Author Contributions
Conceptualization, M.A.R.-R. and G.G.-P.; methodology, M.A.R.-R. and G.G.-P.; validation, D.V.R.-G., A.A.C.-E. and G.G.-P.; formal analysis, D.V.R.-G., A.A.C.-E. and G.G.-P.; investigation, D.V.R.-G., M.A.R.-R., C.E.C.-A., L.B.B.-M., G.A.-R. and J.D.D.-V.; resources, D.V.R.-G., G.C.-G., S.C.-M., G.E.-G. and C.I.; data curation, D.V.R.-G., C.E.C.-A. and G.G.-P.; writing—original draft preparation, D.V.R.-G., A.A.C.-E. and G.G.-P.; writing—review and editing, D.V.R.-G., A.A.C.-E., J.D.D.-V., G.E.-G., C.I. and G.G.-P.; visualization, D.V.R.-G., A.A.C.-E. and G.G.-P.; supervision, D.V.R.-G. and G.G.-P.; project administration, G.G.-P.; funding acquisition, G.G.-P. All authors have read and agreed to the published version of the manuscript.
Figure 1.
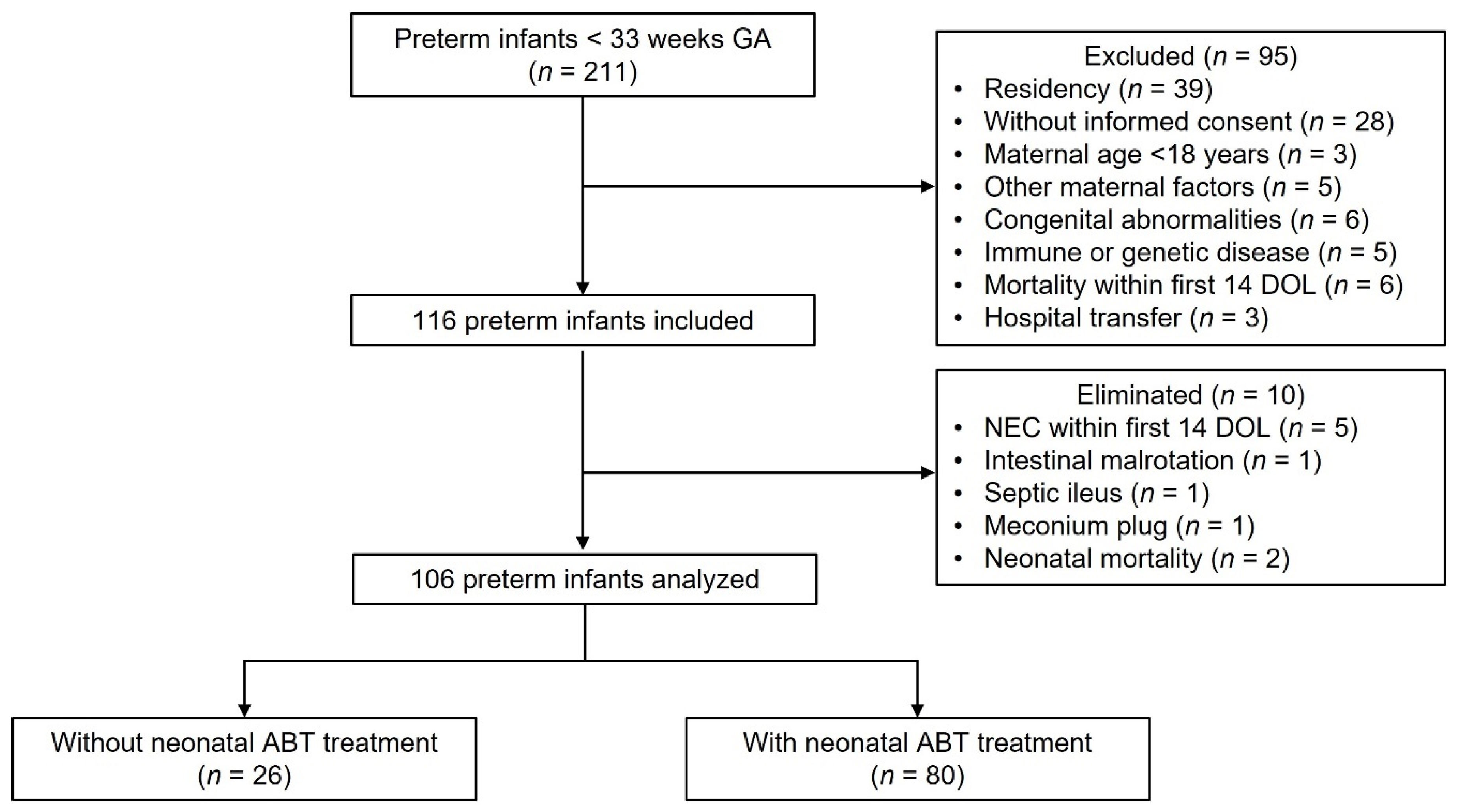
Flow diagram of preterm infants analyzed. Two hundred eleven preterm infants < 33 weeks gestational age were eligible to participate in the study. Ninety-five neonates were excluded, 116 neonates were included, and 10 neonates were eliminated, resulting in 106 neonates analyzed of which 26 were not treated with antibiotics, and 80 were treated with antibiotics during the neonatal period. GA (gestational age), n (number), DOL (day of life), ABT (antibiotic).
Figure 1.
Flow diagram of preterm infants analyzed. Two hundred eleven preterm infants < 33 weeks gestational age were eligible to participate in the study. Ninety-five neonates were excluded, 116 neonates were included, and 10 neonates were eliminated, resulting in 106 neonates analyzed of which 26 were not treated with antibiotics, and 80 were treated with antibiotics during the neonatal period. GA (gestational age), n (number), DOL (day of life), ABT (antibiotic).
Figure 2.
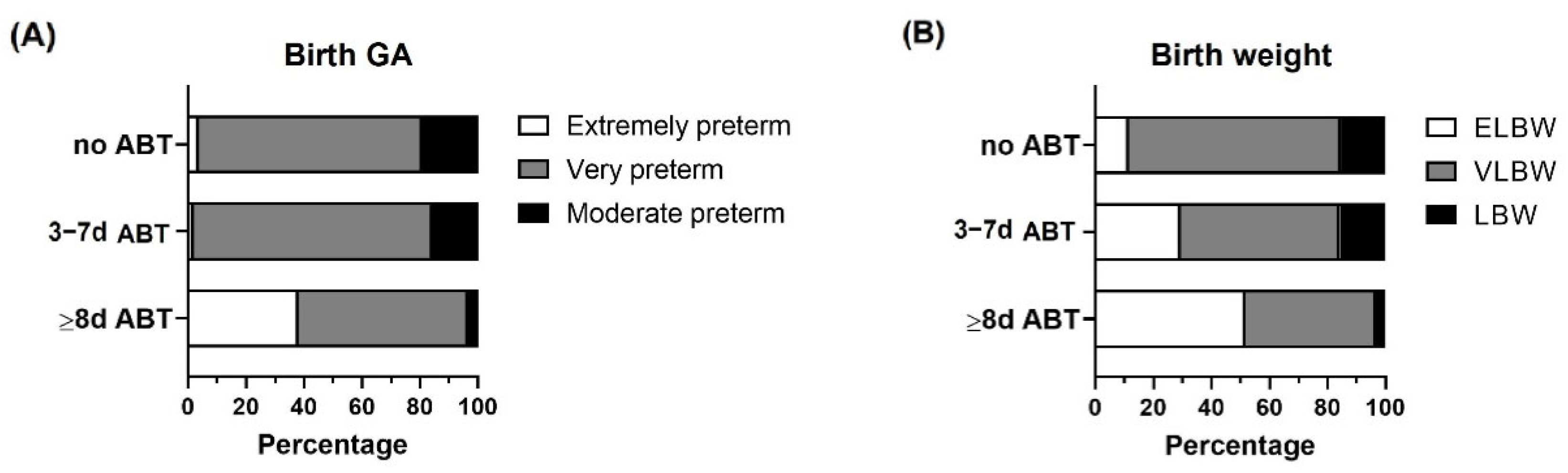
Birth gestational age and birth weight categorization within the study groups. Preterm infants < 33 weeks gestational age (GA) were classified according to neonatal antibiotic treatment days in 3 groups: no antibiotics (no ABT, n = 26), three to seven antibiotic days (3–7 d ABT, n = 51), and eight or more antibiotic days (≥8 d ABT, n = 29). (A) Birth GA classification (extremely preterm, very preterm, moderate preterm), (B) Birth weight classification, extremely low birth weight (ELBW), very low birth weight (VLBW), low birth weight (LBW).
Figure 2.
Birth gestational age and birth weight categorization within the study groups. Preterm infants < 33 weeks gestational age (GA) were classified according to neonatal antibiotic treatment days in 3 groups: no antibiotics (no ABT, n = 26), three to seven antibiotic days (3–7 d ABT, n = 51), and eight or more antibiotic days (≥8 d ABT, n = 29). (A) Birth GA classification (extremely preterm, very preterm, moderate preterm), (B) Birth weight classification, extremely low birth weight (ELBW), very low birth weight (VLBW), low birth weight (LBW).
Figure 3.
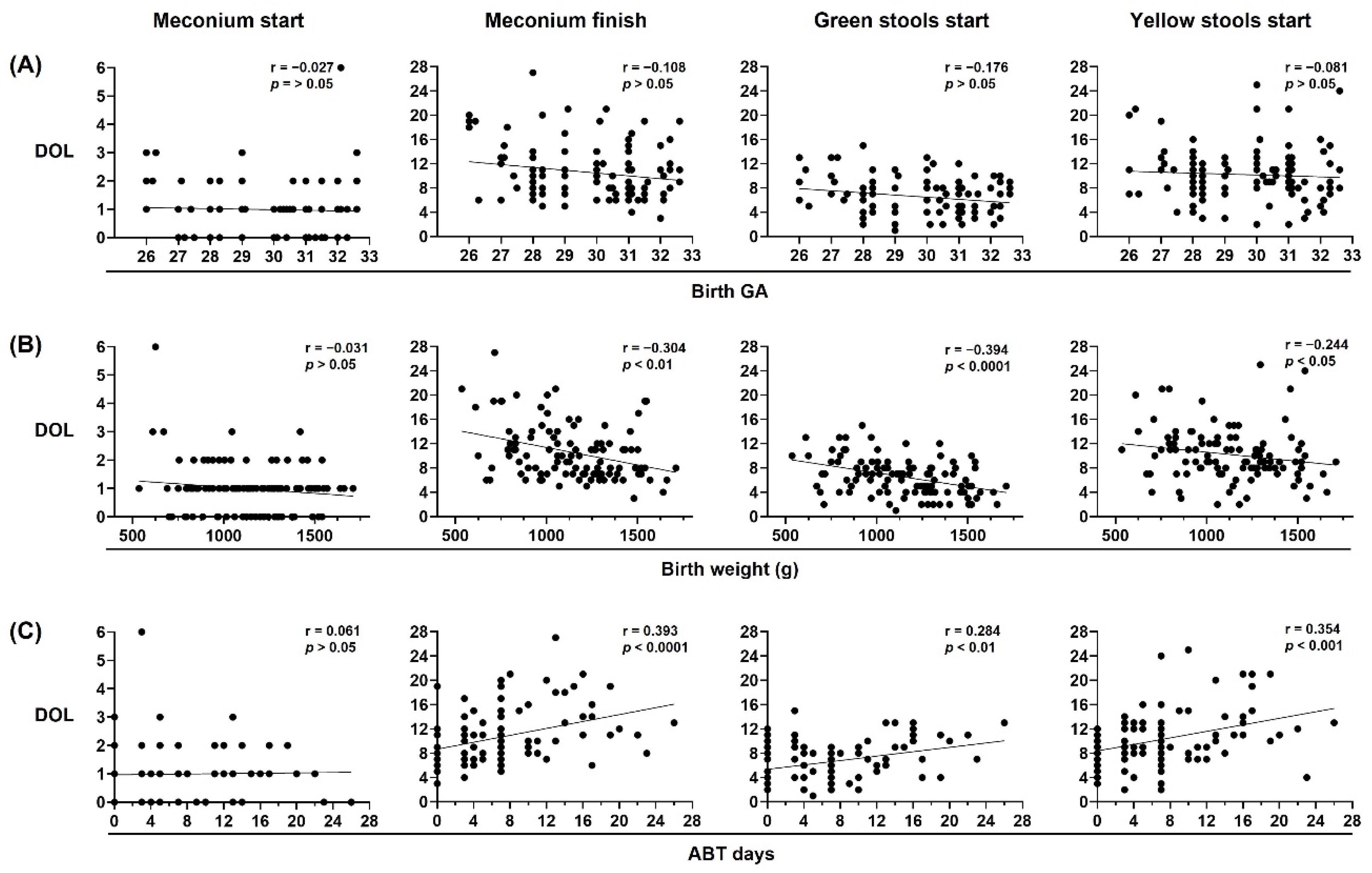
Birth weight and neonatal antibiotic treatment are major contributors to neonatal stool pattern in preterm infants. Neonatal stool pattern from preterm infants < 33 weeks gestational age (GA) at birth (n = 106). Day of life (DOL) on which meconium started, meconium finished, green stools started, and yellow stools started were compared with (A) birth GA, (B) birth weight in grams (g), and (C) antibiotic (ABT) days administered during neonatal period. Simple linear regression analysis; Spearman correlation (r) and p-values (p) are shown.
Figure 3.
Birth weight and neonatal antibiotic treatment are major contributors to neonatal stool pattern in preterm infants. Neonatal stool pattern from preterm infants < 33 weeks gestational age (GA) at birth (n = 106). Day of life (DOL) on which meconium started, meconium finished, green stools started, and yellow stools started were compared with (A) birth GA, (B) birth weight in grams (g), and (C) antibiotic (ABT) days administered during neonatal period. Simple linear regression analysis; Spearman correlation (r) and p-values (p) are shown.
Figure 4.
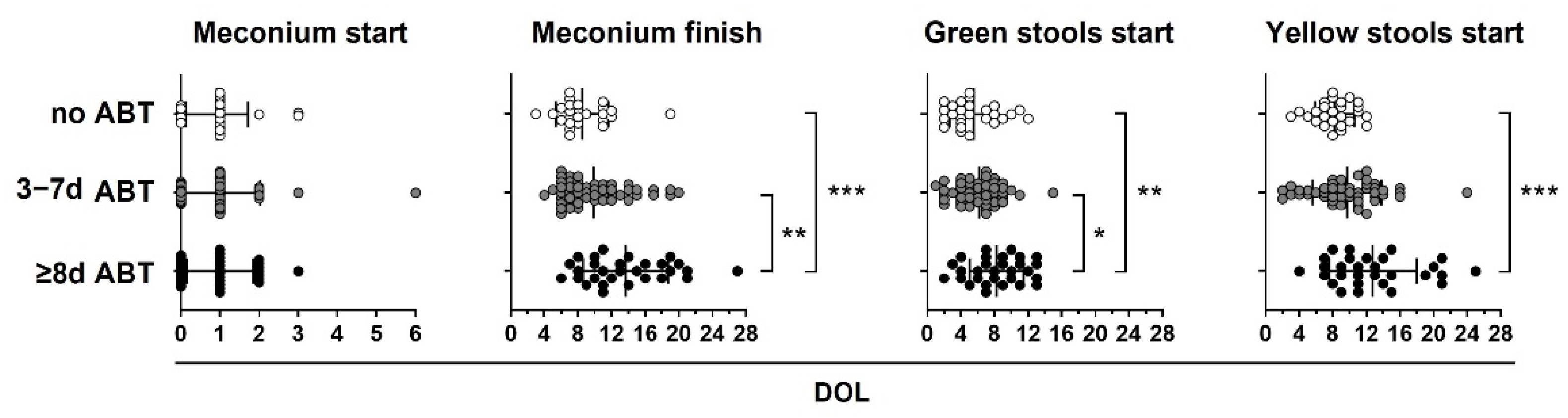
Neonatal antibiotic treatment equal to or greater than 8 days delay in meconium finish, green start, and yellow start stools in preterm infants. Preterm infants < 33 weeks gestational age (GA) were classified according to neonatal antibiotic treatment days in three groups: no antibiotics (no ABT, white circles, n = 26), three to seven antibiotic days (3–7 d ABT, gray circles, n = 51), and eight or more antibiotic days (≥8 d ABT, black circles n = 29). The day of life (DOL) in which meconium started, meconium finished, green stools started, and yellow stools started is shown. Kruskal–Wallis test with Dunn’s post hoc test, p < 0.05 (*), p < 0.01 (**), p < 0.001 (***).
Figure 4.
Neonatal antibiotic treatment equal to or greater than 8 days delay in meconium finish, green start, and yellow start stools in preterm infants. Preterm infants < 33 weeks gestational age (GA) were classified according to neonatal antibiotic treatment days in three groups: no antibiotics (no ABT, white circles, n = 26), three to seven antibiotic days (3–7 d ABT, gray circles, n = 51), and eight or more antibiotic days (≥8 d ABT, black circles n = 29). The day of life (DOL) in which meconium started, meconium finished, green stools started, and yellow stools started is shown. Kruskal–Wallis test with Dunn’s post hoc test, p < 0.05 (*), p < 0.01 (**), p < 0.001 (***).
Figure 5.
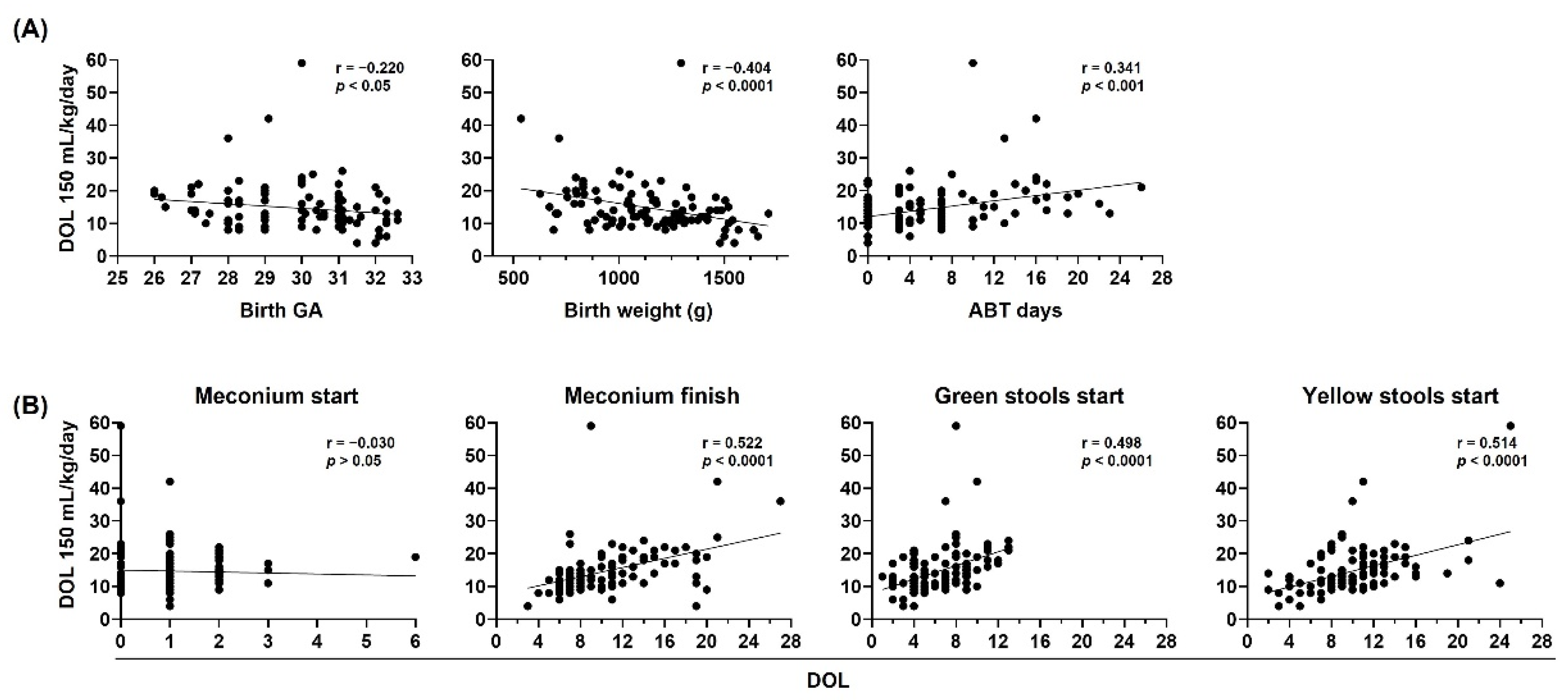
Birth gestational age, birth weight, neonatal antibiotic treatment, and neonatal stool pattern correlate with oral tolerance in preterm infants. The day of life (DOL) to reach 150 mL/kg/day in preterm infants < 33 weeks gestational age (GA) (n = 106) was compared with (A) birth GA (left), birth weight in grams (g) (center), and antibiotic (ATB) days administered during neonatal period (right), (B) DOL on which meconium started, meconium finished, green stools started, and yellow stools started. Simple linear regression analysis, Spearman correlation (r) and p-values (p) are shown.
Figure 5.
Birth gestational age, birth weight, neonatal antibiotic treatment, and neonatal stool pattern correlate with oral tolerance in preterm infants. The day of life (DOL) to reach 150 mL/kg/day in preterm infants < 33 weeks gestational age (GA) (n = 106) was compared with (A) birth GA (left), birth weight in grams (g) (center), and antibiotic (ATB) days administered during neonatal period (right), (B) DOL on which meconium started, meconium finished, green stools started, and yellow stools started. Simple linear regression analysis, Spearman correlation (r) and p-values (p) are shown.
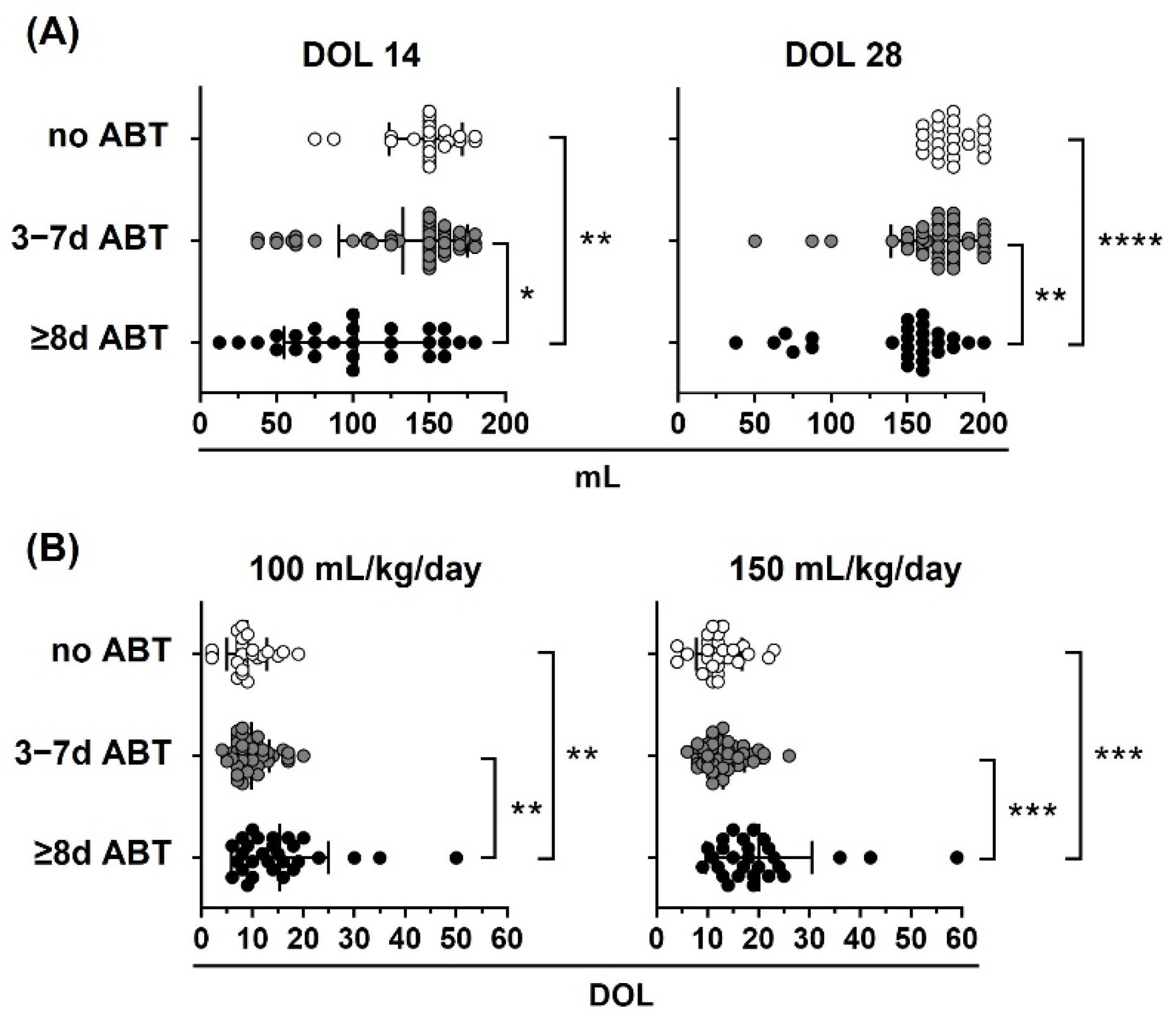
Figure 6.
Neonatal antibiotic treatment affects oral tolerance in preterm infants. Preterm infants < 33 weeks gestational age (GA) were classified according to neonatal antibiotic treatment days in three groups: no antibiotics (no ABT, white circles, n = 26), three to seven antibiotic days (3–7 d ABT, gray circles, n = 51), and eight or more antibiotic days (≥8 d ABT, black circles, n = 29). (A) Oral volume reached at 14 and 28 days of life (DOL), (B) DOL to reach 100 and 150 mL/kg/day. Kruskal–Wallis test with Dunn’s post hoc test; p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****).
Figure 6.
Neonatal antibiotic treatment affects oral tolerance in preterm infants. Preterm infants < 33 weeks gestational age (GA) were classified according to neonatal antibiotic treatment days in three groups: no antibiotics (no ABT, white circles, n = 26), three to seven antibiotic days (3–7 d ABT, gray circles, n = 51), and eight or more antibiotic days (≥8 d ABT, black circles, n = 29). (A) Oral volume reached at 14 and 28 days of life (DOL), (B) DOL to reach 100 and 150 mL/kg/day. Kruskal–Wallis test with Dunn’s post hoc test; p < 0.05 (*), p < 0.01 (**), p < 0.001 (***), p < 0.0001 (****).
Table 1.
Demographic, nutritional, and maternal data from study cohort.
Table 1.
Demographic, nutritional, and maternal data from study cohort.
| Neonatal Variable | No ABT (n = 26) | 3–7 d ABT (n = 51) | ≥8 d ABT (n = 29) | p-Value |
|---|
| Demographic | | | | |
| C-section (%) | 24 (92.3) | 47 (92.2) | 22 (75.9) | 0.102 |
| GA (weeks) | 30.5 ± 1.6 | 30 ± 1.6 | 28.8 ± 1.9 | 0.0008 |
| Female (%) | 13 (50) | 20 (39.2) | 14 (48.3) | 0.589 |
| Singleton (%) | 15 (57.7) | 37 (72.5) | 24 (82.8) | 0.118 |
| Twins (%) | 6 (23.1) | 8 (15.7) | 4 (13.8) | 0.632 |
| Triplets (%) | 5 (19.2) | 6 (11.8) | 1 (3.4) | 0.192 |
| IUGR (%) | 6 (23.1) | 13 (25.5) | 12 (41.4) | 0.236 |
| Birth weight (g) | 1267 ± 221.5 | 1150 ± 268.8 | 998 ± 256 | 0.0007 |
| Birth weight recovery days | 11 ± 3.8 | 10.3 ± 4.3 | 10.3 ± 2.9 | 0.567 |
| Prenatal steroid (%) | 18 (69.2) | 44 (86.3) | 25 (86.2) | 0.145 |
| Postnatal steroid (%) | 4 (15.4) | 14 (27.5) | 14 (48.3) | 0.025 |
| Surfactant (%) | 9 (34.6) | 28 (54.9) | 22 (75.9) | 0.009 |
| Magnesium sulfate (%) | 19 (73.1) | 36 (70.6) | 24 (82.8) | 0.477 |
| NIV (%) | 26 (100) | 50 (98.0) | 29 (100) | 1.0 |
| IMV (%) | 4 (15.4) | 29 (56.9) | 22 (75.9) | <0.0001 |
| Prenatal ABT (%) | 22 (84.6) | 45 (88.2) | 23 (79.3) | 0.566 |
| Neonatal ABT days | 0 | 5.5 ± 1.7 | 14.8 ± 4.5 | - |
| Total ABT days | 3.5 ± 7.4 | 15.6 ± 9.4 | 27.2 ± 19.1 | <0.0001 |
| Hospital stay days | 48.7 ± 19.3 | 64.3 ± 24.2 | 73 ± 28.2 | 0.001 |
| Nutritional | | | | |
| Enteral feeding start day | 1.5 ± 1.2 | 1.5 ± 1.1 | 1.5 ± 1.9 | 0.823 |
| HM (%) | 3 (11.5) | 6 (11.8) | 5 (17.2) | 0.755 |
| HM + FHM (%) | 11 (42.3) | 22 (43.1) | 17 (58.6) | 0.348 |
| HM + FHM + PF (%) | 5 (19.2) | 15 (29.4) | 2 (6.9) | 0.056 |
| HM + PF (%) | 7 (26.9) | 8 (15.7) | 5 (17.2) | 0.474 |
| Neonatal PN days | 11 ± 7.3 | 16.1 ± 8.1 | 19.8 ± 6.5 | 0.0002 |
| Total PN days | 12.5 ± 11.3 | 18.7 ± 13.4 | 24.7 ± 16.1 | 0.0001 |
| Neonatal fast days | 1.2 ± 2.1 | 1.2 ± 1.6 | 2.8 ± 3.1 | 0.025 |
| Maternal | | | | |
| Maternal age (years) | 27.8 ± 6.9 | 28 ± 5.6 | 30.8 ± 6.6 | 0.169 |
| Chorioamnionitis (%) | 2 (7.7) | 6 (11.8) | 5 (17.2) | 0.553 |
| PROM >18 h (%) | 1 (3.8) | 12 (23.5) | 4 (13.8) | 0.087 |
| Healthy weight * (%) | 10 (38.5) | 21 (41.2) | 13 (44.8) | 0.905 |
| Underweight * (%) | 0 | 2 (3.9) | 1 (3.4) | 0.801 |
| Overweight * (%) | 13 (50.0) | 17 (33.3) | 8 (27.6) | 0.195 |
| Obese * (%) | 3 (11.5) | 11 (21.6) | 7 (24.1) | 0.458 |
| Preeclampsia (%) | 13 (50.0) | 18 (35.3) | 16 (55.2) | 0.182 |
| High blood pressure (%) | 4 (15.4) | 4 (7.8) | 7 (24.1) | 0.138 |
| Hypothyroidism (%) | 5 (19.2) | 6 (11.8) | 3 (10.3) | 0.613 |
| Diabetes (%) | 2 (7.7) | 1 (2.0) | 2 (6.9) | 0.372 |
| Renal disease (%) | 3 (11.5) | 2 (3.9) | 1 (3.4) | 0.417 |
| Autoimmune disease (%) | 2 (7.7) | 8 (15.7) | 2 (6.9) | 0.486 |
Table 2.
Preterm infant diseases during hospital stay.
Table 2.
Preterm infant diseases during hospital stay.
| Preterm Infant Disease | No ABT (n = 26) | 3–7 d ABT (n = 51) | ≥8 d ABT (n = 29) | p-Value |
|---|
| Culture-confirmed EOS (%) | 0 | 1 (2.0) | 1 (3.4) | 0.999 |
| Culture-confirmed LOS (%) | 0 | 5 (9.8) | 12 (41.4) | <0.0001 |
| Septic shock (%) | 0 | 1 (2.0) | 5 (17.2) | 0.009 |
| NEC I (%) | 3 (11.5) | 8 (15.7) | 5 (17.2) | 0.883 |
| NEC II (%) | 1 (3.8) | 8 (15.7) | 3 (10.3) | 0.351 |
| Atypical pneumonia (%) | 2 (7.7) | 17 (33.3) | 12 (42.9) | 0.016 |
| Moderate BPD (%) | 5 (19.2) | 21 (41.2) | 17 (58.6) | 0.012 |
| Severe BPD (%) | 3 (11.5) | 8 (15.7) | 4 (13.8) | 0.937 |
| PDA (%) | 6 (23.1) | 16 (31.4) | 14 (50.0) | 0.124 |
| Anemia (%) | 10 (38.5) | 25 (49.0) | 19 (65.5) | 0.125 |
| ROP (%) | 2 (7.7) | 20 (39.2) | 16 (55.2) | <0.001 |
| GERD (%) | 11 (42.3) | 21 (41.2) | 10 (34.5) | 0.799 |
| Oral intolerance (%) | 2 (7.7) | 7 (13.7) | 5 (17.2) | 0.656 |
Table 3.
Neonatal stool pattern from preterm infants <33 weeks GA.
Table 3.
Neonatal stool pattern from preterm infants <33 weeks GA.
| Stool Variable | No ABT (n = 26) | 3–7 d ABT (n = 51) | ≥8 d ABT (n = 29) | p-Value |
|---|
| Meconium start DOL | 0.9 ± 0.8 | 1 ± 1 | 1 ± 0.8 | 0.895 |
| Meconium finish DOL | 8.5 ± 3.1 | 9.9 ± 4 | 13.7 ± 5.1 | 0.0001 |
| Green stools start DOL | 5.5 ± 2.9 | 6.2 ±2.7 | 8.2 ± 3.2 | 0.0027 |
| Yellow stools start DOL | 8.2 ± 2.3 | 9.7 ± 4.1 | 12.8 ± 5.2 | 0.0012 |
| Days without BM | 1.5 ± 2.34 | 1.6 ± 1.96 | 3.2 ± 2.6 | 0.002 |
Table 4.
Multiple linear regression model summary for meconium finish day.
Table 4.
Multiple linear regression model summary for meconium finish day.
| Predictor | B (95% CI) | SE B | Beta | p-Value |
|---|
| Model 1 a | | | | |
| Neonatal ABT | 2.597 (1.33, 3.87) | 0.639 | 0.422 | 0.000 |
| Model 2 b | | | | |
| Neonatal ABT | 2.046 (0.72, 3.38) | 0.669 | 0.332 | 0.003 |
| Model 3 c | | | | |
| Neonatal ABT | 2.291 (1.00, 3.58) | 0.648 | 0.372 | 0.001 |
| PROM >18 h | −3.823 (−6.22, −1.42) | 1.208 | −0.315 | 0.002 |
| Model 4 d | | | | |
| Neonatal ABT | 2.249 (0.79, 3.71) | 0.733 | 0.365 | 0.003 |
| PROM >18 h | −3.862 (−6.35, −1.37) | 1.252 | −0.318 | 0.003 |
Table 5.
Multiple linear regression model summary for green stools start day.
Table 5.
Multiple linear regression model summary for green stools start day.
| Predictor | B (95% CI) | SE B | Beta | p-Value |
|---|
| Model 1 a | | | | |
| Neonatal ABT | 0.955 (0.13, 1.78) | 0.415 | 0.235 | 0.024 |
| Birth weight | −1.556 (−2.61, −0.50) | 0.531 | −0.316 | 0.004 |
| Model 2 b | | | | |
| Neonatal ABT | 0.855 (−0.04, 1.75) | 0.450 | 0.211 | 0.061 |
| Birth weight | −1.476 (−2.61, −0.34) | 0.573 | −0.299 | 0.012 |
| Model 3 c | | | | |
| Neonatal ABT | 0.921 (0.02, 1.82) | 0.453 | 0.227 | 0.045 |
| Birth weight | −1–363 (−2.51, −0.22) | 0.576 | −0.277 | 0.020 |
| Model 4 d | | | | |
| Neonatal ABT | 0.461 (−0.49, 1.41) | 0.476 | 0.114 | 0.336 |
| Birth weight | −0.984 (−2.13, 0.16) | 0.576 | −0.200 | 0.091 |
| Enteral feeding start day | 0.458 (0.001, 0.92) | 0.230 | 0.211 | 0.050 |
Table 6.
Multiple linear regression model summary for yellow stools start day.
Table 6.
Multiple linear regression model summary for yellow stools start day.
| Predictor | B (95% CI) | SE B | Beta | p-Value |
|---|
| Model 1 a | | | | |
| Neonatal ABT | 2.461 (1.23, 3.69) | 0.619 | 0.416 | 0.000 |
| Model 2 b | | | | |
| Neonatal ABT | 2.136 (0.83, 3.44) | 0.656 | 0.361 | 0.002 |
| Model 3 c | | | | |
| Neonatal ABT | 2.330 (1.03, 3.63) | 0.653 | 0.394 | 0.001 |
| Model 4 d | | | | |
| Neonatal ABT | 1.754 (0.30, 3.21) | 0.732 | 0.296 | 0.019 |
Table 7.
Multiple linear regression model summary of DOL to reach 100 mL/kg/day.
Table 7.
Multiple linear regression model summary of DOL to reach 100 mL/kg/day.
| Predictor | B (95% CI) | SE B | Beta | p-Value |
|---|
| Model 1 a | | | | |
| Neonatal ABT | 3.025 (1.26, 4.79) | 0.891 | 0.348 | 0.001 |
| Model 2 b | | | | |
| Neonatal ABT | 2.814 (0.89, 4.73) | 0.967 | 0.323 | 0.005 |
| Model 3 c | | | | |
| Neonatal ABT | 3.098 (1.19, 5.01) | 0.960 | 0.356 | 0.002 |
| Model 4 d | | | | |
| Neonatal ABT | 1.842 (−0.18, 3.86) | 1.017 | 0.212 | 0.074 |
| Neonatal PN days | 0.187 (−0.02, 0.40) | 0.105 | 0.236 | 0.077 |
| Neonatal fast days | 0.578 (−0.09, 1.24) | 0.334 | 0.208 | 0.087 |
Table 8.
Multiple linear regression model summary for DOL to reach 150 mL/kg/day.
Table 8.
Multiple linear regression model summary for DOL to reach 150 mL/kg/day.
| Predictor | B (95% CI) | SE B | Beta | p-Value |
|---|
| Model 1 a | | | | |
| Neonatal ABT | 3.791 (1.77, 5.81) | 1.019 | 0.374 | 0.000 |
| Birth weight | −3.104 (−5.69, −0.51) | 1.304 | −0.252 | 0.019 |
| Model 2 b | | | | |
| Neonatal ABT | 3.726 (1.53, 5.92) | 1.104 | 0.367 | 0.001 |
| Birth weight | −3.248 (−6.04, −0.46) | 1.405 | −0.264 | 0.023 |
| Model 3 c | | | | |
| Neonatal ABT | 4.139 (2.00, 6.27) | 1.075 | 0.408 | 0.000 |
| Birth weight | −2.834 (−5.55, −0.12) | 1.366 | −0.230 | 0.041 |
| Model 4 d | | | | |
| Neonatal ABT | 2.579 (0.30, 4.86) | 1.145 | 0.254 | 0.027 |
| Birth weight | −1.920 (−4.67, 0.83) | 1.385 | −0.156 | 0.169 |
| Neonatal PN days | 0.253 (0.02, 0.49) | 0.118 | 0.273 | 0.035 |
Table 9.
Multiple linear regression model summary for volume of enteral feeds at DOL 14.
Table 9.
Multiple linear regression model summary for volume of enteral feeds at DOL 14.
| Predictor | B (95% CI) | SE B | Beta | p-Value |
|---|
| Model 1 a | | | | |
| Neonatal ABT | −23.194 (−37.61, −8.78) | 7.259 | −0.333 | 0.002 |
| Birth weight | 23.191 (4.74, 41.64) | 9.295 | 0.274 | 0.014 |
| Model 2 b | | | | |
| Neonatal ABT | −20.574 (−36.17, −4.97) | 7.854 | −0.295 | 0.010 |
| Birth weight | 19.936 (0.07, 39.80) | 10.002 | 0.236 | 0.049 |
| Model 3 c | | | | |
| Neonatal ABT | −23.563 (−38.73, −8.40) | 7.634 | −0.338 | 0.003 |
| Birth weight | 16.939 (−2.34, 36.21) | 9.701 | 0.200 | 0.084 |
| Model 4 d | | | | |
| Neonatal ABT | −10.235 (−25.79, 5.32) | 7.824 | −0.147 | 0.194 |
| Birth weight | 6.992 (−11.82, 25.80) | 9.461 | 0.083 | 0.462 |
| Neonatal PN days | −2.889 (−4.49, −1.29) | 0.805 | −0.454 | 0.001 |
Table 10.
Multiple linear regression model summary for volume of enteral feeds at DOL 28.
Table 10.
Multiple linear regression model summary for volume of enteral feeds at DOL 28.
| Predictor | B (95% CI) | SE B | Beta | p-Value |
|---|
| Model 1 a | | | | |
| Neonatal ABT | −16.292 (−32.71, 0.13) | 8.271 | −0.212 | 0.052 |
| Sex | 29.156 (6.87, 51.44) | 11.224 | 0.256 | 0.011 |
| Model 2 b | | | | |
| Neonatal ABT | −14.014 (−31.38, 3.36) | 8.746 | −0.182 | 0.113 |
| Sex | 29.664 (6.99, 52.34) | 11.415 | 0.261 | 0.011 |
| IMV | −29.271 (−57.79, −0.75) | 14.359 | −0.257 | 0.044 |
| Surfactant | 17.066 (−8.37, 42.50) | 12.806 | 0.149 | 0.186 |
| Model 3 c | | | | |
| Neonatal ABT | −17.020 (−33.94, −0.10) | 8.518 | −0.221 | 0.049 |
| Sex | 26.708 (4.53, 48.89) | 11.163 | 0.235 | 0.019 |
| IMV | −30.353 (−58.50, −2.20) | 14.170 | −0.267 | 0.035 |
| Surfactant | 26.031 (0.75, 51.32) | 12.728 | 0.228 | 0.044 |
| Model 4 d | | | | |
| Neonatal ABT | 6.395 (−8.87, 21.66) | 7.680 | 0.083 | 0.407 |
| Sex | 14.822 (−4.09, 33.74) | 9.514 | 0.130 | 0.123 |
| IMV | −26.793 (−50.27, −3.31) | 11.811 | −0.236 | 0.026 |
| Surfactant | 21.865 (0.76, 42.97) | 10.616 | 0.191 | 0.042 |
| Enteral feeding start day | 7.769 (0.39, 15.15) | 3.712 | 0.189 | 0.039 |
| Neonatal PN days | −2.098 (−3.67, −0.53) | 0.790 | −0.299 | 0.009 |
| Neonatal fast days | −12.010 (−17.03, −6.99) | 2.525 | −0.489 | 0.000 |