Intraoperative Circulatory Support in Lung Transplantation: Current Trend and Its Evidence
Abstract
1. Introduction
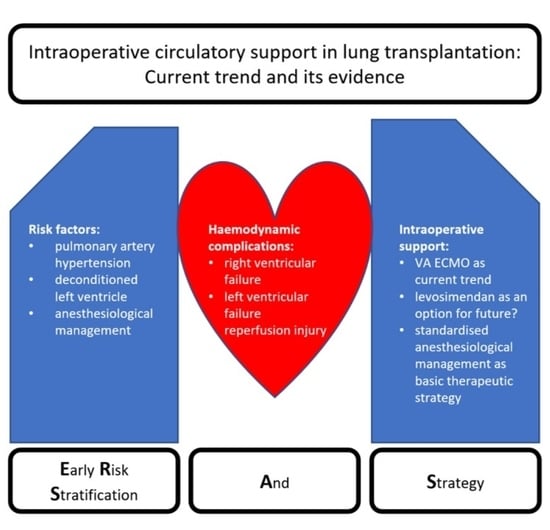
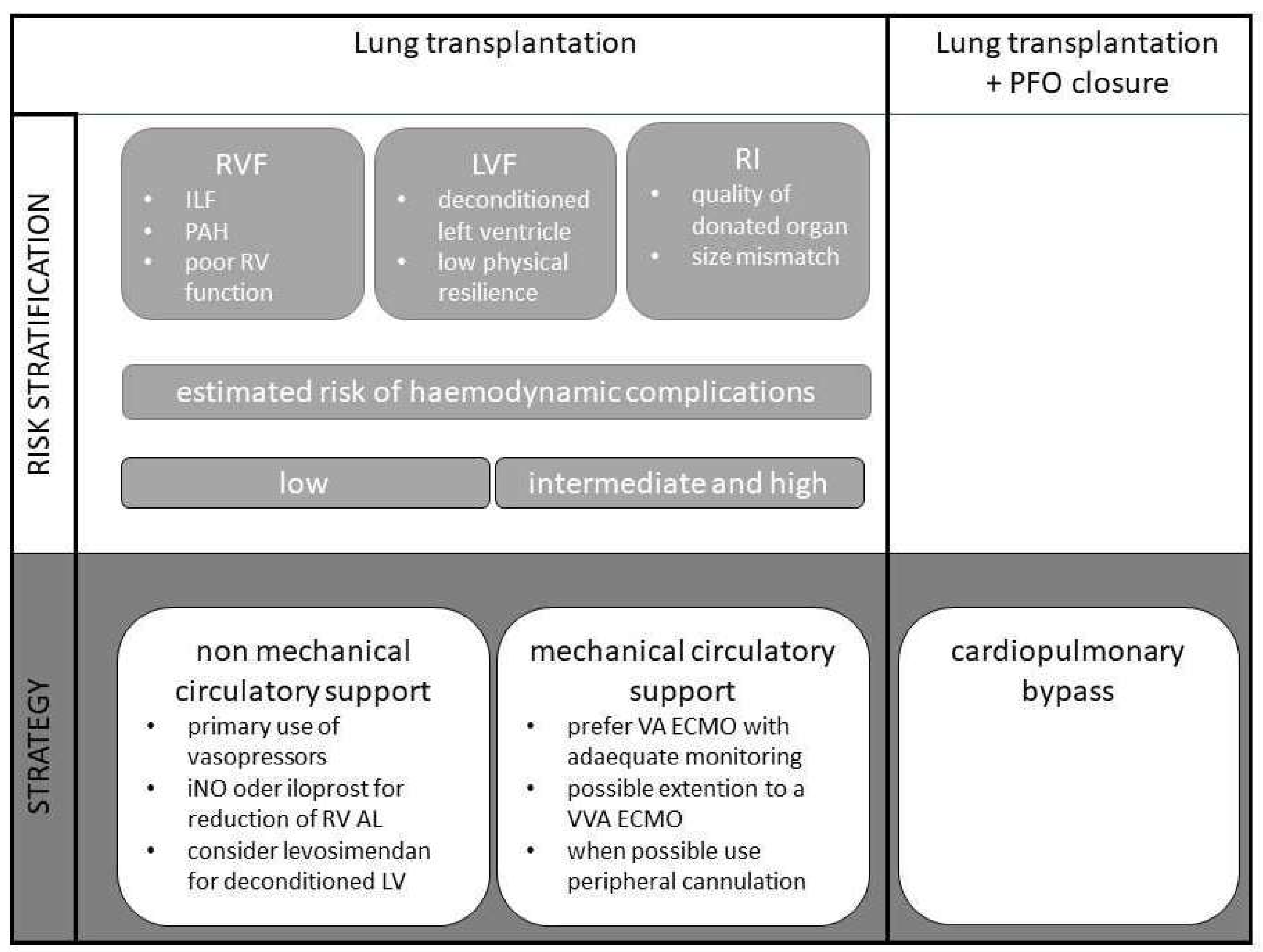
2. Risk Stratification
2.1. Right Ventricular Failure
2.2. Left Ventricular Failure/Diastolic Dysfunction
2.3. Reperfusion Injury
3. Strategy
3.1. Monitoring
3.2. Non-Mechanical Circulatory Support
3.3. Mechanical Circulatory Support
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Con sent Statement
Conflicts of Interest
References
- Panchabhai, T.S.; Chaddha, U.; McCurry, K.R.; Bremner, R.M.; Mehta, A.C. Historical perspectives of lung transplantation: Connecting the dots. J. Thorac. Dis. 2018, 10, 4516–4531. [Google Scholar] [CrossRef]
- Glanville, A.R. Inhaled nitric oxide after lung transplantation: No more cosmesis? Am. J. Respir. Crit. Care Med. 2003, 167, 1463–1464. [Google Scholar] [CrossRef]
- Ohsumi, A.; Date, H. Perioperative circulatory support for lung transplantation. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 631–637. [Google Scholar] [CrossRef]
- Yoo, Y.C. Anesthetic considerations for lung transplantation. Anesth Pain Med. 2019, 14, 241–248. [Google Scholar] [CrossRef]
- Ius, F.; Tudorache, I.; Warnecke, G. Extracorporeal support, during and after lung transplantation: The history of an idea. J. Thorac. Dis. 2018, 10, 5131–5148. [Google Scholar] [CrossRef]
- Kiziltug, H.; Falter, F. Circulatory support during lung transplantation. Curr. Opin. Anaesthesiol. 2020, 33, 37–42. [Google Scholar] [CrossRef]
- Faccioli, E.; Terzi, S.; Pangoni, A.; Lomangino, I.; Rossi, S.; Lloret, A.; Cannone, G.; Marino, C.; Catelli, C.; Dell’Amore, A. Extracorporeal membrane oxygenation in lung transplantation: Indications, techniques and results. World J. Transplant. 2021, 11, 290–302. [Google Scholar] [CrossRef]
- Hoechter, D.J.; von Dossow, V.; Winter, H.; Müller, H.-H.; Meiser, B.; Neurohr, C.; Behr, J.; Guenther, S.; Hagl, C.; Schramm, R. The Munich Lung Transplant Group: Intraoperative Extracorporeal Circulation in Lung Transplantation. Thorac. Cardiovasc. Surg. 2015, 63, 706–714. [Google Scholar] [CrossRef]
- Ius, F.; Sommer, W.; Tudorache, I.; Avsar, M.; Siemeni, T.; Salman, J.; Molitoris, U.; Gras, C.; Juettner, B.; Puntigam, J.; et al. Five-year experience with intraoperative extracorporeal membrane oxygenation in lung transplantation: Indications and midterm results. J. Heart Lung Transplant. 2016, 35, 49–58. [Google Scholar] [CrossRef]
- Hoetzenecker, K.; Benazzo, A.; Stork, T.; Sinn, K.; Schwarz, S.; Schweiger, T.; Klepetko, W. Bilateral lung transplantation on intraoperative extracorporeal membrane oxygenator: An observational study. J. Thorac. Cardiovasc. Surg. 2020, 160, 320–327.e1. [Google Scholar] [CrossRef]
- Geube, M.A.; Perez-Protto, S.E.; McGrath, T.L.; Yang, D.; Sessler, D.I.; Budev, M.M.; Kurz, A.; McCurry, K.R.; Duncan, A.E. Increased Intraoperative Fluid Administration Is Associated with Severe Primary Graft Dysfunction After Lung Transplantation. Anesth. Analg. 2016, 122, 1081–1088. [Google Scholar] [CrossRef]
- Buckwell, E.; Vickery, B.; Sidebotham, D. Anaesthesia for lung transplantation. BJA Educ. 2020, 20, 368–376. [Google Scholar] [CrossRef]
- Castillo, M. Anesthetic management for lung transplantation. Curr. Opin. Anaesthesiol. 2011, 24, 32–36. [Google Scholar] [CrossRef]
- Marczin, N.; de Waal, E.E.C.; Hopkins, P.M.A.; Mulligan, M.S.; Simon, A.; Shaw, A.D.; van Raemdonck, D.; Neyrinck, A.; Gries, C.J.; Algotsson, L.; et al. International consensus recommendations for anesthetic and intensive care management of lung transplantation. An EACTAIC, SCA, ISHLT, ESOT, ESTS, and AST approved document. J. Heart Lung Transplant. 2021, 40, 1327–1348. [Google Scholar] [CrossRef]
- den Hengst, W.A.; Gielis, J.F.; Lin, J.Y.; van Schil, P.E.; de Windt, L.J.; Moens, A.L. Lung ischemia-reperfusion injury: A molecular and clinical view on a complex pathophysiological process. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1283–H1299. [Google Scholar] [CrossRef]
- Fleisher, L.A.; Fleischmann, K.E.; Auerbach, A.D.; Barnason, S.A.; Beckman, J.A.; Bozkurt, B.; Davila-Roman, V.G.; Gerhard-Herman, M.D.; Holly, T.A.; Kane, G.C.; et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 130, e278–e333. [Google Scholar] [CrossRef]
- Kristensen, S.D.; Knuuti, J.; Saraste, A.; Anker, S.; Bøtker, H.E.; de Hert, S.; Ford, I.; Gonzalez-Juanatey, J.R.; Gorenek, B.; Heyndrickx, G.R.; et al. 2014 ESC/ESA Guidelines on non-cardiac surgery: Cardiovascular assessment and management: The Joint Task Force on non-cardiac surgery: Cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur. Heart J. 2014, 35, 2383–2431. [Google Scholar] [CrossRef]
- Gelzinis, T.A. Anesthetic Management of Lung Transplantation: Center Specific Practices and Geographical and Centers Size Differences. J. Cardiothorac. Vasc. Anesth. 2018, 32, 70–72. [Google Scholar] [CrossRef]
- Hinske, L.C.; Hoechter, D.J.; Schröeer, E.; Kneidinger, N.; Schramm, R.; Preissler, G.; Tomasi, R.; Sisic, A.; Frey, L.; von Dossow, V.; et al. Predicting the Necessity for Extracorporeal Circulation During Lung Transplantation: A Feasibility Study. J. Cardiothorac. Vasc. Anesth. 2017, 31, 931–938. [Google Scholar] [CrossRef]
- Taimeh, Z. Assessment and treatment of the failing right heart: Considerations for transplantation referral. J. Thorac. Dis. 2019, 11, S1817–S1820. [Google Scholar] [CrossRef]
- Hoeper, M.M.; Benza, R.L.; Corris, P.; de Perrot, M.; Fadel, E.; Keogh, A.M.; Kühn, C.; Savale, L.; Klepetko, W. Intensive care, right ventricular support and lung transplantation in patients with pulmonary hypertension. Eur. Respir. J. 2019, 53, 1801906. [Google Scholar] [CrossRef]
- Gelzinis, T.; Assaad, S.; Perrino, A.C. Right ventricular function during and after thoracic surgery. Curr. Opin. Anaesthesiol. 2020, 33, 27–36. [Google Scholar] [CrossRef]
- Gorter, T.M.; Verschuuren, E.A.M.; van Veldhuisen, D.J.; Hoendermis, E.S.; Erasmus, M.E.; Bogaard, H.J.; Vonk Noordegraaf, A.; Berger, R.M.F.; van Melle, J.P.; Willems, T.P. Right ventricular recovery after bilateral lung transplantation for pulmonary arterial hypertension. Interact. Cardiovasc. Thorac. Surg. 2017, 24, 890–897. [Google Scholar] [CrossRef]
- Katz, W.E.; Gasior, T.A.; Quinlan, J.J.; Lazar, J.M.; Firestone, L.; Griffith, B.P.; Gorcsan, J. Immediate effects of lung transplantation on right ventricular morphology and function in patients with variable degrees of pulmonary hypertension. J. Am. Coll. Cardiol. 1996, 27, 384–391. [Google Scholar] [CrossRef][Green Version]
- Kusunose, K.; Tsutsui, R.S.; Bhatt, K.; Budev, M.M.; Popović, Z.B.; Griffin, B.P.; Bolen, M.A. Prognostic value of RV function before and after lung transplantation. JACC Cardiovasc. Imaging 2014, 7, 1084–1094. [Google Scholar] [CrossRef]
- Tudorache, I.; Sommer, W.; Kühn, C.; Wiesner, O.; Hadem, J.; Fühner, T.; Ius, F.; Avsar, M.; Schwerk, N.; Böthig, D.; et al. Lung transplantation for severe pulmonary hypertension—Awake extracorporeal membrane oxygenation for postoperative left ventricular remodelling. Transplantation 2015, 99, 451–458. [Google Scholar] [CrossRef]
- Ohsumi, A.; Aoyama, A.; Kinoshita, H.; Yoneda, T.; Yamazaki, K.; Tanaka, S.; Nakajima, D.; Ikeda, T.; Minatoya, K.; Date, H. New strategy to resume and taper epoprostenol after lung transplant for pulmonary hypertension. Gen. Thorac. Cardiovasc. Surg. 2022, 70, 372–377. [Google Scholar] [CrossRef]
- Porteous, M.K.; Ky, B.; Kirkpatrick, J.N.; Shinohara, R.; Diamond, J.M.; Shah, R.J.; Lee, J.C.; Christie, J.D.; Kawut, S.M. Diastolic Dysfunction Increases the Risk of Primary Graft Dysfunction after Lung Transplant. Am. J. Respir. Crit. Care Med. 2016, 193, 1392–1400. [Google Scholar] [CrossRef]
- Avriel, A.; Klement, A.H.; Johnson, S.R.; de Perrot, M.; Granton, J. Impact of Left Ventricular Diastolic Dysfunction on Lung Transplantation Outcome in Patients with Pulmonary Arterial Hypertension. Am. J. Transplant. 2017, 17, 2705–2711. [Google Scholar] [CrossRef]
- Smiseth, O.A. Evaluation of left ventricular diastolic function: State of the art after 35 years with Doppler assessment. J. Echocardiogr. 2018, 16, 55–64. [Google Scholar] [CrossRef]
- Kossaify, A.; Nasr, M. Diastolic Dysfunction and the New Recommendations for Echocardiographic Assessment of Left Ventricular Diastolic Function: Summary of Guidelines and Novelties in Diagnosis and Grading. J. Diagn. Med. Sonogr. 2019, 35, 317–325. [Google Scholar] [CrossRef]
- Khan, S.U.; Salloum, J.; O’Donovan, P.B.; Mascha, E.J.; Mehta, A.C.; Matthay, M.A.; Arroliga, A.C. Acute pulmonary edema after lung transplantation: The pulmonary reimplantation response. Chest 1999, 116, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Hacking, C.; Weerakkody, Y. Post Lung Transplantation Pulmonary Oedema. Available online: https://radiopaedia.org/articles/post-lung-transplantation-pulmonary-oedema (accessed on 3 July 2022).
- Marom, E.M.; Choi, Y.W.; Palmer, S.M.; DeLong, D.M.; Stuart, M.D.; McAdams, H.P. Reperfusion edema after lung transplantation: Effect of daclizumab. Radiology 2001, 221, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.; Herman, S.J.; Winton, T.L. Reperfusion edema after lung transplantation: Radiographic manifestations. Radiology 1998, 206, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Chen-Yoshikawa, T.F. Ischemia-Reperfusion Injury in Lung Transplantation. Cells 2021, 10, 1333. [Google Scholar] [CrossRef] [PubMed]
- Snell, G.I.; Yusen, R.D.; Weill, D.; Strueber, M.; Garrity, E.; Reed, A.; Pelaez, A.; Whelan, T.P.; Perch, M.; Bag, R.; et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading—A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2017, 36, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J.M.; Lee, J.C.; Kawut, S.M.; Shah, R.J.; Localio, A.R.; Bellamy, S.L.; Lederer, D.J.; Cantu, E.; Kohl, B.A.; Lama, V.N.; et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am. J. Respir. Crit. Care Med. 2013, 187, 527–534. [Google Scholar] [CrossRef]
- Kreisel, D.; Krupnick, A.S.; Puri, V.; Guthrie, T.J.; Trulock, E.P.; Meyers, B.F.; Patterson, G.A. Short- and long-term outcomes of 1000 adult lung transplant recipients at a single center. J. Thorac. Cardiovasc. Surg. 2011, 141, 215–222. [Google Scholar] [CrossRef]
- Talaie, T.; DiChiacchio, L.; Prasad, N.K.; Pasrija, C.; Julliard, W.; Kaczorowski, D.J.; Zhao, Y.; Lau, C.L. Ischemia-reperfusion Injury in the Transplanted Lung: A Literature Review. Transplant. Direct 2021, 7, e652. [Google Scholar] [CrossRef]
- Subramaniam, K.; Del Rio, J.M.; Wilkey, B.J.; Kumar, A.; Tawil, J.N.; Subramani, S.; Tani, M.; Sanchez, P.G.; Mandell, M.S. Anesthetic management of lung transplantation: Results from a multicenter, cross-sectional survey by the society for advancement of transplant anesthesia. Clin. Transplant. 2020, 34, e13996. [Google Scholar] [CrossRef]
- Miranda, A.; Zink, R.; McSweeney, M. Anesthesia for lung transplantation. Semin. Cardiothorac. Vasc. Anesth. 2005, 9, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Hoeper, M.M.; Tudorache, I.; Kühn, C.; Marsch, G.; Hartung, D.; Wiesner, O.; Boenisch, O.; Haverich, A.; Hinrichs, J. Extracorporeal membrane oxygenation watershed. Circulation 2014, 130, 864–865. [Google Scholar] [CrossRef] [PubMed]
- Erkılınç, A.; Karaca Baysal, P.; Gürcü, M.E. Anesthetic management in lung transplantation: Our single-center experience. Turk. Gogus Kalp Damar Cerrahisi Derg. 2021, 29, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Hunt, M.F.; Clark, K.T.; Whitman, G.; Choi, C.W.; Geocadin, R.G.; Cho, S.-M. The Use of Cerebral NIRS Monitoring to Identify Acute Brain Injury in Patients With VA-ECMO. J. Intensive Care Med. 2021, 36, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Cannizzaro, G.; Garbin, L.; Clivati, A.; Pesce, L.I. Correction of hypoxia and hypercapnia in COPD patients: Effects on cerebrovascular flow. Monaldi Arch. Chest Dis. 1997, 52, 9–12. [Google Scholar]
- Shoemaker, W.C.; Appel, P.L.; Kram, H.B.; Waxman, K.; Lee, T.S. Prospective trial of supranormal values of survivors as therapeutic goals in high-risk surgical patients. Chest 1988, 94, 1176–1186. [Google Scholar] [CrossRef]
- Hamilton, M.A.; Cecconi, M.; Rhodes, A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth. Analg. 2011, 112, 1392–1402. [Google Scholar] [CrossRef]
- Evans, A.; Dwarakanath, S.; Hogue, C.; Brady, M.; Poppers, J.; Miller, S.; Weiner, M.M. Intraoperative echocardiography for patients undergoing lung transplantation. Anesth. Analg. 2014, 118, 725–730. [Google Scholar] [CrossRef]
- Iyer, M.H.; Bhatt, A.; Kumar, N.; Hussain, N.; Essandoh, M.K. Transesophageal Echocardiography for Lung Transplantation: A New Standard of Care? J. Cardiothorac. Vasc. Anesth. 2020, 34, 741–743. [Google Scholar] [CrossRef]
- Zaidi, A.; Knight, D.S.; Augustine, D.X.; Harkness, A.; Oxborough, D.; Pearce, K.; Ring, L.; Robinson, S.; Stout, M.; Willis, J.; et al. Echocardiographic assessment of the right heart in adults: A practical guideline from the British Society of Echocardiography. Echo Res. Pract. 2020, 7, G19–G41. [Google Scholar] [CrossRef]
- Win, T.T.; Alomari, I.B.; Awad, K.; Ratliff, M.D.; Qualls, C.R.; Roldan, C.A. Transesophageal Versus Transthoracic Echocardiography for Assessment of Left Ventricular Diastolic Function. J. Integr. Cardiol. Open Access 2020, 3. [Google Scholar] [CrossRef]
- Navarro, L.H.C.; Bloomstone, J.A.; Auler, J.O.C.; Cannesson, M.; Della Rocca, G.; Gan, T.J.; Kinsky, M.; Magder, S.; Miller, T.E.; Mythen, M.; et al. Perioperative fluid therapy: A statement from the international Fluid Optimization Group. Perioper. Med. 2015, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Hofer, C.K.; Müller, S.M.; Furrer, L.; Klaghofer, R.; Genoni, M.; Zollinger, A. Stroke volume and pulse pressure variation for prediction of fluid responsiveness in patients undergoing off-pump coronary artery bypass grafting. Chest 2005, 128, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.-L.; Pelosi, P.; Pearse, R.; Payen, D.; Perel, A.; Hoeft, A.; Romagnoli, S.; Ranieri, V.M.; Ichai, C.; Forget, P.; et al. Perioperative cardiovascular monitoring of high-risk patients: A consensus of 12. Crit. Care 2015, 19, 224. [Google Scholar] [CrossRef]
- Pilcher, D.V.; Scheinkestel, C.D.; Snell, G.I.; Davey-Quinn, A.; Bailey, M.J.; Williams, T.J. High central venous pressure is associated with prolonged mechanical ventilation and increased mortality after lung transplantation. J. Thorac. Cardiovasc. Surg. 2005, 129, 912–918. [Google Scholar] [CrossRef]
- McIlroy, D.R.; Pilcher, D.V.; Snell, G.I. Does anaesthetic management affect early outcomes after lung transplant? An exploratory analysis. Br. J. Anaesth. 2009, 102, 506–514. [Google Scholar] [CrossRef]
- Schumann, J.; Henrich, E.C.; Strobl, H.; Prondzinsky, R.; Weiche, S.; Thiele, H.; Werdan, K.; Frantz, S.; Unverzagt, S. Inotropic agents and vasodilator strategies for the treatment of cardiogenic shock or low cardiac output syndrome. Cochrane Database Syst. Rev. 2018, 1, CD009669. [Google Scholar] [CrossRef]
- Overgaard, C.B.; Dzavík, V. Inotropes and vasopressors: Review of physiology and clinical use in cardiovascular disease. Circulation 2008, 118, 1047–1056. [Google Scholar] [CrossRef]
- Mathew, R.; Di Santo, P.; Jung, R.G.; Marbach, J.A.; Hutson, J.; Simard, T.; Ramirez, F.D.; Harnett, D.T.; Merdad, A.; Almufleh, A.; et al. Milrinone as Compared with Dobutamine in the Treatment of Cardiogenic Shock. N. Engl. J. Med. 2021, 385, 516–525. [Google Scholar] [CrossRef]
- Ventetuolo, C.E.; Klinger, J.R. Management of acute right ventricular failure in the intensive care unit. Ann. Am. Thorac. Soc. 2014, 11, 811–822. [Google Scholar] [CrossRef]
- McGovern, J.J.; Cheifetz, I.M.; Craig, D.M.; Bengur, A.R.; Quick, G.; Ungerleider, R.M.; Meliones, J.N. Right ventricular injury in young swine: Effects of catecholamines on right ventricular function and pulmonary vascular mechanics. Pediatr. Res. 2000, 48, 763–769. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Feltracco, P.; Carollo, C.; Ori, C. Levosimendan in lung transplant recipients with difficult weaning from ECMO. Minerva Anestesiol. 2015, 81, 92–93. [Google Scholar] [PubMed]
- Zhuravel, S.V.; Aleksandrova, V.E.; Utkina, I.I.; Kuznetsova, N.K.; Tarabrin, E.A. Levosimendan in lung transplant recipients on VA-ECMO. RJTAO 2020, 22, 118–122. [Google Scholar] [CrossRef]
- Pan, J.; Yang, Y.-M.; Zhu, J.-Y.; Lu, Y.-Q. Multiorgan Drug Action of Levosimendan in Critical Illnesses. Biomed. Res. Int. 2019, 2019, 9731467. [Google Scholar] [CrossRef]
- Boeken, U.; Aubin, H.; Mehdiani, A.; Böttger, C.; Westenfeld, R.; Erbel, S.; Sipahi, F.; Dalyanoglu, H.; Akhyari, P.; Lichtenberg, A. Levosimendan Treatment in Patients with Primary Graft Dysfunction after Heart Transplantation. J. Heart Lung Transplant. 2020, 39, S299. [Google Scholar] [CrossRef]
- Odeyemi, Y.; Dhungana, P.; Dubrock, H. Inotropes for Right Ventricular Failure in Pulmonary Arterial Hypertension. Chest 2018, 154, 1038A. [Google Scholar] [CrossRef]
- Rider, O.J.; Francis, J.M.; Ali, M.K.; Holloway, C.; Pegg, T.; Robson, M.D.; Tyler, D.; Byrne, J.; Clarke, K.; Neubauer, S. Effects of catecholamine stress on diastolic function and myocardial energetics in obesity. Circulation 2012, 125, 1511–1519. [Google Scholar] [CrossRef]
- Condliffe, R.; Kiely, D.G. Critical care management of pulmonary hypertension. BJA Educ. 2017, 17, 228–234. [Google Scholar] [CrossRef]
- Demiselle, J.; Fage, N.; Radermacher, P.; Asfar, P. Vasopressin and its analogues in shock states: A review. Ann. Intensive Care 2020, 10, 9. [Google Scholar] [CrossRef]
- Bonet, L.A.; Guillén, R.V.; Lázaro, I.S.; de La Fuente, C.; Osseyran, F.; Dolz, L.M.; Hernández, M.M.; Sanz, M.P.; Otero, M.R.; Sanz, A.S. Intravenous Sildenafil in Right Ventricular Dysfunction with Pulmonary Hypertension following a Heart Transplant. Heart Int. 2014, 9, 22–25. [Google Scholar] [CrossRef]
- Yoshiyasu, N.; Sato, M.; Nakajima, D.; Tomioka, Y.; Watanabe, Y.; Shiraishi, T.; Funaki, S.; Maeda, S.; Tomoshige, K.; Nakajima, T.; et al. Current status of inhaled nitric oxide therapy for lung transplantation in Japan: A nationwide survey. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Yerebakan, C.; Ugurlucan, M.; Bayraktar, S.; Bethea, B.T.; Conte, J.V. Effects of inhaled nitric oxide following lung transplantation. J. Card. Surg. 2009, 24, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Ghadimi, K.; Cappiello, J.; Cooter-Wright, M.; Haney, J.C.; Reynolds, J.M.; Bottiger, B.A.; Klapper, J.A.; Levy, J.H.; Hartwig, M.G. Inhaled Pulmonary Vasodilator Therapy in Adult Lung Transplant: A Randomized Clinical Trial. JAMA Surg. 2022, 157, e215856. [Google Scholar] [CrossRef] [PubMed]
- Tavare, A.N.; Tsakok, T. Does prophylactic inhaled nitric oxide reduce morbidity and mortality after lung transplantation? Interact. Cardiovasc. Thorac. Surg. 2011, 13, 516–520. [Google Scholar] [CrossRef][Green Version]
- Ri, H.-S.; Son, H.J.; Oh, H.B.; Kim, S.-Y.; Park, J.Y.; Kim, J.Y.; Choi, Y.J. Inhaled nitric oxide therapy was not associated with postoperative acute kidney injury in patients undergoing lung transplantation: A retrospective pilot study. Medicine 2018, 97, e10915. [Google Scholar] [CrossRef]
- Fessler, J.; Godement, M.; Pirracchio, R.; Marandon, J.-Y.; Thes, J.; Sage, E.; Roux, A.; Parquin, F.; Cerf, C.; Fischler, M.; et al. Inhaled nitric oxide dependency at the end of double-lung transplantation: A boosted propensity score cohort analysis. Transpl. Int. 2019, 32, 244–256. [Google Scholar] [CrossRef]
- Wittwer, T.; Franke, U.F.; Sandhaus, T.; Thiene, M.; Groetzner, J.; Strauch, J.T.; Wippermann, J.; Ochs, M.; Muehlfeld, C. Preischemic iloprost application for improvement of graft preservation: Which route is superior in experimental pig lung transplantation: Inhaled or intravenous? Transplant. Proc. 2007, 39, 1345–1349. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.G.; Lee, C.Y.; Kim, N.; Chang, M.-Y.; You, Y.-C.; Kim, H.J.; Paik, H.C.; Oh, Y.J. Effects of intraoperative inhaled iloprost on primary graft dysfunction after lung transplantation: A retrospective single center study. Medicine 2016, 95, e3975. [Google Scholar] [CrossRef]
- Jurmann, M.; Haverich, A.; Demertzis, S.; Schaefers, H.; Wagner, T.; Borst, H. Extracorporeal membrane oxygenation as a bridge to lung transplantation. Eur. J. Cardiothorac. Surg. 1991, 5, 94–98. [Google Scholar] [CrossRef]
- Hoechter, D.J.; Shen, Y.-M.; Kammerer, T.; Günther, S.; Weig, T.; Schramm, R.; Hagl, C.; Born, F.; Meiser, B.; Preissler, G.; et al. Extracorporeal Circulation During Lung Transplantation Procedures: A Meta-Analysis. ASAIO J. 2017, 63, 551–561. [Google Scholar] [CrossRef]
- Magouliotis, D.E.; Tasiopoulou, V.S.; Svokos, A.A.; Svokos, K.A.; Zacharoulis, D. Extracorporeal membrane oxygenation versus cardiopulmonary bypass during lung transplantation: A meta-analysis. Gen. Thorac. Cardiovasc. Surg. 2018, 66, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Sef, D.; Verzelloni Sef, A.; Mohite, P.; Stock, U.; Trkulja, V.; Raj, B.; Garcia Saez, D.; Mahesh, B.; Robertis, F.; de Simon, A. Utilization of extracorporeal membrane oxygenation in DCD and DBD lung transplants: A 2-year single-center experience. Transpl. Int. 2020, 33, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Ruszel, N.; Kiełbowski, K.; Piotrowska, M.; Kubisa, M.; Grodzki, T.; Wójcik, J.; Kubisa, B. Central, peripheral ECMO or CPB? Comparsion between circulatory support methods used during lung transplantation. J. Cardiothorac. Surg. 2021, 16, 341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Xu, Y.; Sang, L.; Chen, S.; Huang, Y.; Nong, L.; Yang, C.; Liu, X.; Liu, D.; Xi, Y.; et al. Factors associated with intraoperative extracorporeal membrane oxygenation support during lung transplantation. Respir. Res. 2020, 21, 85. [Google Scholar] [CrossRef]
- Beaubien-Souligny, W.; Brand, F.Z.A.; Lenoir, M.; Amsallem, M.; Haddad, F.; Denault, A.Y. Assessment of Left Ventricular Diastolic Function by Transesophageal Echocardiography Before Cardiopulmonary Bypass: Clinical Implications of a Restrictive Profile. J. Cardiothorac. Vasc. Anesth. 2019, 33, 2394–2401. [Google Scholar] [CrossRef]
- Shi-Min, Y. Pulmonary reperfusion injury. SV 2017, 13, 14. [Google Scholar] [CrossRef]
- Conti, N.; Gatti, M.; Raschi, E.; Diemberger, I.; Potena, L. Evidence and Current Use of Levosimendan in the Treatment of Heart Failure: Filling the Gap. Drug Des. Dev. Ther. 2021, 15, 3391–3409. [Google Scholar] [CrossRef]
- Feltracco, P. Levosimendan in the management of right ventricle failure early after lung transplant. BJA Br. J. Anaesth. 2012, 109. [Google Scholar] [CrossRef]
- Rajagopal, K.; Hoeper, M.M. State of the Art: Bridging to lung transplantation using artificial organ support technologies. J. Heart Lung Transplant. 2016, 35, 1385–1398. [Google Scholar] [CrossRef]
| Haemodynamic Complications | Risk Factors | Tool for Determination |
|---|---|---|
| RVF | PAH | PAC, TOE |
| tolerance of PA clamping | haemodynamic monitoring | |
| LVF | physical resilience | general preoperative assessments |
| E/e′ | TTE, TOE | |
| RI | donor: hypoxia, hypotension, aspiration, ischemic time | BGA, monitoring, CT scan |
| recipient: size mismatch, BMI | general assessments | |
| amount colloidal volume replacement | protocol based intraoperative care |
| Indication | Parameter | Time of Onset |
|---|---|---|
| idiopathic pulmonary fibrosis | not applicable | preoperative |
| intermediate to severe PAH | systemic and suprasystemic PAP | pre- and intra-operative |
| acute on chronic RVF | increased RVEDD poor RV contractility | after PA clamping |
| acute LVF | CI < 2 L/min/m2 | after reperfusion |
| impaired gas exchange | hypercapnia/hypoxia | intra- and post-operative |
| insufficient non-mechanical circulatory support | increasing need for vasopressors and/or inotorpes decreased CI and/or ScvO2 | any time during treatement |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starke, H.; von Dossow, V.; Karsten, J. Intraoperative Circulatory Support in Lung Transplantation: Current Trend and Its Evidence. Life 2022, 12, 1005. https://doi.org/10.3390/life12071005
Starke H, von Dossow V, Karsten J. Intraoperative Circulatory Support in Lung Transplantation: Current Trend and Its Evidence. Life. 2022; 12(7):1005. https://doi.org/10.3390/life12071005
Chicago/Turabian StyleStarke, Henning, Vera von Dossow, and Jan Karsten. 2022. "Intraoperative Circulatory Support in Lung Transplantation: Current Trend and Its Evidence" Life 12, no. 7: 1005. https://doi.org/10.3390/life12071005
APA StyleStarke, H., von Dossow, V., & Karsten, J. (2022). Intraoperative Circulatory Support in Lung Transplantation: Current Trend and Its Evidence. Life, 12(7), 1005. https://doi.org/10.3390/life12071005