High Glycated Hemoglobin Instead of High Body Mass Index Might Increase the Urine N-Acetyl-β-D-glucosaminidase Con-Centration in Children and Adolescents with Diabetes Mellitus
Abstract
:1. Introduction
2. Experimental Section
2.1. Study Subjects
2.2. Definition of Type 1 and 2 DM
2.3. Clinical Data and Blood Sample Collection
2.4. Renal Injury Marker Measurements
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of Study Subjects
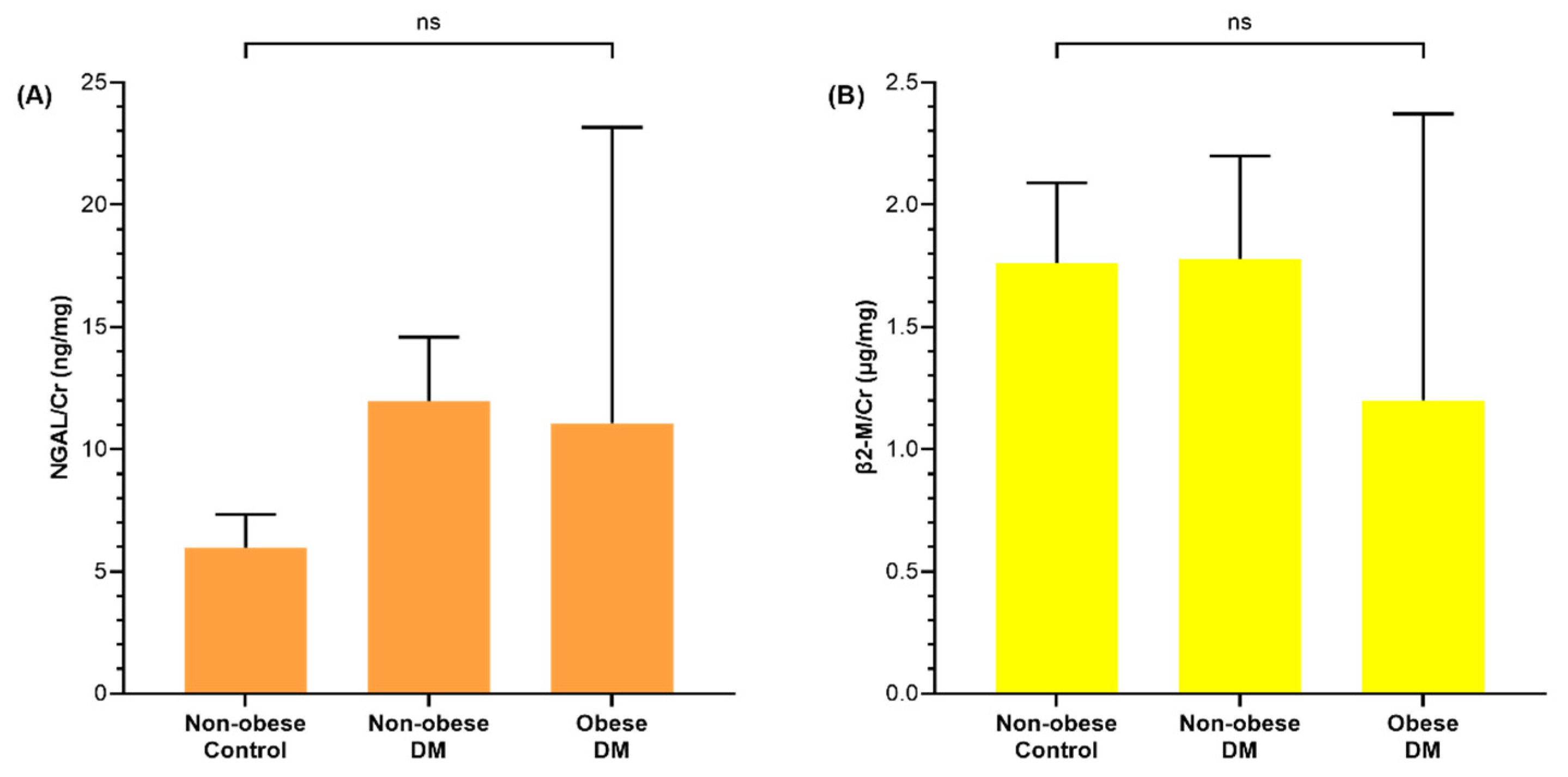
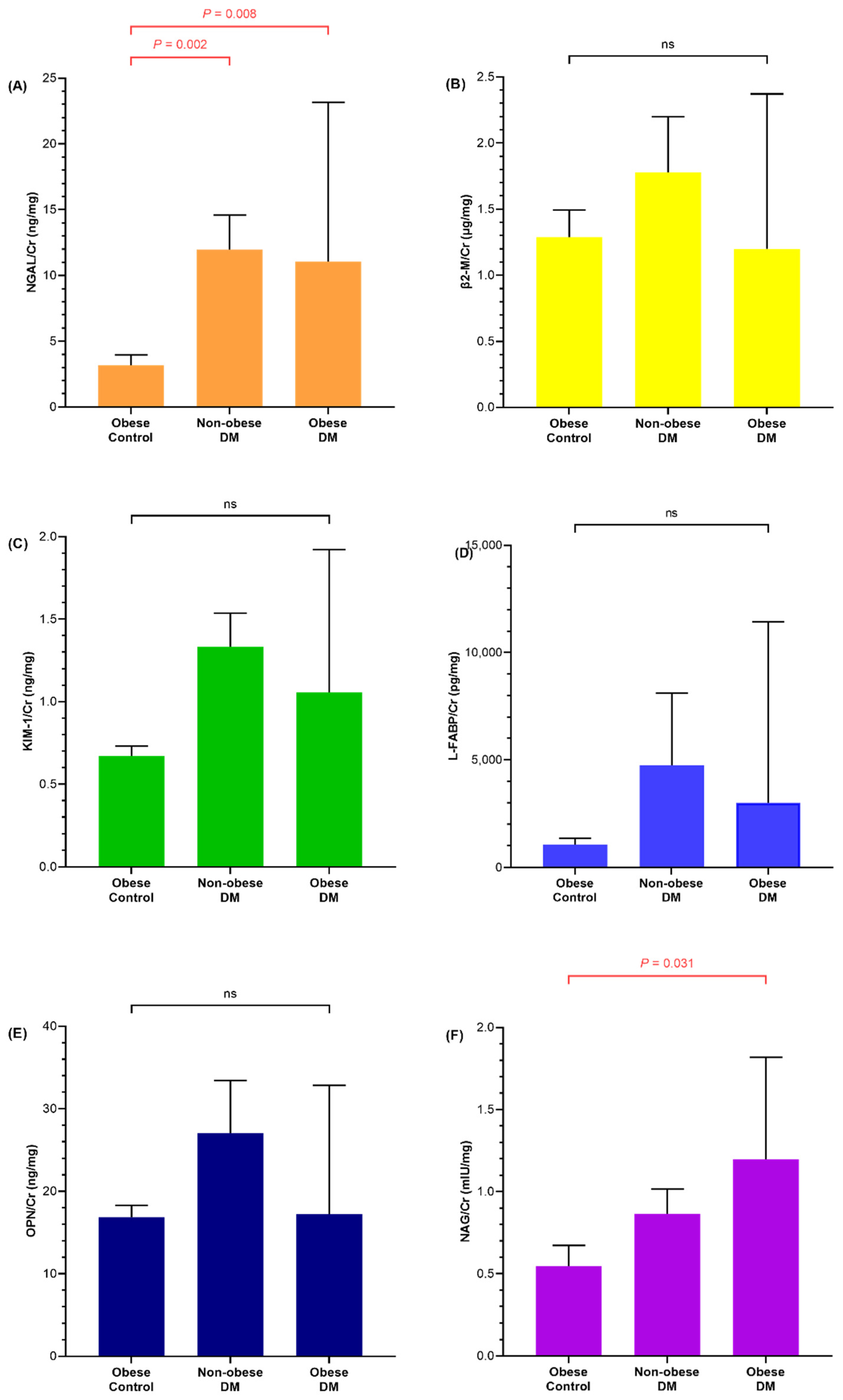
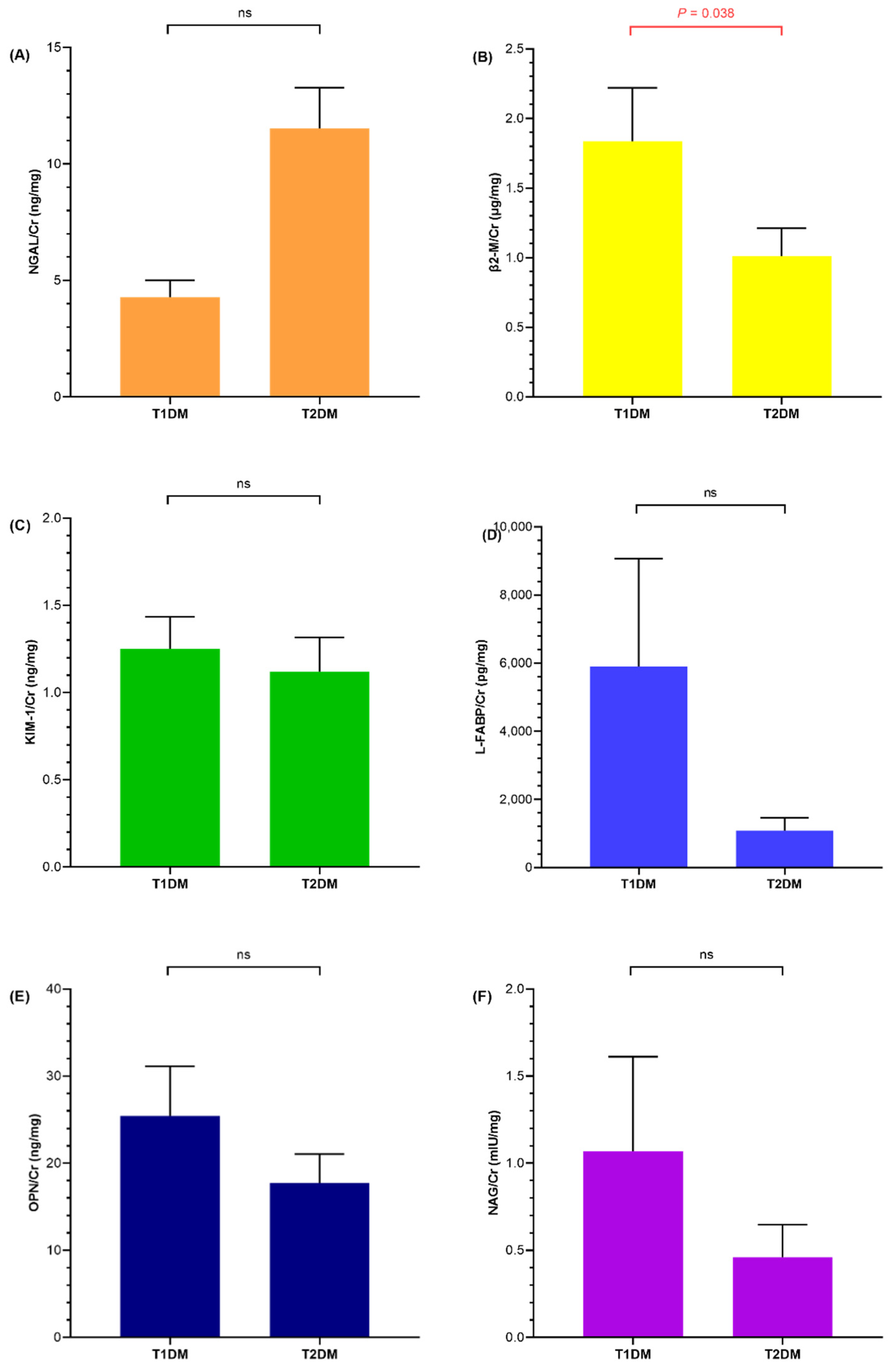
3.2. Renal Injury Markers of Diabetic and Obese Children
3.3. Correlation of Metabolic Parameters with Renal Injury Markers
3.4. Association of Urine NGAL, KIM-1, and NAG with HbA1c
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niba, L.L.; Aulinger, B.; Mbacham, W.F.; Parhofer, K.G. Predictors of glucose control in children and adolescents with type 1 diabetes: Results of a cross-sectional study in Cameroon. BMC Res. Notes 2017, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Mamilly, L.; Mastrandrea, L.D.; Mosquera Vasquez, C.; Klamer, B.; Kallash, M.; Aldughiem, A. Evidence of Early Diabetic Nephropathy in Pediatric Type 1 Diabetes. Front. Endocrinol. 2021, 12, 669954. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yang, F.J.; Tsai, W.Y.; Lee, C.T.; Liu, S.Y.; Yang, W.S.; Tung, Y.C. Serum neutrophil gelatinase-associated lipocalin as a potential biomarker of diabetic kidney disease in patients with childhood-onset type 1 diabetes. J. Formos. Med. Assoc. 2021, 121, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Marcovecchio, M.L.; Colombo, M.; Dalton, R.N.; McKeigue, P.M.; Benitez-Aguirre, P.; Cameron, F.J.; Chiesa, S.T.; Couper, J.J.; Craig, M.E.; Daneman, D.; et al. Biomarkers associated with early stages of kidney disease in adolescents with type 1 diabetes. Pediatric Diabetes 2020, 21, 1322–1332. [Google Scholar] [CrossRef]
- Minges, K.E.; Whittemore, R.; Grey, M. Overweight and obesity in youth with type 1 diabetes. Annu. Rev. Nurs. Res. 2013, 31, 47–69. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed Pharm. 2021, 137, 111315. [Google Scholar] [CrossRef]
- Styne, D.M.; Arslanian, S.A.; Connor, E.L.; Farooqi, I.S.; Murad, M.H.; Silverstein, J.H.; Yanovski, J.A. Pediatric Obesity-Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 709–757. [Google Scholar] [CrossRef]
- Polidori, N.; Giannini, C.; Salvatore, R.; Pelliccia, P.; Parisi, A.; Chiarelli, F.; Mohn, A. Role of urinary NGAL and KIM-1 as biomarkers of early kidney injury in obese prepubertal children. J. Pediatr. Endocrinol. Metab. 2020, 33, 1183–1189. [Google Scholar] [CrossRef]
- Kawada, T. Renal injury in obese children. Ren. Fail 2019, 41, 340. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulou-Marketou, N.; Kanaka-Gantenbein, C.; Marketos, N.; Chrousos, G.P.; Papassotiriou, I. Biomarkers of diabetic nephropathy: A 2017 update. Crit. Rev. Clin. Lab. Sci. 2017, 54, 326–342. [Google Scholar] [CrossRef]
- Sen, S.; Ozalp Kizilay, D.; Taneli, F.; Ozen, C.; Ertan, P.; Ozunan, I.; Yildiz, R.; Ersoy, B. Urinary NGAL is a Potential Biomarker for Early Renal Injury in Insulin Resistant Obese Non-diabetic Children. J. Clin. Res. Pediatr. Endocrinol. 2021, 13, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Piani, F.; Reinicke, T.; Borghi, C.; Tommerdahl, K.L.; Cara-Fuentes, G.; Johnson, R.J.; Bjornstad, P. Acute Kidney Injury in Pediatric Diabetic Kidney Disease. Front. Pediatr. 2021, 9, 668033. [Google Scholar] [CrossRef] [PubMed]
- Aztatzi-Aguilar, O.G.; Sierra-Vargas, M.P.; Ortega-Romero, M.; Jimenez-Corona, A.E. Osteopontin’s relationship with malnutrition and oxidative stress in adolescents. A pilot study. PLoS ONE 2021, 16, e0249057. [Google Scholar] [CrossRef] [PubMed]
- Goknar, N.; Oktem, F.; Ozgen, I.T.; Torun, E.; Kucukkoc, M.; Demir, A.D.; Cesur, Y. Determination of early urinary renal injury markers in obese children. Pediatr. Nephrol. 2015, 30, 139–144. [Google Scholar] [CrossRef]
- Bostan Gayret, O.; Tasdemir, M.; Erol, M.; Tekin Nacaroglu, H.; Zengi, O.; Yigit, O. Are there any new reliable markers to detect renal injury in obese children? Ren. Fail 2018, 40, 416–422. [Google Scholar] [CrossRef] [Green Version]
- Mayer-Davis, E.J.; Kahkoska, A.R.; Jefferies, C.; Dabelea, D.; Balde, N.; Gong, C.X.; Aschner, P.; Craig, M.E. ISPAD Clinical Practice Consensus Guidelines 2018: Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatric Diabetes 2018, 19 (Suppl. 27), 7–19. [Google Scholar] [CrossRef]
- Kim, J.H.; Yun, S.; Hwang, S.S.; Shim, J.O.; Chae, H.W.; Lee, Y.J.; Lee, J.H.; Kim, S.C.; Lim, D.; Yang, S.W.; et al. The 2017 Korean National Growth Charts for children and adolescents: Development, improvement, and prospects. Korean J. Pediatrics 2018, 61, 135–149. [Google Scholar] [CrossRef]
- Marcovecchio, M.L.; Chiesa, S.T.; Armitage, J.; Daneman, D.; Donaghue, K.C.; Jones, T.W.; Mahmud, F.H.; Marshall, S.M.; Neil, H.A.W.; Dalton, R.N.; et al. Renal and Cardiovascular Risk According to Tertiles of Urinary Albumin-to-Creatinine Ratio: The Adolescent Type 1 Diabetes Cardio-Renal Intervention Trial (AdDIT). Diabetes Care 2018, 41, 1963–1969. [Google Scholar] [CrossRef] [Green Version]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Raja, P.; Maxwell, A.P.; Brazil, D.P. The Potential of Albuminuria as a Biomarker of Diabetic Complications. Cardiovasc. Drugs Ther. 2021, 35, 455–466. [Google Scholar] [CrossRef]
- Yoo, J.-J.; Kwon, J.H.; Kim, Y.S.; Nam, S.W.; Park, J.W.; Kim, H.Y.; Kim, C.W.; Shin, S.K.; Chon, Y.E.; Jang, E.S.; et al. The Role of Urinary N-Acetyl-β-d-glucosaminidase in Cirrhotic Patients with Acute Kidney Injury: Multicenter, Prospective Cohort Study. J. Clin. Med. 2021, 10, 4328. [Google Scholar] [CrossRef] [PubMed]
- Phanish, M.K.; Chapman, A.N.; Yates, S.; Price, R.; Hendry, B.M.; Roderick, P.J.; Dockrell, M.E.C. Evaluation of Urinary Biomarkers of Proximal Tubular Injury, Inflammation, and Fibrosis in Patients with Albuminuric and Nonalbuminuric Diabetic Kidney Disease. Kidney Int. Rep. 2021, 6, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- Lou, W.; Cheng, Q.; Liang, Y.; Xia, D. Urinary N-Acetyl-beta-d-Glucosaminidase (NAG) Levels and Risk of Cardiovascular Events in Diabetic Patients. Int. J. Gen. Med. 2021, 14, 10495–10502. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, M.; Lee, Y.H.; Kang, E.S.; Cha, B.S.; Lee, B.W. Renal Tubular Damage Marker, Urinetyl-beta-D-Glucosaminidase, as a Predictive Marker of Hepatic Fibrosis in Type 2 Diabetes Mellitus. Diabetes Metab.J. 2021, 46, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Habashy, S.E.; Adly, A.; Abdel Kader, M.; Ali, S.E. Predictors of future microalbuminuria in children and adolescents with type 1 diabetes mellitus in Egypt. Arch. Med. Sci. Atheroscler. Dis. 2019, 4, e286–e297. [Google Scholar] [CrossRef]
- Mysliwiec, M.; Zorena, K.; Balcerska, A.; Mysliwska, J.; Lipowski, P.; Raczynska, K. The activity of N-acetyl-beta-D-glucosaminidase and tumor necrosis factor-alpha at early stage of diabetic retinopathy development in type 1 diabetes mellitus children. Clin. Biochem. 2006, 39, 851–856. [Google Scholar] [CrossRef]
- Safaeian, B.; Nickavar, A.; Zaeri, H.; Lahootian, L.; Behnampour, N. Utility of Urine N-acetyl-beta-D-glucosaminidase for Prediction of Renal Damage in Obese Children. Saudi J. Kidney Dis. Transplant. Off. Publ. Saudi Cent. Organ Transplant. Saudi Arab. 2021, 32, 699–702. [Google Scholar]
- Kim, S.R.; Lee, Y.H.; Lee, S.G.; Kang, E.S.; Cha, B.S.; Kim, J.H.; Lee, B.W. Urinary N-acetyl-beta-D-glucosaminidase, an early marker of diabetic kidney disease, might reflect glucose excursion in patients with type 2 diabetes. Medicine 2016, 95, e4114. [Google Scholar] [CrossRef]
- American Diabetes Association. 13. Children and Adolescents: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S163–S182. [Google Scholar] [CrossRef] [Green Version]

| Total (n = 96) | |||||||
|---|---|---|---|---|---|---|---|
| Control (n = 43) | T1DM (n = 31) | T2DM (n = 22) | p | ||||
| Non-Obese (n = 17) | Obese (n = 26) | Non-Obese (n = 24) | Obese (n = 7) | Non-Obese (n = 3) | Obese (n = 19) | ||
| Age (years) | 8.83 (8.54, 10.63) | 10.54 (9.42, 12.04) | 14 (9.96, 16.48) | 15 (12.5, 15.83) | 16.58 (15.83, 16.92) | 17.83 (15.75, 18.33) | <0.001 |
| †Sex | 7/10 | 20/6 | 14/10 | 4/3 | 0/3 | 10/9 | 0.075 |
| ‡Institution | 0/17 | 0/26 | 15/9 | 2/5 | 2/1 | 11/8 | <0.001 |
| Height SDS | −0.91 (−1.35, 0.39) | 1.23 (0.4, 1.61) | 0.2 (−0.39. 0.81) | 0.26 (−0.78, 1.57) | 1.81 (−0.88, 2.26) | 0.84 (0.07, 1.81) | <0.001 |
| Weight SDS | −0.09 (−0.92, 0.48) | 2.46 (2.14, 2.99) | −0.33 (−1.05, 0.42) | 234 (1.47, 2.99) | 0.19 (0.14, 1.34) | 3.38 (2.44, 4.26) | <0.001 |
| BMI SDS | 0.17 (−0.59, 0.99) | 2.73 (2.46, 3.24) | −0.68 (−1.44, 0.44) | 2.43 (1.84, 2.93) | 0.31 (−0.77, 0.64) | 3.26 (2.88, 4.79) | <0.001 |
| CRP (mg/dL) | 0.03 (0.03, 0.11) | 0.1 (0.05, 0.19) | 0.3 (0.03, 0.3) | 1.32 (0.25, 3.93) | 1.02 (0.03, 3.39) | 0.73 (0.12, 2.6) | <0.001 |
| Glucose (mg/dL) | 94.5 (90, 97.75) | 94 (91, 97.5) | 109 (100.25, 140) | 224 (161, 248) | 113 (90, 120) | 138 (112, 254) | 0.284 |
| Cr (mg/dL) | 0.46 (0.42, 0.49) | 0.51 (0.44, 0.61) | 0.54 (0.44, 0.71) | 0.48 (0.45, 0.57) | 0.54 (0.53, 0.6) | 0.69 (0.55, 0.81) | 0.003 |
| eGFR (mL/min/1.73 m2) | 119.32 (107.55, 127.56) | 121.62 (104.98, 131.67) | 113.87 (98.35, 131.79) | 138.79 (123.9, 143.09) | 121.33 (116.6, 131.7) | 103.25 (89.89, 133.05) | <0.001 |
| AST (U/L) | 25 (21, 28.75) | 22 (18, 28) | 17 (13.25, 18.75) | 21 (16, 27) | 15 (8, 37) | 34 (13, 59) | 0.611 |
| ALT (U/L) | 14 (9.5, 16.75) | 18 (13, 41) | 10.5 (9, 13.75) | 17 (13, 25) | 10 (4, 65) | 57 (16, 103) | 0.005 |
| TC (mg/dL) | 182.5 (152.25, 225) | 170 (156.5, 196) | 167.5 (148.25, 197.75) | 204 (159, 213) | 193 (177, 207) | 181 (159, 202) | <0.001 |
| TG (mg/dL) | 97.5 (71, 156.75) | 98 (56, 129.5) | 69 (45.25, 111.75) | 174 (109, 290) | 111 (99, 164) | 127 (92, 238) | 0.237 |
| HDL-C (mg/dL) | 54.5 (44, 64.75) | 49 (44, 55.5) | 60 (52, 70) | 58 (48, 72) | 50 (47, 56) | 44 (39, 46) | <0.001 |
| LDL-C (mg/dL) | 108.5 (87.5, 137) | 104 (95.5, 128) | 87.8 (74.8, 125.05) | 98.8 (72.2, 124) | 123.2 (122, 127.2) | 113.6 (90, 122.2) | <0.001 |
| Uric acid (mg/dL) | 4.3 (3.7, 4.95) | 6.35 (4.88, 7.13) | 4.35 (3.58, 5.28) | 4.5 (4, 6) | 4.4 (2.4, 5.7) | 5.5 (4.9, 6.5) | <0.001 |
| HbA1c (%) | 5.2 (5.05, 5.45) | 5.5 (5.38, 5.7) | 7.8 (6.55, 9.18) | 9.5 (8.5, 10.3) | 7.4 (6.8, 7.9) | 8.4 (5.9, 11.7) | <0.001 |
| C-peptide (ng/mL) | 1.91 (1.48, 2.77) | 2.63 (2.18, 3.79) | 0.15 (0.02, 0.6) | 0.11 (0.02, 0.9) | 1.46 (0.94, 4.74) | 3.26 (2.04, 4.57) | <0.001 |
| HOMA-IR | 2.43 (1.73, 5.23) | 4.79 (2.73, 6.5) | 0.74 (0.36, 1.38) | 1.94 (1.62, 11.06) | 1.23 (1.22, 8.68) | 6.59 (4.39, 12.84) | <0.001 |
| HOMA-β (%) | 110.45 (87.06, 246.86) | 229.24 (171.4, 299.24) | 23.83 (9.58, 48.41) | 16.79 (6.17, 26.54) | 31.75 (26.02, 520.53) | 120.26 (67.68, 209.39) | <0.001 |
| Urine ACR (mg/g) | 8.03 (4.14, 12) | 4.12 (3.43, 5.48) | 8.48 (6.54, 13.44) | 20.2 (6.51, 193.43) | 18.5 (4.96, 41.09) | 11.69 (6.99, 25.32) | 0.012 |
| NGAL | β2-M | KIM−1 | L-FABP | OPN | NAG | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rho | p | rho | p | rho | p | rho | p | rho | p | rho | p | |
| Age | 0.116 | 0.259 | −0.369 | <0.001 | 0.015 | 0.885 | −0.296 | 0.003 | −0.135 | 0.19 | −0.398 | <0.001 |
| BMI SDS | −0.094 | 0.363 | −0.166 | 0.106 | −0.019 | 0.854 | −0.058 | 0.573 | −0.16 | 0.12 | −0.137 | 0.182 |
| CRP | 0.193 | 0.059 | −0.072 | 0.489 | 0.104 | 0.312 | −0.068 | 0.509 | −0.047 | 0.65 | −0.264 | 0.009 |
| AST | −0.042 | 0.687 | 0.106 | 0.305 | −0.108 | 0.293 | 0.065 | 0.529 | −0.15 | 0.143 | 0.062 | 0.546 |
| ALT | −0.108 | 0.297 | −0.011 | 0.915 | −0.094 | 0.36 | −0.071 | 0.493 | −0.187 | 0.068 | −0.066 | 0.523 |
| TC | 0.103 | 0.317 | 0.075 | 0.465 | 0.144 | 0.163 | 0.019 | 0.853 | 0.041 | 0.694 | 0.155 | 0.13 |
| TG | 0.123 | 0.233 | −0.005 | 0.962 | −0.064 | 0.537 | −0.008 | 0.939 | −0.059 | 0.565 | 0.074 | 0.472 |
| HDL-C | 0.043 | 0.676 | 0.058 | 0.573 | −0.007 | 0.946 | 0.061 | 0.554 | −0.006 | 0.951 | −0.064 | 0.534 |
| LDL-C | 0.023 | 0.827 | −0.025 | 0.808 | 0.172 | 0.094 | −0.057 | 0.584 | 0.004 | 0.965 | 0.102 | 0.321 |
| Uric acid | −0.24 | 0.018 | −0.2 | 0.051 | −0.099 | 0.335 | −0.195 | 0.057 | −0.044 | 0.672 | −0.132 | 0.2 |
| HbA1c | 0.21 | 0.04 | −0.1 | 0.332 | 0.253 | 0.013 | −0.027 | 0.797 | −0.072 | 0.484 | 0.26 | 0.01 |
| C-peptide | −0.241 | 0.018 | −0.155 | 0.131 | −0.103 | 0.318 | −0.103 | 0.318 | −0.045 | 0.662 | −0.038 | 0.712 |
| HOMA-IR | −0.159 | 0.122 | −0.095 | 0.356 | −0.034 | 0.745 | −0.053 | 0.608 | 0.007 | 0.948 | 0.015 | 0.881 |
| HOMA-β | −0.313 | 0.002 | −0.066 | 0.521 | −0.214 | 0.036 | −0.057 | 0.583 | 0.017 | 0.869 | −0.04 | 0.701 |
| Urine ACR | 0.278 | 0.006 | −0.424 | <0.001 | 0.22 | 0.032 | 0.304 | 0.003 | 0.217 | 0.033 | 0.438 | <0.001 |
| HbA1c | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-Obese Control | Obese Control | Non-Obese Diabetic | Obese Diabetic | ||||||
| rho | p | rho | p | rho | p | rho | p | ||
| NGAL | Non-obese control | −0.044 | 0.868 | 0.072 | 0.784 | 0.113 | 0.666 | 0.27 | 0.294 |
| Obese control | 0.115 | 0.661 | −0.116 | 0.574 | −0.135 | 0.512 | 0.166 | 0.417 | |
| Non-obese diabetic | −0.13 | 0.62 | 0.108 | 0.601 | −0.081 | 0.687 | −0.423 | 0.031 | |
| Obese diabetic | 0.029 | 0.913 | −0.06 | 0.772 | 0.193 | 0.346 | 0.267 | 0.187 | |
| KIM−1 | Non-obese control | −0.231 | 0.373 | 0.647 | 0.005 | 0.322 | 0.208 | 0.140 | 0.592 |
| Obese control | 0.052 | 0.842 | −0.248 | 0.222 | 0.119 | 0.561 | 0.159 | 0.438 | |
| Non-obese diabetic | 0.277 | 0.282 | 0.069 | 0.738 | −0.125 | 0.533 | −0.294 | 0.145 | |
| Obese diabetic | −0.439 | 0.078 | −0.152 | 0.459 | 0.125 | 0.543 | 0.279 | 0.168 | |
| NAG | Non-obese control | −0.029 | 0.913 | 0.041 | 0.841 | 0.228 | 0.262 | 0.419 | 0.033 |
| Obese control | −0.121 | 0.644 | 0.058 | 0.779 | 0.259 | 0.201 | 0.032 | 0.877 | |
| Non-obese diabetic | 0.108 | 0.679 | 0.346 | 0.173 | 0.143 | 0.004 | 0.056 | 0.018 | |
| Obese diabetic | −0.175 | 0.503 | −0.189 | 0.355 | 0.105 | 0.032 | 0.096 | 0.002 | |
| NGAL | KIM-1 | NAG | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HbA1c | OR (95% CI) | SE | p | OR (95% CI) | SE | p | OR (95% CI) | SE | p |
| Unadjusted | 0.228 (−1.43–1.88) | 0.825 | 0.784 | 0.019 (−0.106–0.145) | 0.063 | 0.753 | 0.366 (0.074–0.658) | 0.145 | 0.015 |
| Adjusted | |||||||||
| Model 1 | 0.631 (−0.899–2.16) | 0.76 | 0.411 | 0.035 (−0.086–0.156) | 0.06 | 0.56 | 0.355 (0.081–0.629) | 0.136 | 0.012 |
| Model 2 | 0.522 (−1.08–2.13) | 0.797 | 0.516 | 0.057 (−0.068–0.182) | 0.062 | 0.363 | 0.378 (0.091–0.665) | 0.143 | 0.011 |
| Model 3 | 0.442 (−112–2) | 0.772 | 0.569 | 0.056 (−0.07–0.183) | 0.063 | 0.375 | 0.353 (0.101–0.605) | 0.125 | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suh, J.-S.; Cho, K.S.; Kim, S.K.; Kim, S.-H.; Cho, W.K.; Jung, M.H.; Ahn, M.B. High Glycated Hemoglobin Instead of High Body Mass Index Might Increase the Urine N-Acetyl-β-D-glucosaminidase Con-Centration in Children and Adolescents with Diabetes Mellitus. Life 2022, 12, 879. https://doi.org/10.3390/life12060879
Suh J-S, Cho KS, Kim SK, Kim S-H, Cho WK, Jung MH, Ahn MB. High Glycated Hemoglobin Instead of High Body Mass Index Might Increase the Urine N-Acetyl-β-D-glucosaminidase Con-Centration in Children and Adolescents with Diabetes Mellitus. Life. 2022; 12(6):879. https://doi.org/10.3390/life12060879
Chicago/Turabian StyleSuh, Jin-Soon, Kyoung Soon Cho, Seul Ki Kim, Shin-Hee Kim, Won Kyoung Cho, Min Ho Jung, and Moon Bae Ahn. 2022. "High Glycated Hemoglobin Instead of High Body Mass Index Might Increase the Urine N-Acetyl-β-D-glucosaminidase Con-Centration in Children and Adolescents with Diabetes Mellitus" Life 12, no. 6: 879. https://doi.org/10.3390/life12060879
APA StyleSuh, J.-S., Cho, K. S., Kim, S. K., Kim, S.-H., Cho, W. K., Jung, M. H., & Ahn, M. B. (2022). High Glycated Hemoglobin Instead of High Body Mass Index Might Increase the Urine N-Acetyl-β-D-glucosaminidase Con-Centration in Children and Adolescents with Diabetes Mellitus. Life, 12(6), 879. https://doi.org/10.3390/life12060879






