Botox Therapy for Hypertrophy of the Masseter Muscle Causes a Compensatory Increase of Stiffness of Other Muscles of Masticatory Apparatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
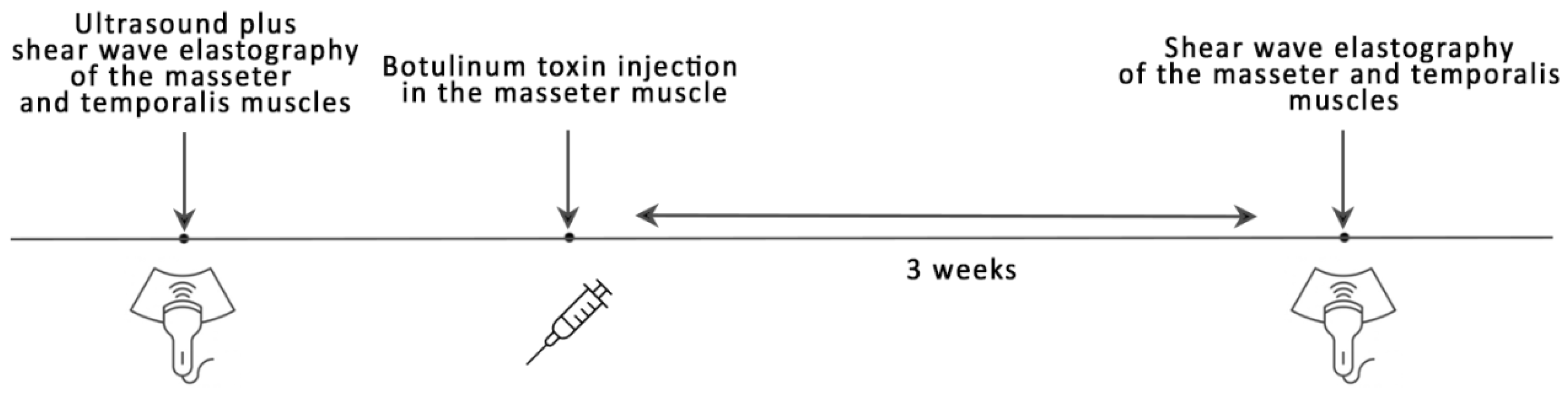
2.2. Study Procedure
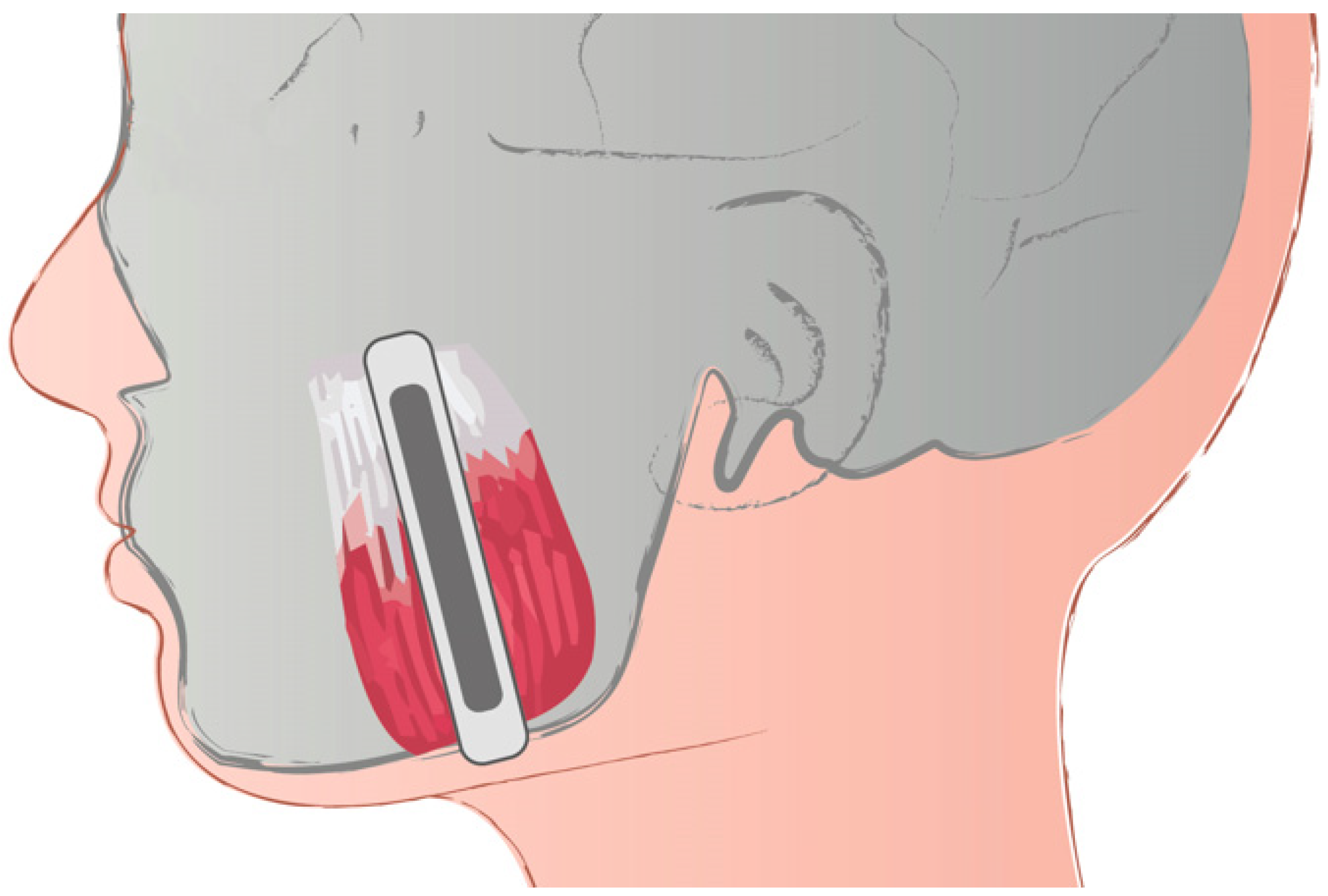
2.3. Botox Treatment
2.4. Shear Wave Elastography
2.5. Statistical Analysis
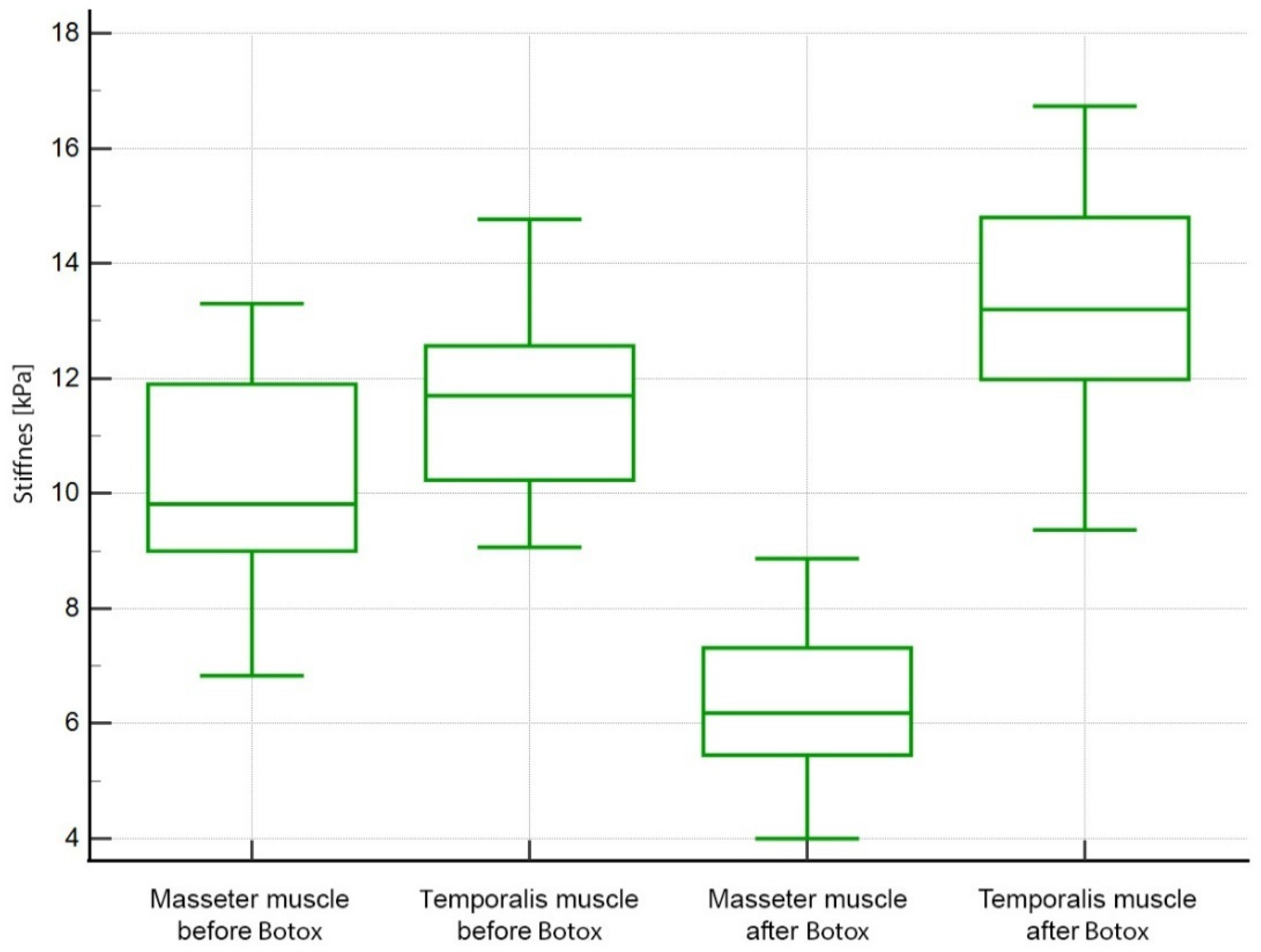
3. Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graziano, P.; Dell’Aversana Orabona, G.; Astarita, F.; Ponzo, L.M.; Nunziata, R.; Salzano, G.; Maglitto, F.; Solari, D.; Santella, A.; Cappabianca, M.; et al. Bilateral hypertrophy of masseteric and temporalis muscles, our fifteen patients and review of literature. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 7–11. [Google Scholar] [PubMed]
- Guruprasad, R.; Rishi, S.; Nair, P.P.; Thomas, S. Masseter and medial pterygoid muscle hypertrophy. BMJ Case Rep. 2011, 2011, bcr0720114557. [Google Scholar] [PubMed]
- Kebede, B.; Megersa, S. Idiopathic masseter muscle hypertrophy. Ethiop. J. Health Sci. 2011, 21, 209–212. [Google Scholar]
- Han, H.S.; Park, J.W.; Youn, C.S.; Ahn, J.Y.; Cho, S.; Park, K.Y. Validation of the novel masseter muscle hypertrophy scale in Asian population. J. Cosmet. Dermatol. 2021, 20, 1948–1950. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, E.C.; Kabala, J.; Guest, P.G. Magnetic resonance imaging in the diagnosis of asymmetrical bilateral masseteric hypertrophy. Dentomaxillofac. Radiol. 1999, 28, 52–54. [Google Scholar] [CrossRef] [PubMed]
- Rispoli, D.Z.; Camargo, P.M.; Pires, J.L.; Fonseca, V.R.; Mandelli, K.K.; Pereira, M.A.C. Benign masseter muscle hypertrophy. Braz. J. Otorhinolaryngol. 2008, 74, 790–793. [Google Scholar] [CrossRef]
- Boltshauser, E. Hypertrophy of temporalis muscles due to chewing gum “abuse”. J. Child. Neurol. 1996, 11, 210. [Google Scholar] [CrossRef]
- Vasileva, R. Masseter muscle hypertrophy in dentistry. Scr. Sci. Med. Dent. 2018, 4, 15–19. [Google Scholar] [CrossRef]
- Băbțan, A.M.; Boșca, B.A.; Crișan, M.; Lenghel, M.; Câmpian, R.S.; Ilea, A. Ultrasound Investigation in Congenital Masseter Hypertrophy. Biomed. J. Sci. Tech. Res. 2018, 6, 5262–5268. [Google Scholar]
- Furdui-Carr, G.; Mandel, L. Unilateral masseteric hypertrophy. A case report. N. Y. State Dent. J. 2010, 76, 46–48. [Google Scholar]
- Sharma, M.M.; Marya, J.; Thakur, A. Unilateral masseter hypertrophy with unusual twitching—A case report. J. Adv. Med. Dent. Sci. Res. 2017, 5, 58–60. [Google Scholar]
- Olchowy, C.; Grzech-Leśniak, K.; Hadzik, J.; Olchowy, A.; Łasecki, M. Monitoring of Changes in Masticatory Muscle Stiffness after Gum Chewing Using Shear Wave Elastography. J. Clin. Med. 2021, 10, 2480. [Google Scholar] [CrossRef]
- Roncević, R. Masseter muscle hypertrophy. Aetiology and therapy. J. Maxillofac. Surg. 1986, 14, 344–348. [Google Scholar] [CrossRef]
- Ganguly, J.; Kulshreshtha, D.; Almotiri, M.; Jog, M. Muscle Tone Physiology and Abnormalities. Toxins 2021, 13, 282. [Google Scholar] [CrossRef]
- Ranasinghe, J.C.; Wickramasinghe, C.; Rodrigo, G. Isolated unilateral temporalis muscle hypertrophy in a child: A case report with literature review. BMC Pediatr. 2018, 18, 71. [Google Scholar] [CrossRef]
- Bocchialini, G.; Castellani, A.; Negrini, S.; Rossi, A. New Management in Bilateral Masseter Muscle Hypertrophy. Craniomaxillofac. Trauma Reconstr. 2017, 10, 325–328. [Google Scholar] [CrossRef]
- Fedorowicz, Z.; van Zuuren, E.J.; Schoones, J. Botulinum toxin for masseter hypertrophy. Cochrane Database Syst. Rev. 2013, 2013, Cd007510. [Google Scholar] [CrossRef] [PubMed]
- Olchowy, C.; Olchowy, A.; Hadzik, J.; Dąbrowski, P.; Mierzwa, D. Dentists can provide reliable shear wave elastography measurements of the stiffness of masseter muscles: A possible scenario for a faster diagnostic process. Adv. Clin. Exp. Med. 2021, 30, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Bercoff, J.; Tanter, M.; Fink, M. Supersonic shear imaging: A new technique for soft tissue elasticity mapping. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 396–409. [Google Scholar] [CrossRef]
- Olchowy, A.; Więckiewicz, M.; Malysa, A.; Olchowy, C. Determination of Reference Values of the Masseter Muscle Stiffness in Healthy Adults Using Shear Wave Elastography. Int. J. Environ. Res. Public Health 2021, 18, 9371. [Google Scholar] [CrossRef]
- Olchowy, C.; Olchowy, A.; Pawluś, A.; Więckiewicz, M.; Sconfienza, L.M. Stiffness of the Masseter Muscle in Children-Establishing the Reference Values in the Pediatric Population Using Shear-Wave Elastography. Int. J. Environ. Res. Public Health 2021, 18, 9619. [Google Scholar] [CrossRef]
- Rauso, R.; Tartaro, G.; Rugge, L.; Chirico, F.; Zerbinati, N. Remodeling the neck and the lower jaw with dehoxycholate injections. J. Biol. Regul. Homeost. Agents 2018, 32, 1279–1283. [Google Scholar]
- von Lindern, J.J.; Niederhagen, B.; Appel, T.; Bergé, S.; Reich, R.H. Type A botulinum toxin for the treatment of hypertrophy of the masseter and temporal muscles: An alternative treatment. Plast. Reconstr. Surg. 2001, 107, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Parente, E.V.; Silvares, M.G.; Naegele, M.M.; Ribeiro, D.P.B.; Andrade, M. Surgical treatment of bilateral temporalis and masseteric hypertrophy: Report of a case. Open J. Stomatol. 2013, 3, 99–102. [Google Scholar] [CrossRef][Green Version]
- Schiffman, E.; Ohrbach, R. Executive summary of the Diagnostic Criteria for Temporomandibular Disorders for clinical and research applications. J. Am. Dent. Assoc. 2016, 147, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Park, R.H.; Park, J.B. Botulinum toxin type A for the treatment of hypertrophy of the masseter muscle. Plast. Reconstr. Surg. 2010, 125, 1693–1705. [Google Scholar] [CrossRef]
- Olchowy, C.; Więckiewicz, M.; Sconfienza, L.M.; Łasecki, M.; Seweryn, P.; Smardz, J.; Hnitecka, S.; Dominiak, M.; Olchowy, A. Potential of Using Shear Wave Elastography in the Clinical Evaluation and Monitoring of Changes in Masseter Muscle Stiffness. Pain Res. Manag. 2020, 2020, 4184268. [Google Scholar] [CrossRef] [PubMed]
- Smyth, A.G. Botulinum toxin treatment of bilateral masseteric hypertrophy. Br. J. Oral Maxillofac. Surg. 1994, 32, 29–33. [Google Scholar] [CrossRef]
- Melfa, F.; Siragusa, D.; Caruso, D.G.; Tunesi, L.; Zerbinati, N.; Chirico, F.; Lo Faro, C.; Rauso, R. An Italian experience of a new personalized injective protocol (Botutouch) for botulinum toxin application in aesthetic medicine. Dermatol. Ther. 2020, 33, e14395. [Google Scholar] [CrossRef]
- Hong, J.Y.; Jeong, G.J.; Kwon, T.R.; Kim, J.H.; Li, K.; Kim, B.J. Efficacy and Safety of a Novel Botulinum Toxin A for Masseter Reduction: A Randomized, Double-Blind, Placebo-Controlled, Optimal Dose-Finding Study. Dermatol. Surg. 2021, 47, e5–e9. [Google Scholar] [CrossRef]
- Wei, J.; Xu, H.; Dong, J.; Li, Q.; Dai, C. Prolonging the duration of masseter muscle reduction by adjusting the masticatory movements after the treatment of masseter muscle hypertrophy with botulinum toxin type A injection. Dermatol. Surg. 2015, 41 (Suppl. 1), S101–S109. [Google Scholar] [CrossRef]
- Bhattacharjee, K.; Singh, M.; Bhattacharjee, H. Extended effect after a single dose of type A botulinum toxin for asymmetric masseter muscle hypertrophy. Indian J. Plast. Surg. 2015, 48, 196–199. [Google Scholar] [CrossRef]
- Shome, D.; Khare, S.; Kapoor, R. Efficacy of Botulinum Toxin in Treating Asian Indian Patients with Masseter Hypertrophy: A 4-Year Follow-Up Study. Plast. Reconstr. Surg. 2019, 144, 390e–396e. [Google Scholar] [CrossRef]
- No, Y.A.; Ahn, B.H.; Kim, B.J.; Kim, M.N.; Hong, C.K. Three-dimensional CT might be a potential evaluation modality in correction of asymmetrical masseter muscle hypertrophy by botulinum toxin injection. J. Cosmet. Laser Ther. 2016, 18, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Klein, F.H.; Brenner, F.M.; Sato, M.S.; Robert, F.M.; Helmer, K.A. Lower facial remodeling with botulinum toxin type A for the treatment of masseter hypertrophy. An. Bras. Dermatol. 2014, 89, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.T.; Peng, J.H.; Peng, H.P. Literature review of the adverse events associated with botulinum toxin injection for the masseter muscle hypertrophy. J. Cosmet. Dermatol. 2018, 17, 675–687. [Google Scholar] [CrossRef]
- Olchowy, A.; Wieckiewicz, M.; Winocur, E.; Dominiak, M.; Dekkers, I.; Łasecki, M.; Olchowy, C. Great potential of ultrasound elastography for the assessment of the masseter muscle in patients with temporomandibular disorders. A systematic review. Dentomaxillofac. Radiol. 2020, 49, 20200024. [Google Scholar] [CrossRef]
- Taş, S.; Onur, M.R.; Yılmaz, S.; Soylu, A.R.; Korkusuz, F. Shear Wave Elastography Is a Reliable and Repeatable Method for Measuring the Elastic Modulus of the Rectus Femoris Muscle and Patellar Tendon. J. Ultrasound Med. 2017, 36, 565–570. [Google Scholar] [CrossRef]
- Cho, G.-H.; Lee, Y. Analysis of Masticatory Muscle Activity Based on Presence of Temporomandibular Joint Disorders. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e921337. [Google Scholar] [CrossRef]
| N = 23 | Before Botox | After Botox | p-Value |
|---|---|---|---|
| Masseter muscle | |||
| Left masseter | 10.16 ± 1.74 | 6.38 ± 1.36 | <0.0001 |
| Right masseter | 10.20 ± 1.65 | 6.37 ± 1.34 | <0.0001 |
| p-value | 0.6302 | 1.000 | |
| Temporalis muscle | |||
| Left temporalis | 11.57 ± 1.55 | 13.12 ± 1.96 | 0.0002 |
| Right temporalis | 11.62 ± 1.57 | 13.09 ± 1.92 | 0.0013 |
| p-value | 0.9813 | 0.9813 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mierzwa, D.; Olchowy, C.; Olchowy, A.; Nawrot-Hadzik, I.; Dąbrowski, P.; Chobotow, S.; Grzech-Leśniak, K.; Kubasiewicz-Ross, P.; Dominiak, M. Botox Therapy for Hypertrophy of the Masseter Muscle Causes a Compensatory Increase of Stiffness of Other Muscles of Masticatory Apparatus. Life 2022, 12, 840. https://doi.org/10.3390/life12060840
Mierzwa D, Olchowy C, Olchowy A, Nawrot-Hadzik I, Dąbrowski P, Chobotow S, Grzech-Leśniak K, Kubasiewicz-Ross P, Dominiak M. Botox Therapy for Hypertrophy of the Masseter Muscle Causes a Compensatory Increase of Stiffness of Other Muscles of Masticatory Apparatus. Life. 2022; 12(6):840. https://doi.org/10.3390/life12060840
Chicago/Turabian StyleMierzwa, Dorota, Cyprian Olchowy, Anna Olchowy, Izabela Nawrot-Hadzik, Paweł Dąbrowski, Sławomir Chobotow, Kinga Grzech-Leśniak, Paweł Kubasiewicz-Ross, and Marzena Dominiak. 2022. "Botox Therapy for Hypertrophy of the Masseter Muscle Causes a Compensatory Increase of Stiffness of Other Muscles of Masticatory Apparatus" Life 12, no. 6: 840. https://doi.org/10.3390/life12060840
APA StyleMierzwa, D., Olchowy, C., Olchowy, A., Nawrot-Hadzik, I., Dąbrowski, P., Chobotow, S., Grzech-Leśniak, K., Kubasiewicz-Ross, P., & Dominiak, M. (2022). Botox Therapy for Hypertrophy of the Masseter Muscle Causes a Compensatory Increase of Stiffness of Other Muscles of Masticatory Apparatus. Life, 12(6), 840. https://doi.org/10.3390/life12060840








