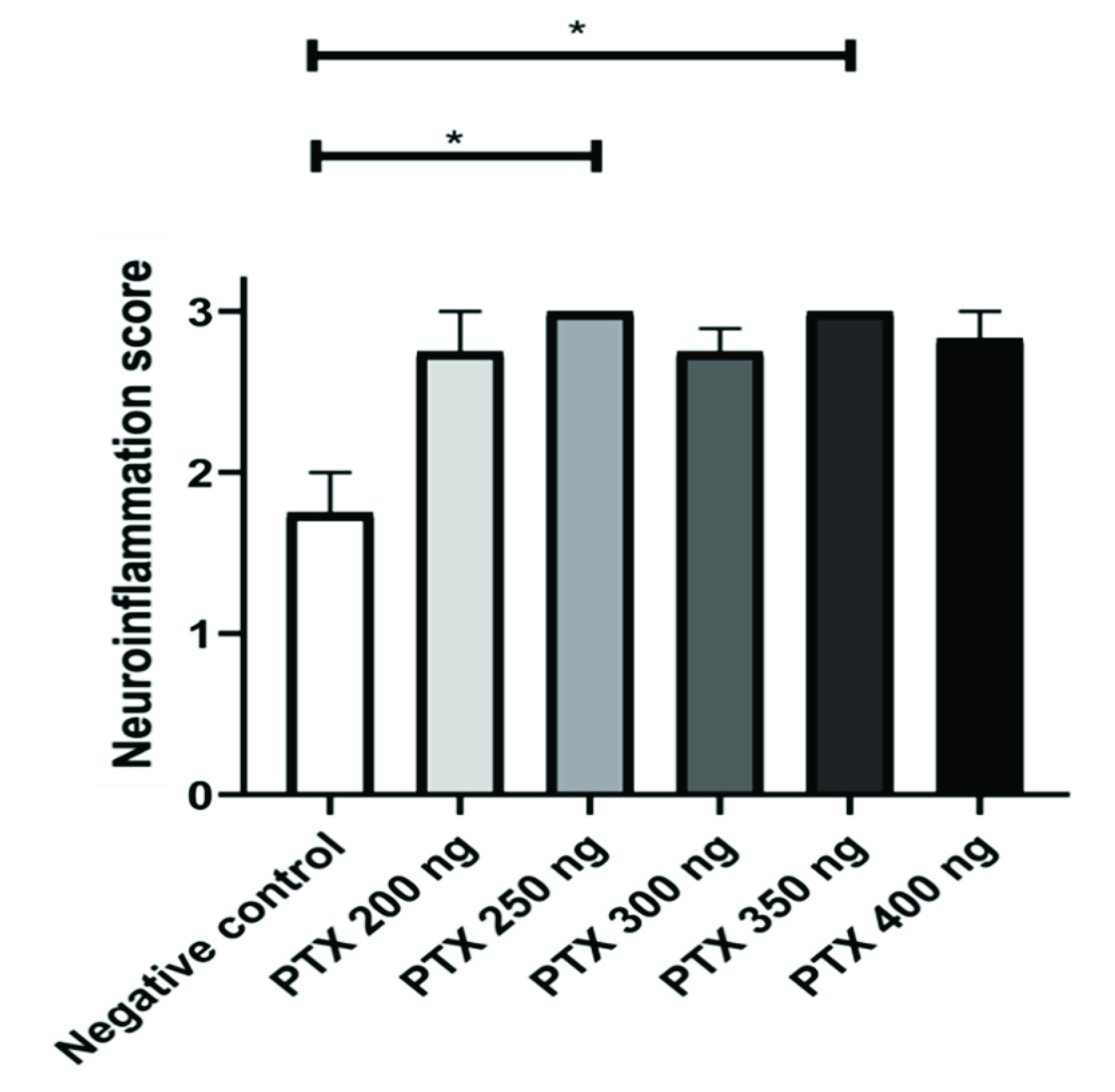
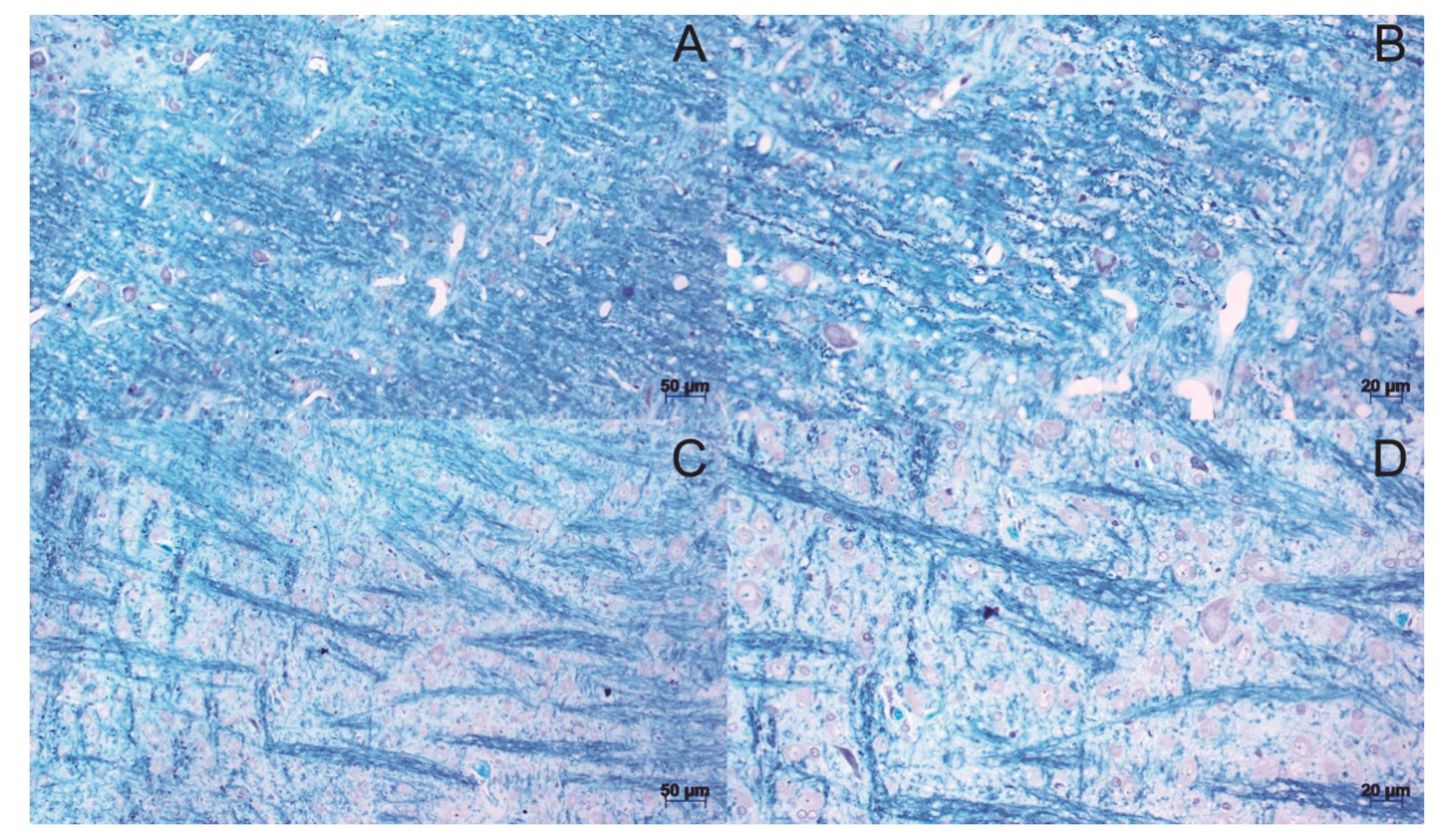
3.3. Histopathological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lemus, H.N.; Warrington, A.E.; Rodriguez, M. Multiple Sclerosis. Neurol. Clin. 2018, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Al Jumah, M.A.; Abumaree, M.H. The Immunomodulatory and Neuroprotective Effects of Mesenchymal Stem Cells (MSCs) in Experimental Autoimmune Encephalomyelitis (EAE): A Model of Multiple Sclerosis (MS). Int. J. Mol. Sci. 2012, 13, 9298–9331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, A.J.; Baranzini, S.E.; Geurts, J.; Hemmer, B.; Ciccarelli, O. Multiple sclerosis. Lancet 2018, 391, 1622–1636. [Google Scholar] [CrossRef]
- Browne, P.; Chandraratna, D.; Angood, C.; Tremlett, H.; Baker, C.; Taylor, B.V.; Thompson, A.J. Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology 2014, 83, 1022–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waubant, E.; Lucas, R.; Mowry, E.; Graves, J.; Olsson, T.; Alfredsson, L.; Langer-Gould, A. Environmental and genetic risk factors for MS: An integrated review. Ann. Clin. Transl. Neurol. 2019, 6, 1905–1922. [Google Scholar] [CrossRef] [PubMed]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Lublin, F.D. New Multiple Sclerosis Phenotypic Classification. Eur. Neurol. 2014, 72, 1–5. [Google Scholar] [CrossRef]
- Faissner, S.; Plemel, J.R.; Gold, R.; Yong, W. Progressive multiple sclerosis: From pathophysiology to therapeutic strategies. Nat. Rev. Drug Discov. 2019, 18, 905–922. [Google Scholar] [CrossRef]
- Biotti, D.; Ciron, J. First-line therapy in relapsing remitting multiple sclerosis. Rev. Neurol. 2018, 174, 419–428. [Google Scholar] [CrossRef]
- Maillart, E. Treatment of progressive multiple sclerosis: Challenges and promising perspectives. Rev. Neurol. 2018, 174, 441–448. [Google Scholar] [CrossRef]
- Baker, D.; Amor, S. Experimental autoimmune encephalomyelitis is a good model of multiple sclerosis if used wisely. Mult. Scler. Relat. Disord. 2014, 3, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, C.S.; Farooqi, N.; O’Brien, K.; Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS): EAE as model for MS. Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef] [PubMed]
- Bjelobaba, I.; Begovic-Kupresanin, V.; Pekovic, S.; Lavrnja, I. Animal models of multiple sclerosis: Focus on experimental autoimmune encephalomyelitis. J. Neurosci. Res. 2018, 96, 1021–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, S.; Doering, A.; Letiembre, M.; Liu, Y.; Hao, W.; Diem, R.; Bernreuther, C.; Glatzel, M.; Engelhardt, B.; Fassbender, K. The LPS receptor, CD14, in experimental autoimmune encephalomyelitis and multiple sclerosis. Cell. Physiol. Biochem. 2006, 17, 167–172. [Google Scholar] [CrossRef]
- Janova, H.; Böttcher, C.; Holtman, I.R.; Regen, T.; van Rossum, D.; Götz, A.; Ernst, A.-S.; Fritsche, C.; Gertig, U.; Saiepour, N.; et al. CD14 is a key organizer of microglial responses to CNS infection and injury. Glia 2016, 64, 635–649. [Google Scholar] [CrossRef]
- Prinz, M.; Garbe, F.; Schmidt, H.; Mildner, A.; Gutcher, I.; Wolter, K.; Piesche, M.; Schroers, R.; Weiss, E.; Kirschning, C.J.; et al. Innate immunity mediated by TLR9 modulates pathogenicity in an animal model of multiple sclero-sis. J. Clin. Investig. 2006, 116, 456–464. [Google Scholar] [CrossRef] [Green Version]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 1–22. [Google Scholar] [CrossRef]
- Giladi, A.; Wagner, L.K.; Li, H.; Dörr, D.; Medaglia, C.; Paul, F.; Shemer, A.; Jung, S.; Yona, S.; Mack, M.; et al. Cxcl10+ monocytes define a pathogenic subset in the central nervous system during autoimmune neuroinflammation. Nat. Immunol. 2020, 21, 525–534. [Google Scholar] [CrossRef]
- Lando, Z.; Teitelbaum, D.; Arnon, R. Induction of experimental allergic encephalomyelitis in genetically resistant strains of mice. Nature 1980, 287, 551–552. [Google Scholar] [CrossRef]
- Hofstetter, H.H.; Shive, C.L.; Forsthuber, T.G. Pertussis toxin modulates the immune response to neuroantigens injected in incomplete Freund’s adjuvant: Induction of Th1 cells and experimental autoimmune encephalomyelitis in the presence of high frequencies of Th2 cells. J. Immunol. 2002, 169, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Kerfoot, S.M.; Kubes, P. Overlapping Roles of P-Selectin and 4 Integrin to Recruit Leukocytes to the Central Nervous System in Experimental Autoimmune Encephalomyelitis. J. Immunol. Res. 2002, 169, 1000–1006. [Google Scholar] [CrossRef] [Green Version]
- Racke, M.K.; Quigley, L.; Cannella, B.; Raine, C.S.; McFarlin, D.R.; Scott, D.E. Superantigen modulation of experimental allergic encephalomyelitis: Activation of energy determines outcome. J. Immunol. 1994, 152, 2051–2059. [Google Scholar] [PubMed]
- Curtis, A.D., II; Taslim, N.; Reece, S.P.; Grebenciucova, E.; Ray, R.H.; Rosenbaum, M.D.; Wardle, R.L.; Scott, M.R.V.; Mannie, M.D. The Extracellular Domain of Myelin Oligodendrocyte Glycoprotein Elicits Atypical Experimental Autoimmune Encephalomyelitis in Rat and Macaque Species. PLoS ONE 2015, 9, e0117878. [Google Scholar] [CrossRef]
- Li, Z.; Liu, F.; He, X.; Yang, X.; Shan, F.; Feng, J. Exosomes derived from mesenchymal stem cells attenuate inflammation and demyelination of the central nervous system in EAE rats by regulating the polarization of microglia. Int. Immunopharmacol. 2019, 67, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Rosenling, T.; Stoop, M.P.; Attali, A.; van Aken, H.; Suidgeest, E.; Christin, C.; Stingl, C.; Suits, F.; Horvatovich, P.; Hintzen, R.Q.; et al. Profiling and Identification of Cerebrospinal Fluid Proteins in a Rat EAE Model of Multiple Sclerosis. J. Proteome Res. 2012, 11, 2048–2060. [Google Scholar] [CrossRef]
- Palumbo, S.; Pellegrini, S. Experimental in vivo models of multiple sclerosis: State of the art. In Multiple Sclerosis: Perspectives in Treatment and Pathogenesis; Zagon, I.S., McLaughlin, P.J., Eds.; Codon Publications: Brisbane, QLD, Australia, 2017; pp. 173–183. [Google Scholar] [CrossRef] [Green Version]
- Glatigny, S.; Bettelli, E. Experimental Autoimmune Encephalomyelitis (EAE) as Animal Models of Multiple Sclerosis (MS). Cold. Spring Harb. Perspect. Med. 2018, 8, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Mohajeri, M.; Sadeghizadeh, M.; Javan, M. Pertussis toxin promotes relapsing–remitting experimental autoimmune encephalomyelitis in Lewis rats. J. Neuroimmunol. 2015, 289, 105–110. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Y.; Lian, Q.; Yang, B.; Ma, Y.; Wu, X.; Sun, S.; Liu, Y.; Sun, B. Differential IL-10 production by DCs determines the distinct adjuvant effects of LPS and PTX in EAE induction. Eur. J. Immunol. 2014, 44, 1352–1362. [Google Scholar] [CrossRef]
- Ronchi, F.; Basso, C.; Preite, S.; Preite, S.; Reboldi, A.; Baumjohann, D.; Perlini, L.; Lanzavecchia, A.; Sallusto, F. Experimental priming of encephalitogenic Th1/Th17 cells requires pertussis toxin-driven IL-1β production by myeloid cells. Nat. Commun. 2016, 7, 11541. [Google Scholar] [CrossRef]
- Toader, L.E.; Rosu, G.C.; Catalin, B.; Tudorica, V.; Pirici, I.; Taisescu, O.; Muresanu, D.F. Clinical and Histopathological Assessment on an Animal Model with Experimental Autoimmune Encephalomyelitis. Curr. Health Sci. J. 2018, 44, 280–287. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, H.; Cui, L. Activation of astrocytes and expression of inflammatory cytokines in rats with experimental autoimmune encephalomyelitis. Exp. Ther. Med. 2018, 16, 4401–4406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gibson-Corley, K.N.; Boyden, A.W.; Leidinger, M.R.; Lambertz, A.M.; Ofori-Amanfo, G.; Naumann, P.W.; Goeken, J.A.; Karandikar, N.J. A method for histopathological study of the multifocal nature of spinal cord lesions in murine experimental autoimmune encephalomyelitis. PeerJ 2016, 4, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J.; Vidal-Jornada, A.; Montalban, X. Multiple sclerosis: Clinical aspects. Curr. Opin. Neurol. 2018, 31, 752–759. [Google Scholar] [CrossRef] [PubMed]


| Score | Motor Clinical Signs |
|---|---|
| 0 | No signs |
| 1 | Tail paralysis |
| 2 | Tail paralysis and weakness in hind legs |
| 3 | Hind leg paralysis |
| 4 | Hind leg paralysis and weakness in the anterior legs |
| 5 | Total paralysis of the animal |
| Score | Histopathological Findings |
|---|---|
| 0 | No inflammatory cells |
| 1 | A few scattered inflammatory cells |
| 2 | Organization of inflammatory infiltrates around blood vessels |
| 3 | Extensive perivascular cuffing with extension into adjacent parenchyma, or parenchymal infiltration without obvious cuffing |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perussolo, M.C.; Mogharbel, B.F.; Saçaki, C.S.; Dziedzic, D.S.M.; Nagashima, S.; de Meira, L.F.; Guarita-Souza, L.C.; de Noronha, L.; Athayde Teixeira de Carvalho, K. Demyelination Lesions Do Not Correlate with Clinical Manifestations by Bordetella pertussis Toxin Concentrations. Life 2022, 12, 962. https://doi.org/10.3390/life12070962
Perussolo MC, Mogharbel BF, Saçaki CS, Dziedzic DSM, Nagashima S, de Meira LF, Guarita-Souza LC, de Noronha L, Athayde Teixeira de Carvalho K. Demyelination Lesions Do Not Correlate with Clinical Manifestations by Bordetella pertussis Toxin Concentrations. Life. 2022; 12(7):962. https://doi.org/10.3390/life12070962
Chicago/Turabian StylePerussolo, Maiara Carolina, Bassam Felipe Mogharbel, Claudia Sayuri Saçaki, Dilcele Silva Moreira Dziedzic, Seigo Nagashima, Leanderson Franco de Meira, Luiz Cesar Guarita-Souza, Lúcia de Noronha, and Katherine Athayde Teixeira de Carvalho. 2022. "Demyelination Lesions Do Not Correlate with Clinical Manifestations by Bordetella pertussis Toxin Concentrations" Life 12, no. 7: 962. https://doi.org/10.3390/life12070962
APA StylePerussolo, M. C., Mogharbel, B. F., Saçaki, C. S., Dziedzic, D. S. M., Nagashima, S., de Meira, L. F., Guarita-Souza, L. C., de Noronha, L., & Athayde Teixeira de Carvalho, K. (2022). Demyelination Lesions Do Not Correlate with Clinical Manifestations by Bordetella pertussis Toxin Concentrations. Life, 12(7), 962. https://doi.org/10.3390/life12070962






