Obstetrical and Fertility Outcomes Following Transcatheter Pelvic Arterial Embolization for Postpartum Hemorrhage: A Cohort Follow-Up Study
Abstract
:1. Introduction
2. Materials and Methods
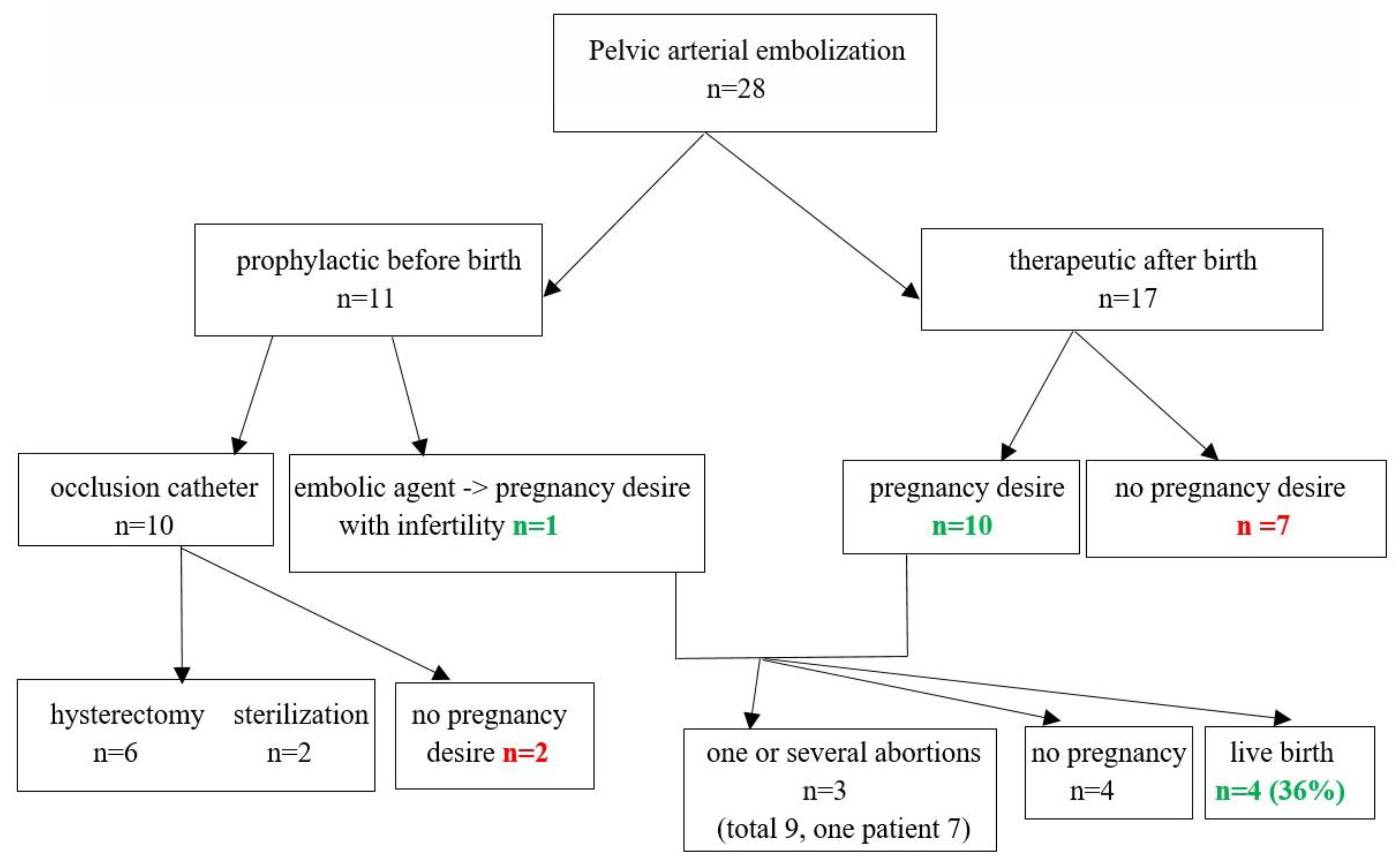
3. Results
Follow-Up Results
4. Case Report
5. Discussion
| Author, Year of Publication | Size of Cohort (n) | Study Period (Years) | Efficacy of TAE (%) | Number of Subsequent Deliveries | Mode of Delivery | Reccurence of PPH in Subsequent Pregnancy |
|---|---|---|---|---|---|---|
| Salomon et al., 2003 [13] | 28 | 5 | 100% | 4 | NA | 100% |
| Shim et al., 2006 [19] | 43 | 3 | 86% | 6 | 5 vaginal, 1 CS | NA |
| Fiori et al., 2009 [21] | 56 | 10 | 100% | 12 | 12 vaginal | 8% |
| Hardemann et al., 2010 [26] | 53 | 6 | 100% | 13 | 9 vaginal, 4 CS | 18.1% |
| Poggi et al., 2015 [31] | 103 | 3 | NA | 17 | 7 vaginal, 10 CS | 58.8% |
| Radan et al., 2022 | 28 | 10 | 100% | 4 | 1 vaginal, 3 CS | 50% |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ART | assisted reproductive technology |
| CS | cesarean section |
| FFP | fresh frozen plasma |
| ICU | intensive care unit |
| PLT | platelets |
| PPH | postpartum hemorrhage |
| RBCU | red blood cell unit |
| rhFVIIIa | recombinant human factor seven |
| SD | standard deviation |
| TAE | transcatheter arterial embolization |
| TXA | tranexamic acid |
References
- Cheng, H.-H.; Tsang, L.L.-C.; Hsu, T.-Y.; Kung, C.-T.; Ou, C.-Y.; Chang, C.-D.; Tsai, C.-C.; Cheng, Y.-F.; Kung, F.-T. Transcatheter arterial embolization as first-line rescue in intractable primary postpartum hemorrhage: Assessment, outcome, and subsequent fertility. J. Formos. Med. Assoc. 2017, 116, 380–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHO. The World Health Report: 2005 Make Every Mother and Child Count; World Health Organization (WHO): Geneva, Switzerland, 2005. [Google Scholar]
- Ledee, N.; Ville, Y.; Musset, D.; Mercier, F.; Frydman, R.; Fernandez, H. Management in intractable obstetric haemorrhage: An audit study on 61 cases. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 94, 189–196. [Google Scholar] [CrossRef]
- Goffinet, F. Hemorrhage during delivery: Management in France and value of prostaglandins. J. Gynecol. Obstet. Biol. Reprod. 1997, 26, 26–33. [Google Scholar]
- Ekin, A.; Gezer, C.; Solmaz, U.; Taner, C.E.; Dogan, A.; Ozeren, M. Predictors of severity in primary postpartum hemorrhage. Arch. Gynecol. Obstet. 2015, 292, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.J.; Heaston, D.K.; Poulson, A.M.; Gabert, H.A.; Mineau, D.E.; Miller, F.J. Uncontrollable postpartum bleeding: A new approach to hemostasis through angiographic arterial embolization. Obstet. Gynecol. 1979, 54, 361–365. [Google Scholar] [PubMed]
- Spreu, A.; Abgottspon, F.; Baumann, M.U.; Kettenbach, J.; Surbek, D. Efficacy of pelvic artery embolisation for severe postpartum hemorrhage. Arch. Gynecol. Obstet. 2017, 296, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Obstetricians and Gynaecologists. The Role of Emergency and Elective Interventional Radiology in Postpartum Hemorrhage. Royal College of Obstetricians and Gynaecologists Good Practice Guideline No. 6; Royal College of Obstetricians and Gynaecologists: London, UK, 2007. [Google Scholar]
- Deux, J.F.; Bazot, M.; Le Blanche, A.F.; Tassart, M.; Khalil, A.; Berkane, N.; Uzan, S.; Boudghene, F. Is selective embolization of uterine arteries a safe alternative to hysterectomy in patients with postpartum hemorrhage? Am. J. Roentgenol. 2001, 177, 145–149. [Google Scholar] [CrossRef]
- Pelage, J.P.; Le Dref, O.; Jacob, D.; Soyer, P.; Herbreteau, D.; Rymer, R. Selective arterial embolization of the uterine arteries in the management of intractable post-partum hemorrhage. Acta Obstet. Gynecol. Scand. 1999, 78, 698–703. [Google Scholar] [CrossRef]
- Merland, J.; Houdart, E.; Herbreteau, D.; Trystram, D.; Ledref, O.; Aymard, A.; Bouret, J.; Ravina, J. Place of emergency arterial embolisation in obstetric haemorrhage about 16 personal cases. Eur. J. Obstet. Gynecol. Reprod. Biol. 1996, 65, 141–143. [Google Scholar] [CrossRef]
- Committee on Practice Bulletins-Obstetrics. Practice Bulletin No. 183: Postpartum Hemorrhage. Obstet. Gynecol. 2017, 130, e168–e186. [Google Scholar] [CrossRef]
- Escobar, M.F.; Nassar, A.H.; Theron, G.; Barnea, E.R.; Nicholson, W.; Ramasauskaite, D.; Lloyd, I.; Chandraharan, E.; Miller, S.; Burke, T.; et al. FIGO recommendations on the management of postpartum hemorrhage 2022. Int. J. Gynecol. Obstet. 2022, 157, 3–50. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.K.; Wolanske, K.A.; Hwang, G.L.; Sze, D.Y.; Kee, S.T.; Dake, M.D. Angiographic Classification of Ovarian Artery–to–Uterine Artery Anastomoses: Initial Observations in Uterine Fibroid Embolization. Radiology 2002, 224, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Salomon, L.J.; de Tayrac, R.; Castagine-Meary, V.; Audibert, F.; Musset, D.; Ciorascu, R.; Frydman, R.; Fernandez, H. Fertility and pregnancy outcome following pelvic arterial embolization for severe post-partum hemorrhage. A cohort study. Hum. Reprod. 2003, 18, 849–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hardeman, S.; Decroisette, E.; Marin, B.; Vincelot, A.; Aubard, Y.; Pouquet, M.; Maubon, A. Fertility after embolization of the uterine arteries to treat obstetrical hemorrhage: A review of 53 cases. Fertil. Steril. 2010, 94, 2574–2579. [Google Scholar] [CrossRef] [PubMed]
- Picone, O.; Salomon, L.; Ville, Y.; Kadoch, J.; Frydman, R.; Fernandez, H. Fetal growth and Doppler assessment in patients with a history of bilateral internal iliac artery embolization. J. Matern. Fetal Neonatal Med. 2003, 13, 305–308. [Google Scholar] [CrossRef]
- Sentilhes, L.; Gromez, A.; Clavier, E.; Resch, B.; Verspyck, E.; Marpeau, L. Fertility and pregnancy following pelvic arterial embolization for postpartum hemorrhage. BJOG Int. J. Obstet. Gynaecol. 2010, 117, 84–93. [Google Scholar] [CrossRef]
- Ornan, D.; White, R.; Pollak, J.; Tal, M. Pelvic embolization for intractable postpartum hemorrhage: Long-term follow up and implications for fertility. Obstet. Gynecol. 2003, 102, 904–910. [Google Scholar] [CrossRef]
- Descargues, G.; Tinlot, F.M.; Douvrin, F.; Clavier, E.; Lemoine, J.P.; Marpau, L. Menses, fertility and pregnancy and arterial embolization for the control of postpartum hemorrhage. Hum. Reprod. 2004, 19, 339–343. [Google Scholar] [CrossRef] [Green Version]
- Shim, J.-Y.; Yoon, H.-K.; Won, H.-S.; Kim, S.-K.; Lee, P.R.; Kim, A. Angiographic embolization for obstetrical hemorrhage: Effectiveness and follow-up outcome of fertility. Acta Obstet. Gynecol. Scand. 2006, 85, 815–820. [Google Scholar] [CrossRef]
- Boulleret, C.; Chahid, T.; Gallot, D.; Mofid, R.; Hai, D.T.; Ravel, A. Hypogastric arterial selective and superselective embolization for severe postpartum hemorrhage: A retrospective review of 36 cases. Cardiovasc. Interv. Radiol. 2004, 27, 344–348. [Google Scholar] [CrossRef]
- Fiori, O.; Deux, J.-F.; Kambale, J.-C.; Uzan, S.; Bougdhene, F.; Berkane, N. Impact of pelvic arterial embolization for intractable postpartum hemorrhage on fertility. Am. J. Obstet. Gynecol. 2009, 200, 384.e1–384.e4. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, A.J.; Weinberg, C.R.; O’Connor, J.F. Incidence of early loss of pregnancy. N. Engl. J. Med. 1988, 319, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Mohan, P.P.; Hamblin, M.H.; Vogelzang, R.L. Uterine artery embolization and its effect on fertility. J. Vasc. Interv. Radiol. 2013, 24, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Gizzo, S.; Saccardi, C.; Patrelli, T.S.; Di Gangi, S.; Breda, E.; Fagherazzi, S.; Noventa, M.; D‘Antona, D.; Nardelli, G.B. Fertility rate and subsequent pregnancy outcomes after conservative surgical techniques in postpartum hemorrhage: 15 years of literature. Fertil. Steril. 2013, 99, 2097–2107. [Google Scholar] [CrossRef]
- Doumouchtsis, S.K.; Nikolopoulos, K.; Talaulikar, V.; Krishna, A.; Arulkumaran, S. Menstrual and fertility outcomes following the surgical management of postpartum haemorrhage: A systematic review. BJOG Int. J. Obstet. Gynaecol. 2014, 121, 382–388. [Google Scholar] [CrossRef] [Green Version]
- Holub, Z.; Mara, M.; Kuzel, D.; Jabor, A.; Maskova, J.; Eim, J. Pregnancy outcomes after uterine artery occlusion: Prospective multicentric study. Fertil. Steril. 2008, 90, 1886–1891. [Google Scholar] [CrossRef]
- Labarta, F.R.; Recarte, M.P.; Luque, A.A.; Prieto, L.J.; Martín, L.P.; Leyte, M.G.; Abizanda, F.P.; Ramirez, F.M.; Corral, A.P.; Quinatana, L.O.; et al. Outcomes of pelvic arterial embolization in the management of postpartum haemorrhage: A case series study and systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 206, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Radan, A.P.; Polowy, J.A.; Heverhagen, A.; Simillion, C.; Baumann, M.; Raio, L.; Schleussner, E.; Mueller, M.; Surbek, D. Cervico-vaginal placental α-macroglobulin-1 combined with cervical length for the prediction of preterm birth in women with threatened preterm labor. Acta Obstet. Gynecol. Scand. 2019, 99, 357–363. [Google Scholar] [CrossRef]
- Eriksson, L.-G.; Mulic-Lutvica, A.; Jangland, L.; Nyman, R. Massive postpartum hemorrhage treated with transcatheter arterial embolization: Technical aspects and long-term effects on fertility and menstrual cycle. Acta Radiol. 2007, 48, 635–642. [Google Scholar] [CrossRef]
- Cordonnier, C.; Ha-Vien, D.E.; Depret, S.; Houfflin-Debarge, V.; Provost, N.; Subtil, D. Foetal growth restriction in the next pregnancy after uterine artery embolization for post-partum haemorrhage. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002, 103, 183–184. [Google Scholar] [CrossRef]
- Poggi, S.H.; Yaeger, A.; Wahdan, Y.; Ghidini, A. Outcome of pregnancies after pelvic artery embolization for postpartum hemorrhage: Retrospective cohort study. Am. J. Obstet. Gynecol. 2015, 213, 576.e1–576.e5. [Google Scholar] [CrossRef] [PubMed]

| Embolization | |
|---|---|
| 17 (61%) |
| - Prophylactic (n, %) | 11 (39%) |
| TAE site (artery) | |
| - uterine artery (uni- or bilateral) (n, %) | 16 (57%) |
| - common iliac artery (bilateral) (n, %) | 3 (11%) |
| - internal iliac artery (bilateral) (n, %) | 7 (25%) |
| - aneurism of the left uterine artery (n, %) | 1 (4%) |
| - left pudendal artery (n, %) | 1 (4%) |
| Method | |
| - embolic agents (Embozene®/BioSphere®, Gelfoam®/Spongostan®, Coils VortX®) (n, %) | 18 (64%) |
| - Occlusion catheter (n, %) | 10 (36%) |
| Age in years (median, +/− SD) | 34 (+/− 5) |
| Nulliparous (n, %) | 11 (39%) |
| Multipara (n, %) | 17 (61%) |
| Previous CS (n, %) | 11 (39%) |
| Previous curettage (n, %) | 12 (43%) |
| Previous PPH (n, %) | 6 (21%) |
| Uterine fibromas (n, %) | 2 (7%) |
| Previous preeclampsia/eclampsia/HELLP | 5 (18%) |
| Congenital bleeding disorder (n, %) | 2 (7%) |
| Acquired coagulopathy (n, %) | 1 (4%) |
| Histologically confirmed abnormal placental implantation (increta/accreta/percreta) (n, %) | 13 (46%) |
| Placenta previa (n, %) | 10 (38%) |
| Intake of low dose aspirin (n, %) | 3 (11%) |
| Vaginal delivery (n, %) | 6 (21%) |
| Vaginal-operative delivery (n, %) | 4 (14%) |
| Cesarean section (n, %) | 18 (64%) |
| Gestational age at birth, weeks (median, +/− SD) | 36.4 (4.42) |
| Secondary PPH (n, %) | 1 (4%) |
| Blood loss (mL, median, +/− SD) | 3446 (2749) |
| Placental abruption (n, %) | 1 (4%) |
| Prolonged labour (n, %) | 3 (11%) |
| Uterine rupture (n, %) | 1 (4%) |
| Conservative Intervention | |
|---|---|
| Tranexamic acid (n, %) | 10 (36%) |
| Uterotonics (carbetocin, oxytoxin, sulprostone) (n, %) | 14 (50%) |
| Cell saver (n, %) | 3 (11%) |
| Blood transfusion (n, %) | 22 (78%) |
| Fresh frozen plasma (n, %) | 16 (57%) |
| Platelet transfusion (n, %) | 8 (29%) |
| Fibrinogen (n, %) | 7 (25%) |
| Activated recombinant human factor seven (rhFVIIIa) (n, %) | 4 (14%) |
| Bakri®-Balloon (n, %) | 7 (25%) |
| Surgical Intervention | |
| Cervix suture (n, %) | 4 (14%) |
| Compression sutures (n, %) | 3 (11%) |
| Vessel ligatures (n, %) | 2 (7%) |
| Curettage (n, %) | 10 (36%) |
| Manual placenta delivery (n, %) | 1 (4%) |
| Hysterectomy (n, %) | 6 (21%) |
| Suture uterus rupture (n, %) | 1 (4%) |
| Gender | Week of Gestation at Birth | Delivery Mode | Birth Weight (g) | Arterial pH | Venous pH | 5-Min APGAR Score |
|---|---|---|---|---|---|---|
| male | 39 + 6 | Vaginal | 3345 | 7.38 | 7.39 | 9 |
| female | 36 + 2 | I° CS | 2620 | 7.33 | 7.42 | 9 |
| female | 40 + 3 | I° Repeat-CS | 3095 | 7.18 | 7.32 | 10 |
| female | 36 + 1 | I° Repeat-CS | 2710 | 7.30 | 7.35 | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radan, A.-P.; Schneider, S.; Zdanowicz, J.A.; Raio, L.; Mertineit, N.; Heverhagen, J.T.; Surbek, D.V. Obstetrical and Fertility Outcomes Following Transcatheter Pelvic Arterial Embolization for Postpartum Hemorrhage: A Cohort Follow-Up Study. Life 2022, 12, 892. https://doi.org/10.3390/life12060892
Radan A-P, Schneider S, Zdanowicz JA, Raio L, Mertineit N, Heverhagen JT, Surbek DV. Obstetrical and Fertility Outcomes Following Transcatheter Pelvic Arterial Embolization for Postpartum Hemorrhage: A Cohort Follow-Up Study. Life. 2022; 12(6):892. https://doi.org/10.3390/life12060892
Chicago/Turabian StyleRadan, Anda-Petronela, Sophie Schneider, Jarmila A. Zdanowicz, Luigi Raio, Nando Mertineit, Johannes Thomas Heverhagen, and Daniel V. Surbek. 2022. "Obstetrical and Fertility Outcomes Following Transcatheter Pelvic Arterial Embolization for Postpartum Hemorrhage: A Cohort Follow-Up Study" Life 12, no. 6: 892. https://doi.org/10.3390/life12060892
APA StyleRadan, A.-P., Schneider, S., Zdanowicz, J. A., Raio, L., Mertineit, N., Heverhagen, J. T., & Surbek, D. V. (2022). Obstetrical and Fertility Outcomes Following Transcatheter Pelvic Arterial Embolization for Postpartum Hemorrhage: A Cohort Follow-Up Study. Life, 12(6), 892. https://doi.org/10.3390/life12060892






