In Vitro Characterization of the Human Skeletal Stem Cell-like Properties of Primary Bone-Derived Mesenchymal Stem/Stromal Cells in Patients with Late and Early Hip Osteoarthritis
Abstract
:1. Introduction
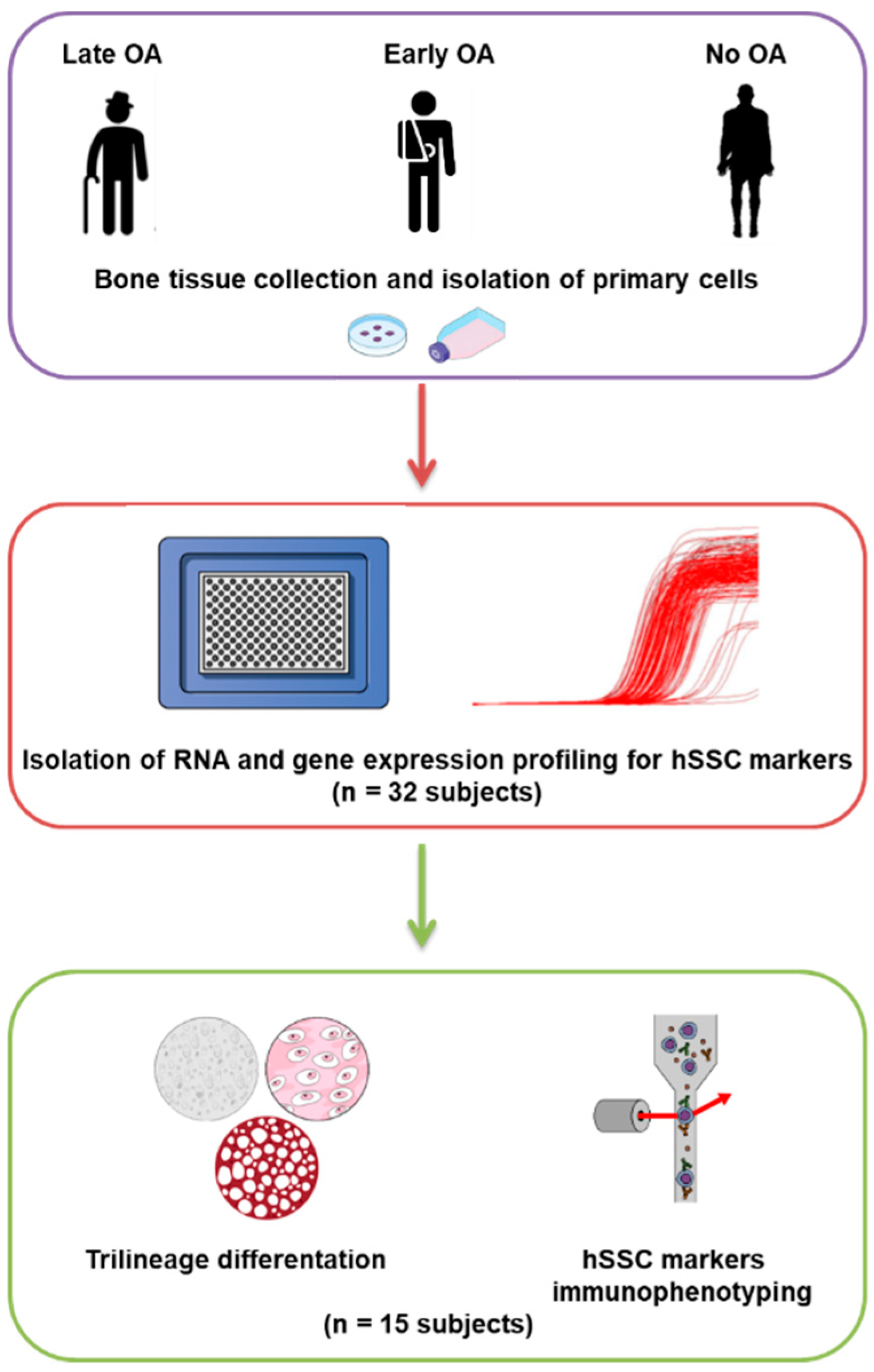
2. Methods
2.1. Donor Inclusion, Tissue Harvesting, and Primary Cell Isolation
2.2. RNA Isolation, Reverse Transcription, and Gene Expression Profiling
2.3. Trilineage Differentiation
2.4. Immunophenotyping
2.5. Statistical Analysis
3. Results
3.1. Study Subjects
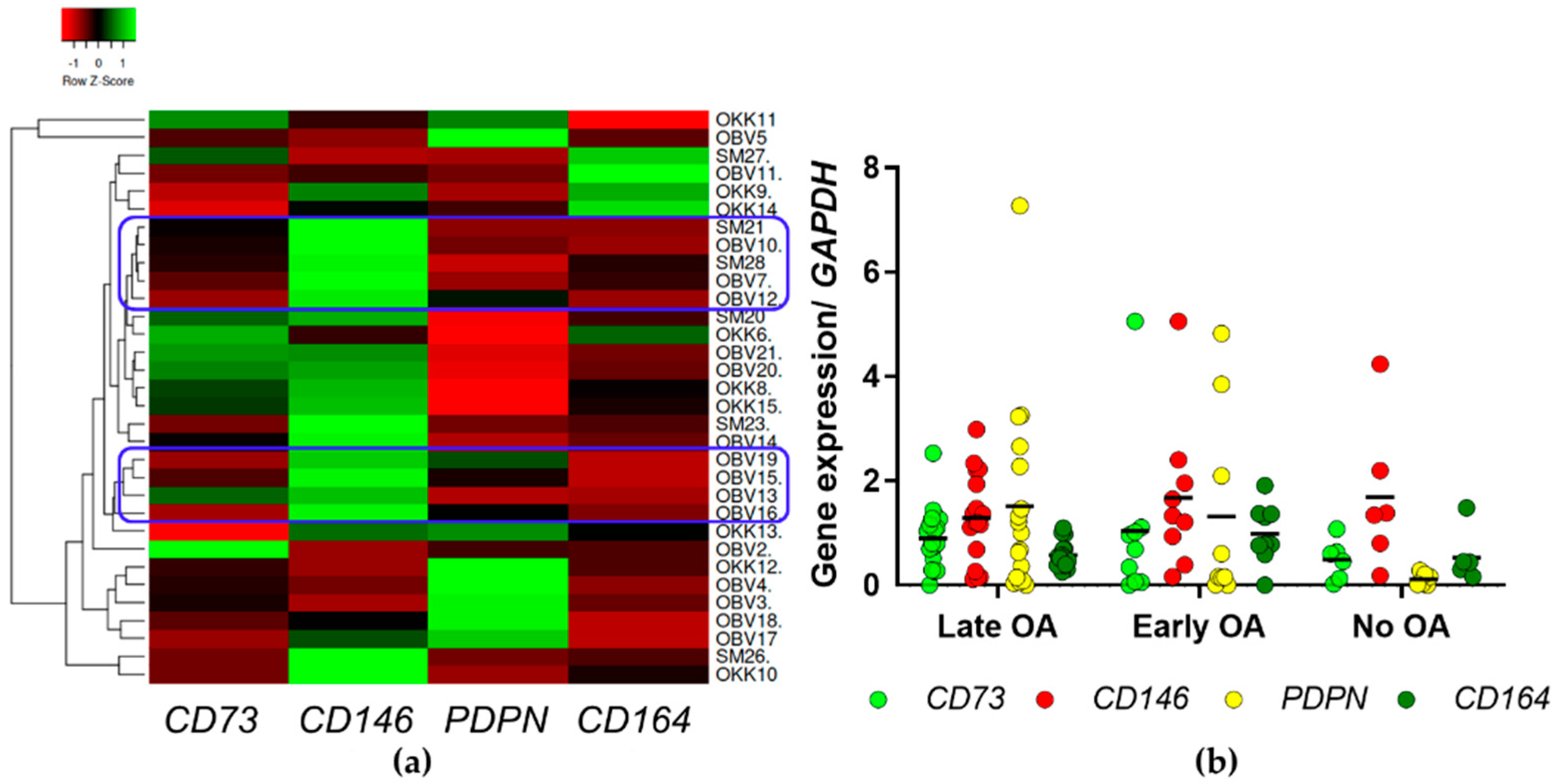
3.2. Gene Expression Profiling Identified Clusters of Samples from Late OA Patients with Low Expression of hSSC Markers
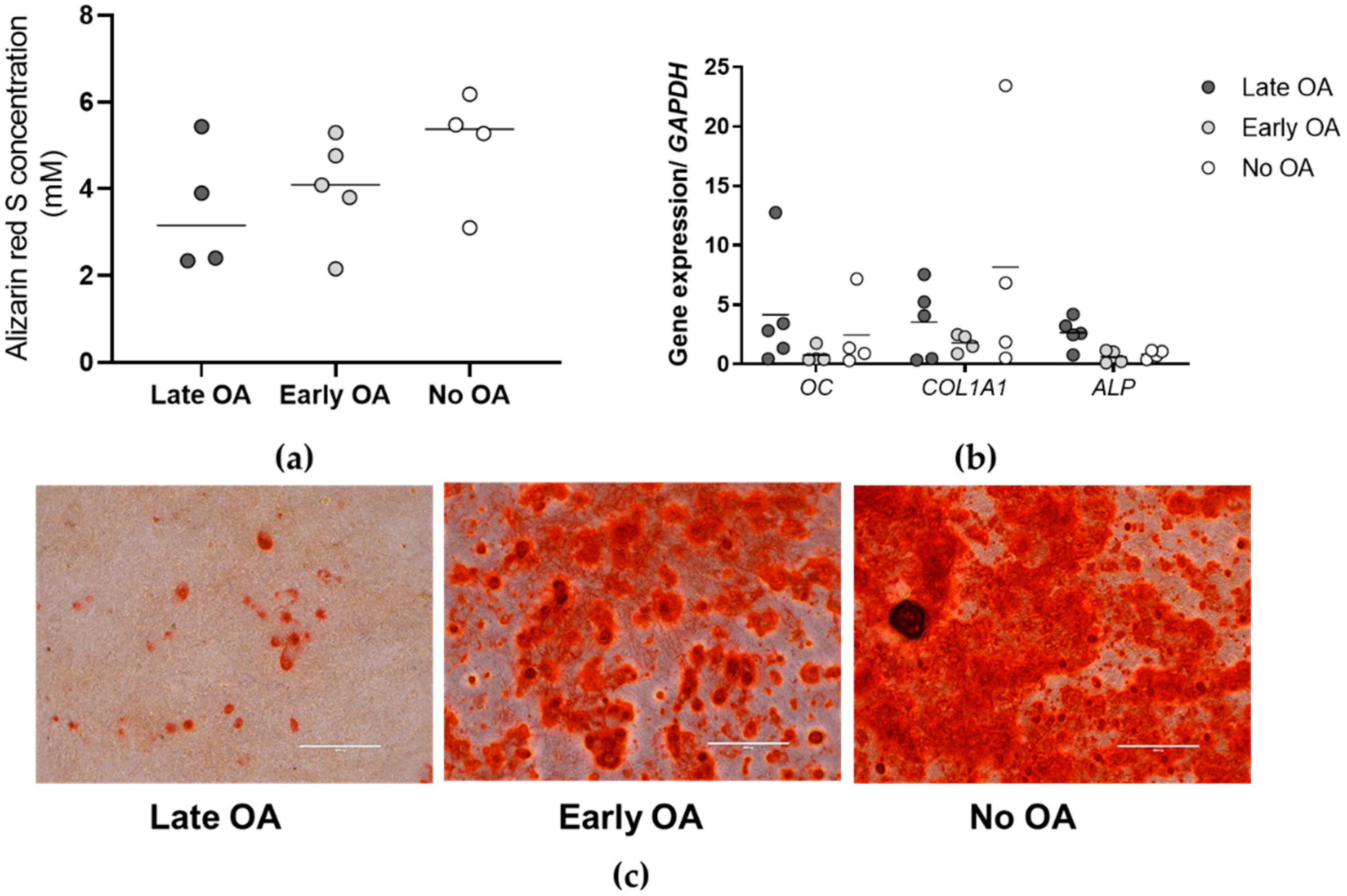
3.3. The Primary Bone-Derived Cells Show Similar Osteogenic Potential between the Tested Groups of Donors
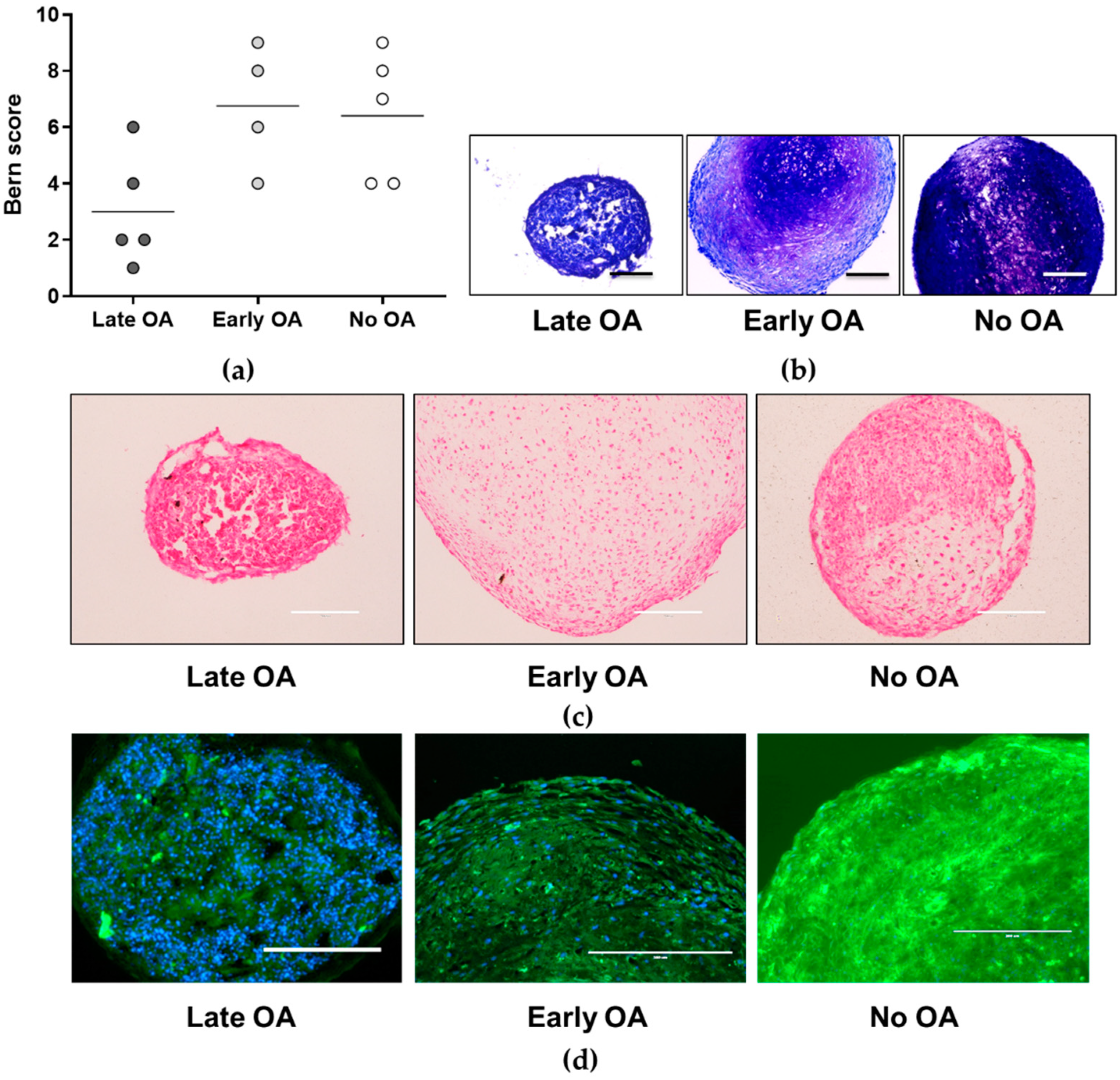
3.4. No Differences in Chondrogenic Potential of the Primary Bone-Derived Cells between the Tested Groups of Donors
3.5. Similar Adipogenic Potential of the Primary Bone-Derived Cells between the Tested Groups of Donors
3.6. The Primary Bone-Derived Cells from Early OA Patients Show Lower Percentage of the Positive Marker CD164 in Comparison with the Late OA Patients and No OA Donors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Felson, D.T.; Hodgson, R. Identifying and treating pre-clinical and early osteoarthritis. Rheum. Dis. Clin. N. Am. 2014, 40, 699. [Google Scholar] [CrossRef] [Green Version]
- Čamernik, K.; Barlič, A.; Drobnič, M.; Marc, J.; Jeras, M.; Zupan, J. Mesenchymal stem cells in the musculoskeletal system: From animal models to human tissue regeneration? Stem Cell Rev. Rep. 2018, 14, 346–369. [Google Scholar] [CrossRef] [PubMed]
- Nurul, A.A.; Azlan, M.; Ahmad Mohd Zain, M.R.; Sebastian, A.A.; Fan, Y.Z.; Fauzi, M.B. Mesenchymal stem cells: Current concepts in the management of inflammation in osteoarthritis. Biomedicines 2021, 9, 785. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, J.; Xu, H.; Lin, Z.; Chang, H.; Liu, W.; Kong, L. Mesenchymal stem cells in knee osteoarthritis treatment: A systematic review and meta-analysis. J. Orthop. Transl. 2020, 24, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Maleitzke, T.; Elazaly, H.; Festbaum, C.; Eder, C.; Karczewski, D.; Perka, C.; Duda, G.N.; Winkler, T. Mesenchymal stromal cell-based therapy—An alternative to arthroplasty for the treatment of osteoarthritis? A state of the art review of clinical trials. J. Clin. Med. 2020, 9, 2062. [Google Scholar] [CrossRef] [PubMed]
- Čamernik, K.; Mihelič, A.; Mihalič, R.; Marolt Presen, D.; Janež, A.; Trebše, R.; Marc, J.; Zupan, J. Increased exhaustion of the subchondral bone-derived mesenchymal stem/ stromal cells in primary versus dysplastic osteoarthritis. Stem Cell Rev. Rep. 2020, 16, 742–754. [Google Scholar] [CrossRef]
- Campbell, T.M.; Churchman, S.M.; Gomez, A.; Mcgonagle, D.; Conaghan, P.G.; Ponchel, F.; Jones, E. Mesenchymal stem cell alterations in bone marrow lesions in patients with hip osteoarthritis. Arthritis Rheum. 2016, 68, 1648–1659. [Google Scholar] [CrossRef]
- Murphy, J.M.; Dixon, K.; Beck, S.; Fabian, D.; Feldman, A.; Barry, F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002, 46, 704–713. [Google Scholar] [CrossRef]
- Zupan, J.; Tang, D.; Oreffo, R.O.C.; Redl, H.; Marolt Presen, D. Bone-marrow-derived mesenchymal stromal cells: From basic biology to applications in bone tissue engineering and bone regeneration. In Cell Engineering and Regeneration; Springer International Publishing: Berlin, Germany, 2020; pp. 139–192. [Google Scholar]
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the global challenges of ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef]
- Murphy, M.P.; Koepke, L.S.; Lopez, M.T.; Tong, X.; Ambrosi, T.H.; Gulati, G.S.; Marecic, O.; Wang, Y.; Ransom, R.C.; Hoover, M.Y.; et al. Articular cartilage regeneration by activated skeletal stem cells. Nat. Med. 2020, 26, 1583–1592. [Google Scholar] [CrossRef]
- Chan, C.K.F.; Gulati, G.S.; Sinha, R.; Tompkins, J.V.; Lopez, M.; Carter, A.C.; Ransom, R.C.; Reinisch, A.; Wearda, T.; Murphy, M.; et al. Identification of the human skeletal stem cell. Cell 2018, 175, 43–56.e21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Čamernik, K.; Mihelič, A.; Mihalič, R.; Haring, G.; Herman, S.; Marolt Presen, D.; Janež, A.; Trebše, R.; Marc, J.; Zupan, J. Comprehensive analysis of skeletal muscle- and bone-derived mesenchymal stem/stromal cells in patients with osteoarthritis and femoral neck fracture. Stem Cell Res. Ther. 2020, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Tönnis, D.; Heinecke, A. Acetabular and femoral anteversion: Relationship with osteoarthritis of the hip. J. Bone Jt. Surg.-Ser. A 1999, 81, 1747–1770. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Čamernik, K.; Mihelič, A.; Mihalič, R.; Marolt Presen, D.; Janež, A.; Trebše, R.; Marc, J.; Zupan, J. Skeletal-muscle-derived mesenchymal stem/stromal cells from patients with osteoarthritis show superior biological properties compared to bone-derived cells. Stem Cell Res. 2019, 38, 101465. [Google Scholar] [CrossRef]
- Haring, G.; Zupan, J. Knee and peri-knee tissues of post mortem donors are strategic sources of mesenchymal stem/stromal cells for regenerative procedures. Int. J. Mol. Sci. 2022, 23, 3170. [Google Scholar] [CrossRef]
- Imbert, A.M.; Garulli, C.; Choquet, E.; Koubi, M.; Aurrand-Lions, M.; Chabannon, C. CD146 expression in human breast cancer cell lines induces phenotypic and functional changes observed in epithelial to mesenchymal transition. PLoS ONE 2012, 7, e43752. [Google Scholar] [CrossRef]
- Huang, A.F.; Chen, M.W.; Huang, S.M.; Kao, C.L.; Lai, H.C.; Chan, J.Y.H. CD164 regulates the tumorigenesis of ovarian surface epithelial cells through the SDF-1α/CXCR4 axis. Mol. Cancer 2013, 12, 115. [Google Scholar] [CrossRef] [Green Version]
- Ariizumi, T.; Ogose, A.; Kawashima, H.; Hotta, T.; Li, G.; Xu, Y.; Umezu, H.; Sugai, M.; Endo, N. Expression of podoplanin in human bone and bone tumors: New marker of osteogenic and chondrogenic bone tumors. Pathol. Int. 2010, 60, 193–202. [Google Scholar] [CrossRef]
- Cappellari, A.R.; Rockenbach, L.; Dietrich, F.; Clarimundo, V.; Glaser, T.; Braganhol, E.; Abujamra, A.L.; Roesler, R.; Ulrich, H.; Liveira Battastini, A.M. Characterization of ectonucleotidases in human medulloblastoma cell lines: Ecto-5′NT/CD73 in metastasis as potential prognostic factor. PLoS ONE 2012, 7, e47468. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Grogan, S.P.; Barbero, A.; Winkelmann, V.; Fitzsimmons, J.; O’Driscoll, S.; Martin, I.; Mainil-Varlet, P. Development of a visual histological grading system to evaluate tissue engineered cartilage. In Proceedings of the 51st Annual Meeting of the Orthopaedic Research Society, Washington, DC, USA, 20–23 February 2005; p. 1768. [Google Scholar]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef] [PubMed]
- March, L.; Cross, M.; Arden, N.; Hawker, G. Osteoarthritis: A Serious Disease. Available online: https://oarsi.org/education/oarsi-resources/oarsi-white-paper-oa-serious-disease (accessed on 1 June 2022).
- Hamood, R.; Tirosh, M.; Fallach, N.; Chodick, G.; Eisenberg, E.; Lubovsky, O. Prevalence and incidence of osteoarthritis: A population-based retrospective cohort study. J. Clin. Med. 2021, 10, 4282. [Google Scholar] [CrossRef] [PubMed]
- Watt, S.M.; Bühring, H.J.; Simmons, P.J.; Zannettino, A.W.C. The stem cell revolution: On the role of CD164 as a human stem cell marker. NPJ Regen. Med. 2021, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, A.J.; Kania, K.; Rafipay, A.J.; Sambale, M.; Kuwahara, S.T.; Collins, F.L.; Smeeton, J.; Serowoky, M.A.; Rowley, L.; Wang, H.; et al. Identification of the skeletal progenitor cells forming osteophytes in osteoarthritis. Ann. Rheum. Dis. 2020, 79, 1625–1634. [Google Scholar] [CrossRef]
- Roelofs, A.J.; Zupan, J.; Riemen, A.H.K.; Kania, K.; Ansboro, S.; White, N.; Clark, S.M.; De Bari, C. Joint morphogenetic cells in the adult mammalian synovium. Nat. Commun. 2017, 8, 15040. [Google Scholar] [CrossRef] [Green Version]
- Haydont, V.; Neiveyans, V.; Perez, P.; Busson, É.; Lataillade, J.J.; Asselineau, D.; Fortunel, N.O. Fibroblasts from the human skin dermo-hypodermal junction are distinct from dermal papillary and reticular fibroblasts and from mesenchymal stem cells and exhibit a specific molecular profile related to extracellular matrix organization and modeling. Cells 2020, 9, 368. [Google Scholar] [CrossRef] [Green Version]
- Ward, L.S.C.; Sheriff, L.; Marshall, J.L.; Manning, J.E.; Brill, A.; Nash, G.B.; McGettrick, H.M. Podoplanin regulates the migration of mesenchymal stromal cells and their interaction with platelets. J. Cell Sci. 2019, 132, jcs222067. [Google Scholar] [CrossRef] [Green Version]
- Lv, F.J.; Tuan, R.S.; Cheung, K.M.C.; Leung, V.Y.L. Concise review: The surface markers and identity of human mesenchymal stem cells. Stem Cells 2014, 32, 1408–1419. [Google Scholar] [CrossRef]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guimarães-Camboa, N.; Cattaneo, P.; Sun, Y.; Moore-Morris, T.; Gu, Y.; Dalton, N.D.; Rockenstein, E.; Masliah, E.; Peterson, K.L.; Stallcup, W.B.; et al. Pericytes of multiple organs do not behave as mesenchymal stem cells in vivo. Cell Stem Cell 2017, 20, 345–359.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Bari, C.; Dell’Accio, F.; Luyten, F.P. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 2001, 44, 85–95. [Google Scholar] [CrossRef]



| Donor Group | N | Age (Years) | M/F | BMI (m/kg2) |
|---|---|---|---|---|
| Late OA | 17 | 76 ± 11 ****/*** | 6/11 | 28.2 ± 4.6 |
| Early OA | 9 | 43 ± 14 **** | 4/5 | 25.0 ± 2.7 |
| No OA | 6 | 53 ± 18 *** | 3/3 | 28.2 ± 4.7 |
| Donor Group | N | Age (Years) | M/F | BMI (m/kg2) |
|---|---|---|---|---|
| Late OA | 5 | 80 ± 6 **/* | 3/2 | 24.9 ± 2.5 |
| Early OA | 5 | 48 ± 4 ** | 1/4 | 25.6 ± 2.7 |
| No OA | 5 | 54 ± 20 * | 2/3 | 27.1 ± 4.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasenc, L.; Stražar, K.; Mihelič, A.; Mihalič, R.; Trebše, R.; Haring, G.; Jeras, M.; Zupan, J. In Vitro Characterization of the Human Skeletal Stem Cell-like Properties of Primary Bone-Derived Mesenchymal Stem/Stromal Cells in Patients with Late and Early Hip Osteoarthritis. Life 2022, 12, 899. https://doi.org/10.3390/life12060899
Jasenc L, Stražar K, Mihelič A, Mihalič R, Trebše R, Haring G, Jeras M, Zupan J. In Vitro Characterization of the Human Skeletal Stem Cell-like Properties of Primary Bone-Derived Mesenchymal Stem/Stromal Cells in Patients with Late and Early Hip Osteoarthritis. Life. 2022; 12(6):899. https://doi.org/10.3390/life12060899
Chicago/Turabian StyleJasenc, Lara, Klemen Stražar, Anže Mihelič, Rene Mihalič, Rihard Trebše, Gregor Haring, Matjaž Jeras, and Janja Zupan. 2022. "In Vitro Characterization of the Human Skeletal Stem Cell-like Properties of Primary Bone-Derived Mesenchymal Stem/Stromal Cells in Patients with Late and Early Hip Osteoarthritis" Life 12, no. 6: 899. https://doi.org/10.3390/life12060899
APA StyleJasenc, L., Stražar, K., Mihelič, A., Mihalič, R., Trebše, R., Haring, G., Jeras, M., & Zupan, J. (2022). In Vitro Characterization of the Human Skeletal Stem Cell-like Properties of Primary Bone-Derived Mesenchymal Stem/Stromal Cells in Patients with Late and Early Hip Osteoarthritis. Life, 12(6), 899. https://doi.org/10.3390/life12060899








