CCL2, CCR2 Gene Variants and CCL2, CCR2 Serum Levels Association with Age-Related Macular Degeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
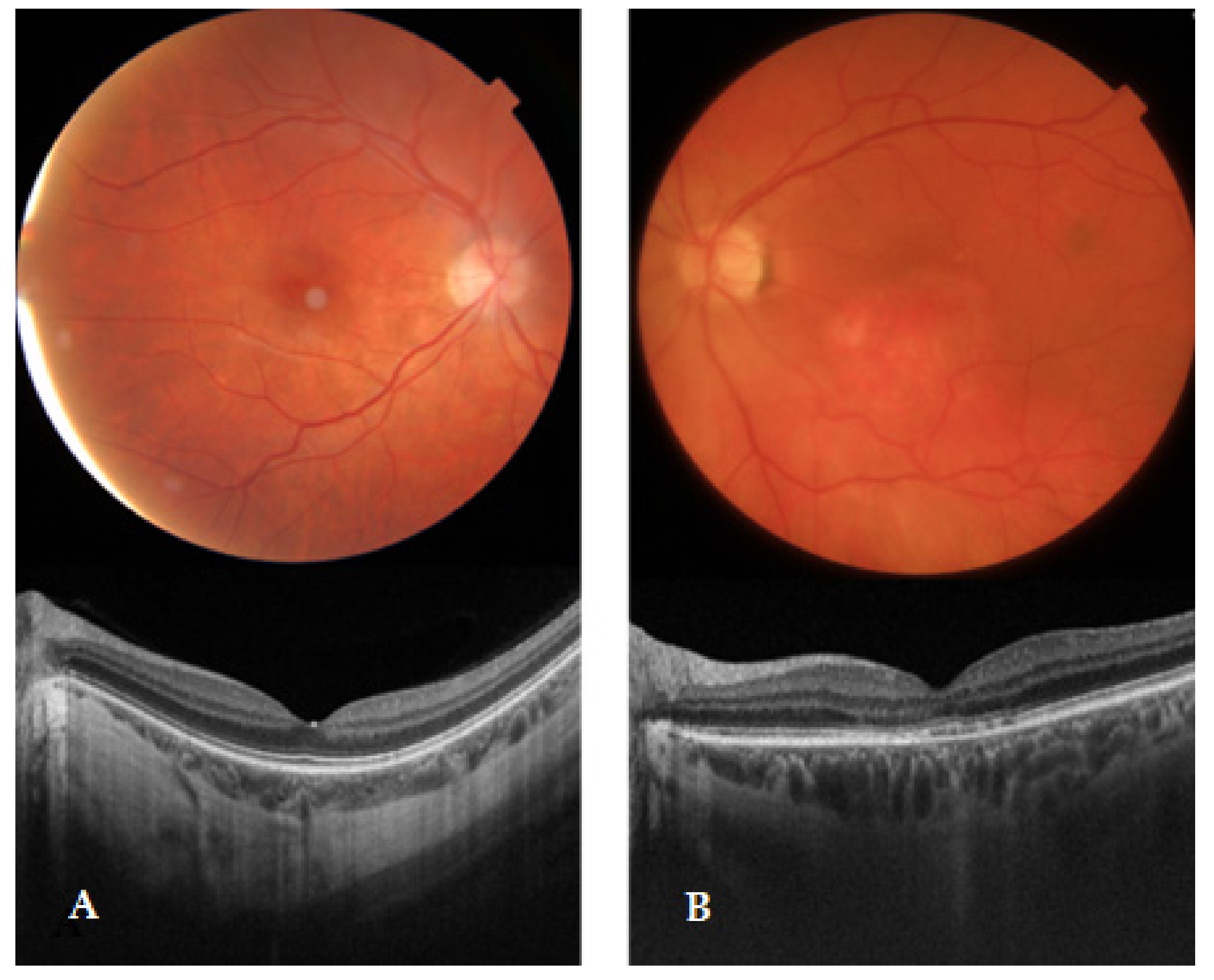
2.2. Ophthalmological Evaluation
2.3. DNA Extraction and Genotyping
2.4. Genotyping Quality Control
2.5. Total Protein Estimation
2.6. Statistical Analysis
3. Results
3.1. Haplotype Associations with AMD
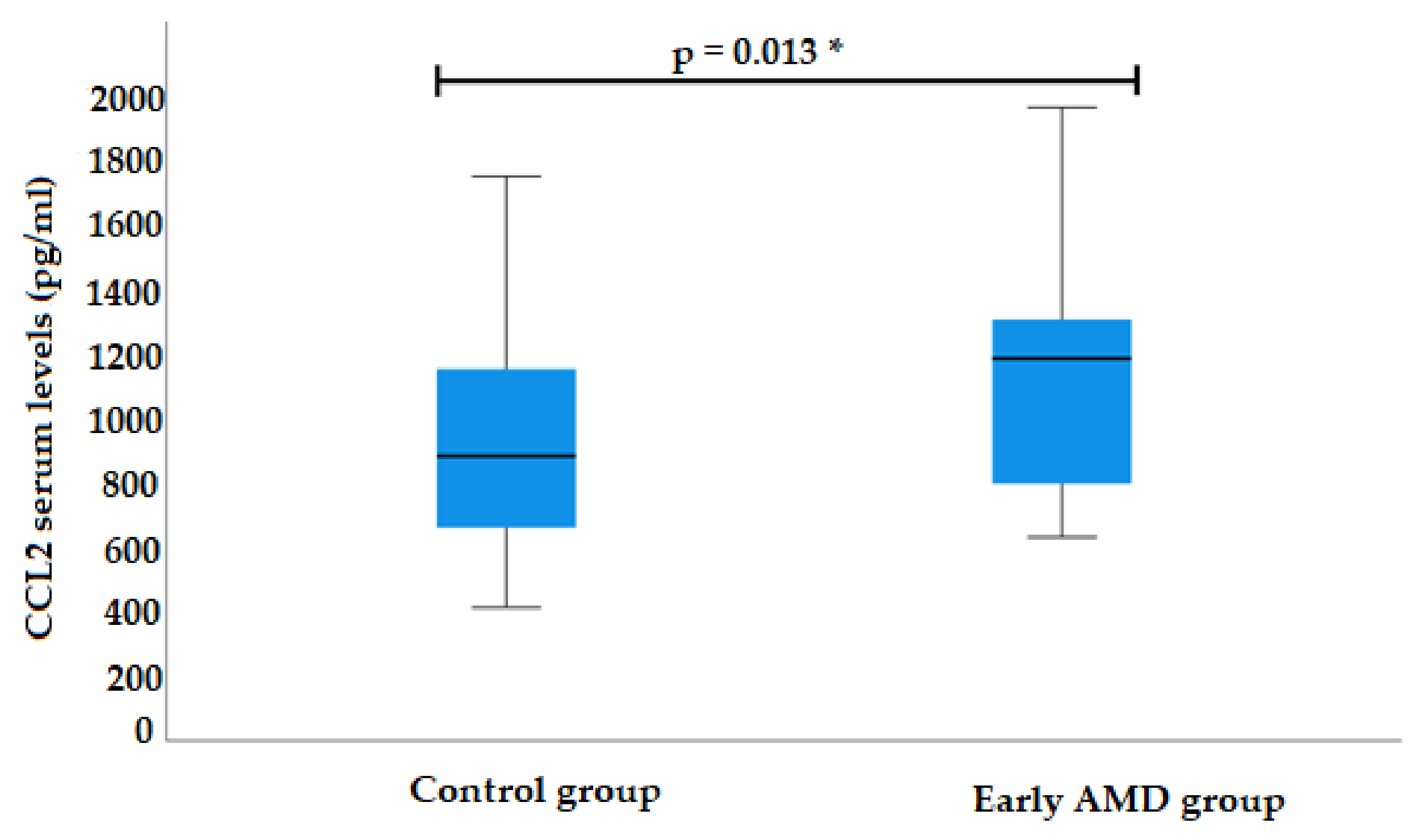
3.2. CCL2 Serum Levels
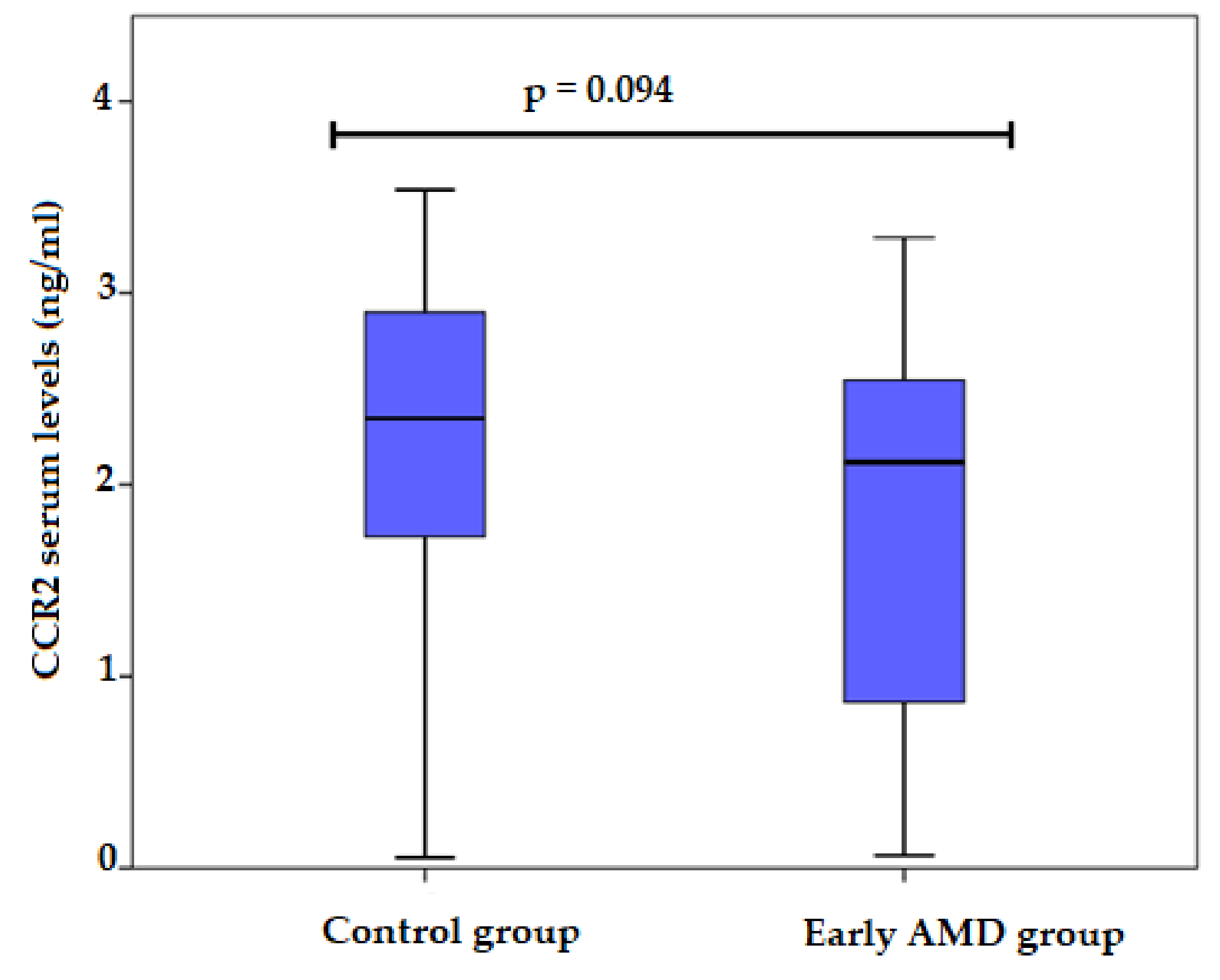
3.3. CCR2 Serum Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klein, R.; Peto, T.; Bird, A.; Vannewkirk, M.R. The epidemiology of age-related macular degeneration. Am. J. Ophthalmol. 2004, 137, 486–495. [Google Scholar] [CrossRef] [PubMed]
- García-Layana, A.; Cabrera-López, F.; García-Arumí, J.; Arias-Barquet, L.; Ruiz-Moreno, J.M. Early and intermediate age-related macular degeneration: Update and clinical review. Clin. Interv. Aging 2017, 12, 1579–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonas, J.B.; Forster, T.M.; Steinmetz, P.; Schlichtenbrede, F.C.; Harder, B.C. Choroidal thickness in age-related macular degeneration. Retina 2014, 34, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Owen, C.G.; Jarrar, Z.; Wormald, R.; Cook, D.G.; Fletcher, A.E.; Rudnicka, A.R. The estimated prevalence and incidence of late stage age related macular degeneration in the UK. Br. J. Ophthalmol. 2012, 96, 752–756. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, R.; Yasuda, M.; Song, S.J.; Chen, S.-J.; Jonas, J.B.; Wang, J.J.; Mitchell, P.; Wong, T.Y. The prevalence of age-related macular degeneration in Asians: A systematic review and meta-analysis. Ophthalmology 2010, 117, 921–927. [Google Scholar] [CrossRef]
- Rudnicka, A.R.; Jarrar, Z.; Wormald, R.; Cook, D.G.; Fletcher, A.; Owen, C.G. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: A meta-analysis. Ophthalmology 2012, 119, 571–580. [Google Scholar] [CrossRef]
- Klaver, C.C.W.; Assink, J.J.M.; Van Leeuwen, R.; Wolfs, R.C.W.; Vingerling, J.R.; Stijnen, T.; Hofman, A.; De Jong, P.T. Incidence and progression rates of age-related maculopathy: The Rotterdam Study. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2237–2241. [Google Scholar]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Hyman, L.; Neborsky, R. Risk factors for age-related macular degeneration: An update. Curr. Opin. Ophthalmol. 2002, 13, 171–175. [Google Scholar] [CrossRef]
- Joachim, N.; Colijn, J.M.; Kifley, A.; Lee, K.E.; Buitendijk, G.H.S.; Klein, B.E.K.; Myers, C.E.; Meuer, S.M.; Tan, A.G.; Holliday, E.G.; et al. Five-year progression of unilateral age-related macular degeneration to bilateral involvement: The Three Continent AMD Consortium report. Br. J. Ophthalmol. 2018, 101, 1185–1192. [Google Scholar] [CrossRef] [Green Version]
- Modenese, A.; Gobba, F. Macular degeneration and occupational risk factors: A systematic review. Int. Arch. Occup. Environ. Health 2019, 92, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Fritsche, L.G.; Fariss, R.N.; Stambolian, D.; Abecasis, R.; Curcio, C.A.; Swaroop, A. Age-related macular degeneration: Genetics and biology coming together. Annu. Rev. Genom. Hum. Genet. 2014, 15, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Mullins, R.F.; Russell, S.R.; Anderson, D.H.; Hageman, G.S. Drusen associated with aging and age-related macular degeneration contain proteins common to extracellular deposits associated with atherosclerosis, elastosis, amyloidosis, and dense deposit disease. FASEB J. 2000, 14, 835–846. [Google Scholar] [CrossRef]
- Forrester, J.V. Macrophages eyed in macular degeneration. Nat. Med. 2003, 9, 2. [Google Scholar] [CrossRef]
- Nussenblatt, R.B.; Ferris, F. Age-related macular degeneration and the immune response: Implications for therapy. Am. J. Ophthalmol. 2007, 144, 618–626. [Google Scholar] [CrossRef] [Green Version]
- Penfold, P.L.; Killingsworth, M.C.; Sarks, S.H. Senile macular degeneration: The involvement of immunocompetent cells. Graefe’s Arch. Clin. Exp. Ophthalmol. 1985, 223, 69–76. [Google Scholar] [CrossRef]
- Kersten, E.; Paun, C.C.; Schellevis, R.L.; Hoyng, C.B.; Delcourt, C.; Lengyel, I.; Peto, T.; Ueffing, M.; Klaver, C.C.; Dammeier, S.; et al. Systemic and ocular fluid compounds as potential biomarkers in age-related macular degeneration. Surv. Ophthalmol. 2018, 63, 9–39. [Google Scholar] [CrossRef] [Green Version]
- Anand, A.; Sharma, N.K.; Gupta, A.; Prabhakar, S.; Sharma, S.K.; Singh, R.; Gupta, P.K. Single nucleotide polymorphisms in MCP-1 and its receptor are associated with the risk of age related macular degeneration. PLoS ONE 2012, 7, e49905. [Google Scholar] [CrossRef] [Green Version]
- Cochran, B.H.; Reffel, A.C.; Stiles, C.D. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell 1983, 33, 939–947. [Google Scholar] [CrossRef]
- Barna, B.P.; Pettay, J.; Barnett, G.H.; Zhou, P.; Iwasaki, K.; Estes, M.L. Regulation of monocyte chemoattractant protein-1 expression in adult human non-neoplastic astrocytes is sensitive to tumor necrosis factor (TNF) or antibody to the 55-kDa TNF receptor. J. Neuroimmunol. 1994, 50, 101–107. [Google Scholar] [CrossRef]
- Yoshimura, T.; Yuhki, N.; Moore, S.K.; Appella, E.; Lerman, M.I.; Leonard, E.J. Human monocyte chemoattractant protein-1 (MCP-1) Full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989, 244, 487–493. [Google Scholar] [CrossRef] [Green Version]
- NCBI. Gene_Result. NCBI. 2018. Available online: https://www.ncbi.nlm.nih.gov/gene/7054#top (accessed on 5 May 2022).
- Soria, G.; Ben-baruch, A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008, 267, 271–285. [Google Scholar] [CrossRef]
- Bianconi, V.; Sahebkar, A.; Atkin, S.L.; Pirro, M. The regulation and importance of monocyte chemoattractant protein-1. Curr. Opin. Hematol. 2018, 25, 44–51. [Google Scholar] [CrossRef]
- Conductier, G.; Blondeau, N.; Guyon, A.; Nahon, J.-L.; Rovere, C. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J. Neuroimmunol. 2010, 224, 93–100. [Google Scholar] [CrossRef]
- Hernandez-Aguilera, A.; Fibla, M.; Cabre, N.; Luciano-Mateo, F.; Camps, J.; Fernandez-Arroyo, S.; Martín-Paredero, V.; Menendez, J.A.; Sirvent, J.J.; Joven, J. Chemokine (C-C motif) ligand 2 and coronary artery disease: Tissue expression of functional and atypical receptors. Cytokine 2019, 126, 154923. [Google Scholar] [CrossRef]
- Rana, A.K.; Li, Y.; Dang, Q.; Yang, F. Monocytes in rheumatoid arthritis: Circulating precursors of macrophages and osteoclasts and, their heterogeneity and plasticity role in RA pathogenesis. Int. Immunopharmacol. 2018, 65, 348–359. [Google Scholar] [CrossRef]
- Rossi, D.; Zlotnik, A. The biology of chemokines and their receptors. Annu. Rev. Immunol. 2000, 18, 217–242. [Google Scholar] [CrossRef]
- Raoul, W.; Auvynet, C.; Camelo, S.; Guillonneau, X.; Feumi, C.; Combadière, C.; Sennlaub, F. CCL2/CCR2 and CX3CL1/CX3CR1 chemokine axes and their possible involvement in age-related macular degeneration. J. Neuroinflamm. 2010, 7, 87. Available online: http://www.jneuroinflammation.com/content/7/1/87 (accessed on 12 April 2022). [CrossRef] [PubMed] [Green Version]
- Du, Z.; Wu, X.; Song, M.; Li, P.; Wang, L. Oxidative damage induces MCP-1 secretion and macrophage aggregation in age-related macular degeneration (AMD). Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 2469–2476. [Google Scholar] [CrossRef]
- Lechner, J.; Chen, M.; Hogg, R.E.; Toth, L.; Silvestri, G.; Chakravarthy, U.; Xu, H. Peripheral blood mononuclear cells from neovascular age-related macular degeneration patients produce higher levels of chemokines CCL2 (MCP-1) and CXCL8 (IL-8). J. Neuroinflamm. 2017, 14, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grunin, M.; Burstyn-Cohen, T.; Hagbi-Levi, S.; Peled, A.; Chowers, I. Chemokine receptor expression in peripheral blood monocytes from patients with neovascular age-related macular degeneration. Investig. Opthalmol. Vis. Sci. 2012, 53, 5292–5300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falk, M.K.; Singh, A.; Faber, C.; Nissen, M.H.; Hviid, T.; Sorensen, T.L. CX3CL1/CX3CR1 and CCL2/CCR2 chemokine/chemokine receptor complex in patients with AMD. PLoS ONE 2014, 9, e112473. [Google Scholar]
- Sharma, N.K.; Sharma, K.; Singh, R.; Sharma, S.K.; Anand, A. CCL2 single nucleotide polymorphism of rs1024611 implicates prominence of inflammatory cascade by univariate modeling in Indian AMD. PLoS ONE 2018, 13, e0193423. [Google Scholar] [CrossRef] [Green Version]
- Bonyadi, M.; Foruzandeh, Z.; Mohammadian, T.; Fotouhi, N.; Soheilian, M.; Jabbarpoor Bonyadi, M.H.; Javadzadeh, A.; Moein, H.; Yaseri, M. Evaluation of CC-cytokine ligand 2 and complementary factor H Y402H polymorphisms and their interactional association with age-related macular degeneration. Acta Ophthalmol. 2016, 94, e779–e785. [Google Scholar] [CrossRef]
- Saddala, M.S.; Lennikov, A.; Mukwaya, A.; Fan, L.; Hu, Z.; Huang, H. Transcriptome-wide analysis of differentially expressed chemokine receptors, SNPs, and SSRs in the age-related macular degeneration. Hum. Genom. 2019, 13, 15. [Google Scholar] [CrossRef] [Green Version]
- Despriet, D.D.G.; Bergen, A.A.B.; Merriam, J.E.; Zernant, J.; Barile, G.R.; Smith, R.T.; Barbazetto, I.A.; van Soest, S.; Bakker, A.; de Jong, P.T.V.M.; et al. Comprehensive analysis of the candidate genes CCL2, CCR2, and TLR4 in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2009, 49, 364–371. [Google Scholar] [CrossRef] [Green Version]
- Hossein, M.; Bonyadi, J.; Mohammadian, T. Evaluation of C-reactive protein and CC-cytokine ligand 2 polymorphism interaction for age-related macular degeneration. Ophthalmic Genet. 2016, 37, 465–467. [Google Scholar] [CrossRef]
- Nyquist, P.A.; Winkler, C.A.; McKenzie, L.M.; Yanek, L.R.; Becker, L.C.; Becker, D.M. Single nucleotide polymorphisms in monocyte chemoattractant protein-1 and its receptor act synergistically to increase the risk of carotid atherosclerosis. Cerebrovasc. Dis. 2009, 28, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Yun, D.H.; Kim, S.K.; Chung, J.-H.; Lee, J.S.; Park, H.-K.; Chon, J.; Kim, D.H.; Yoo, S.D.; Kim, H.-S. Association of CXCL1 promoter polymorphism with ischaemic stroke in Korean population. Int. J. Immunogenet. 2012, 40, 306–310. [Google Scholar] [CrossRef]
- Solé, X.; Guinó, E.; Valls, J.; Iniesta, R.; Moreno, V. SNPStats: A web tool for the analysis of association studies. Bioinformatics 2006, 22, 1928–1929. [Google Scholar] [CrossRef] [Green Version]
- Fernandez, A.B.; Panza, G.A.; Cramer, B.; Chatterjee, S. Age-related macular degeneration and incident stroke: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0142968. [Google Scholar] [CrossRef]
- Duan, Y.; Mo, J.; Klein, R.; Scott, I.U. Age-related macular degeneration is associated with incident myocardial infarction among elderly Americans. Ophthalmology 2007, 114, 732–737. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, G. Association between Monocyte Chemotactic Protein 1 variants and age-related macular degeneration onset among Chinese people. Med. Sci. Monit. 2020, 26, e921584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zor, R.K.; Erşan, S.; Küçük, E.; Yıldırım, G.; Sari, İ. Serum malondialdehyde, monocyte chemoattractant protein-1, and vitamin C levels in wet type age-related macular degeneration patients. Ther. Adv. Ophthalmol. 2020, 12, 2515841420951682. [Google Scholar] [CrossRef]
- Xie, P.; Kamei, M.; Suzuki, M.; Matsumura, N.; Nishida, K.; Sakimoto, S.; Sakaguchi, H.; Nishida, K. Suppression and regression of choroidal neovascularization in mice by a novel CCR2 antagonist, INCB3344. PLoS ONE 2011, 6, e28933. [Google Scholar] [CrossRef] [Green Version]
- Georgakis, M.K.; Gill, D.; Rannikmäe, K.; Traylor, M.; Anderson, C.D.; Lee, J.M.; Kamatani, Y.; Hopewell, J.C.; Worrall, B.B.; MEGASTROKE consortium of the International Stroke Genetics Consortium (ISGC); et al. Genetically determined levels of circulating cytokines and risk of stroke: Role of Monocyte Chemoattractant Protein-1. Circulation 2019, 139, 256–268. [Google Scholar] [CrossRef]
- Guymer, R.H.; Tao, L.W.; Goh, J.K.; Liew, D.; Ischenko, O.; Robman, L.D.; Aung, K.; Cipriani, T.; Cain, M.; Richardson, A.J.; et al. Identification of urinary biomarkers for age-related macular degeneration. Investig. Opthalmol. Vis. Sci. 2011, 52, 4639–4644. [Google Scholar] [CrossRef] [Green Version]
- Kramer, M.; Hasanreisoglu, M.; Feldman, A.; Axer-Siegel, R.; Sonis, P.; Maharshak, I.; Monselise, Y.; Gurevich, M.; Weinberger, D. Monocyte chemoattractant protein-1 in the aqueous humour of patients with age-related macular degeneration. Clin. Exp. Ophthalmol. 2011, 40, 617–625. [Google Scholar] [CrossRef]
- Jonas, J.B.; Tao, Y.; Neumaier, M.; Findeisen, P. Monocyte chemoattractant protein 1, intercellular adhesion molecule 1, and vascular cell adhesion molecule 1 in exudative age-related macular degeneration. Arch. Ophthalmol. 2010, 128, 1281–1286. [Google Scholar] [CrossRef] [Green Version]
- Lubowicka, E.; Przylipiak, A.; Zajkowska, M.; Piskór, B.M.; Malinowski, P.; Fiedorowicz, W.; Ławicki, S. Plasma chemokine CCL2 and its receptor CCR2 concentrations as diagnostic biomarkers for breast cancer patients. BioMed Res. Int. 2018, 2018, 2124390. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Yuzhalin, A.E.; Gordon-weeks, A.N.; Muschel, R.J. Targeting the CCL2-CCR2 signaling axis in cancer metastasis. Oncotarget 2016, 7, 28697–28710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristic | Early AMD (n = 310) | Control (n = 384) | p Value | |
|---|---|---|---|---|
| Gender, n (%) | Male | 96 (31) | 119 (31) | 0.995 |
| Female | 214 (69) | 265 (69) | ||
| Age (years), median (IQR) | 76 (12) | 75 (9) | 0.097 | |
| Gene/Marker | Genotype/Allele | Early AMD, n (%) | Control Group, n (%) | p Value |
|---|---|---|---|---|
| CCR2 rs1799865 | CC | 26 (8.4) | 26 (6.8) | 0.723 0.577 |
| CT | 121 (39) | 152 (39.6) | ||
| TT | 163 (52.6) | 206 (53.6) | ||
| C | 173 (27.9) | 204 (26.6) | ||
| T | 447 (72.1) | 564 (73.4) | ||
| CCL2 rs4586 | CC | 41 (13.2) | 44 (11.5) | 0.757 0.589 |
| CT | 139 (44.8) | 179 (46.6) | ||
| TT | 130 (41.9) | 161 (41.9) | ||
| C | 221(35.6) | 267 (34.8) | ||
| T | 399 (64.4) | 501 (65.2) | ||
| CCL2 rs1024611 | AA | 152 (49) | 216 (56.3) | 0.078 0.032 |
| AG | 135 (43.5) | 151 (39.3) | ||
| GG | 23 (7.4) | 17 (4.4) | ||
| A | 439 (70.8) | 583 (75.9) | ||
| G | 181 (29.2) | 185 (24.1) | ||
| CCL2 rs2857656 | CC | 23 (7.4) | 17 (4.4) | 0.087 0.037 |
| CG | 135 (43.5) | 152 (39.6) | ||
| GG | 152 (49) | 215 (56) | ||
| C | 181 (29.2) | 186 (24.2) | ||
| G | 439 (70.8) | 582 (75.8) |
| SNP | Early AMD Model/Allele | OR; 95% CI; p Value |
|---|---|---|
| rs1024611 | Additive G | 1.323; 1.032–1.697; 0.027 |
| rs2857656 | Additive C | 1.314; 1.025–1.684; 0.031 |
| rs4586 (D′; r2) | rs1024611 (D′; r2) | rs2857656 (D′; r2) | |
|---|---|---|---|
| rs4586 (D’; r2) | - | 0.995; 0.654 | 0.991; 0.651 |
| rs1024611 (D’; r2) | - | - | 0.996; 0.989 |
| rs2857656 (D’; r2) | - | - | - |
| Haplotype | rs4586 | rs1024611 | rs2857656 | Frequency AMD Group | Frequency Control Group | OR (95% CI) | p Value |
|---|---|---|---|---|---|---|---|
| 1 | T | A | G | 0.64 | 0.65 | 1 | - |
| 2 | C | A | G | 0.066 | 0.106 | 0.65 (0.44–0.96) | 0.031 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gudauskiene, G.; Vilkeviciute, A.; Gedvilaite, G.; Liutkeviciene, R.; Zaliuniene, D. CCL2, CCR2 Gene Variants and CCL2, CCR2 Serum Levels Association with Age-Related Macular Degeneration. Life 2022, 12, 1038. https://doi.org/10.3390/life12071038
Gudauskiene G, Vilkeviciute A, Gedvilaite G, Liutkeviciene R, Zaliuniene D. CCL2, CCR2 Gene Variants and CCL2, CCR2 Serum Levels Association with Age-Related Macular Degeneration. Life. 2022; 12(7):1038. https://doi.org/10.3390/life12071038
Chicago/Turabian StyleGudauskiene, Gaile, Alvita Vilkeviciute, Greta Gedvilaite, Rasa Liutkeviciene, and Dalia Zaliuniene. 2022. "CCL2, CCR2 Gene Variants and CCL2, CCR2 Serum Levels Association with Age-Related Macular Degeneration" Life 12, no. 7: 1038. https://doi.org/10.3390/life12071038
APA StyleGudauskiene, G., Vilkeviciute, A., Gedvilaite, G., Liutkeviciene, R., & Zaliuniene, D. (2022). CCL2, CCR2 Gene Variants and CCL2, CCR2 Serum Levels Association with Age-Related Macular Degeneration. Life, 12(7), 1038. https://doi.org/10.3390/life12071038







