Current Status of Biparametric MRI in Prostate Cancer Diagnosis: Literature Analysis
Abstract
:1. Introduction
1.1. Role of I.V. Contrast (DCE Imaging) in Prostate Cancer Imaging and Controversies
1.2. Prior Reviews Comparing mpMRI against bpMRI (without DCE)
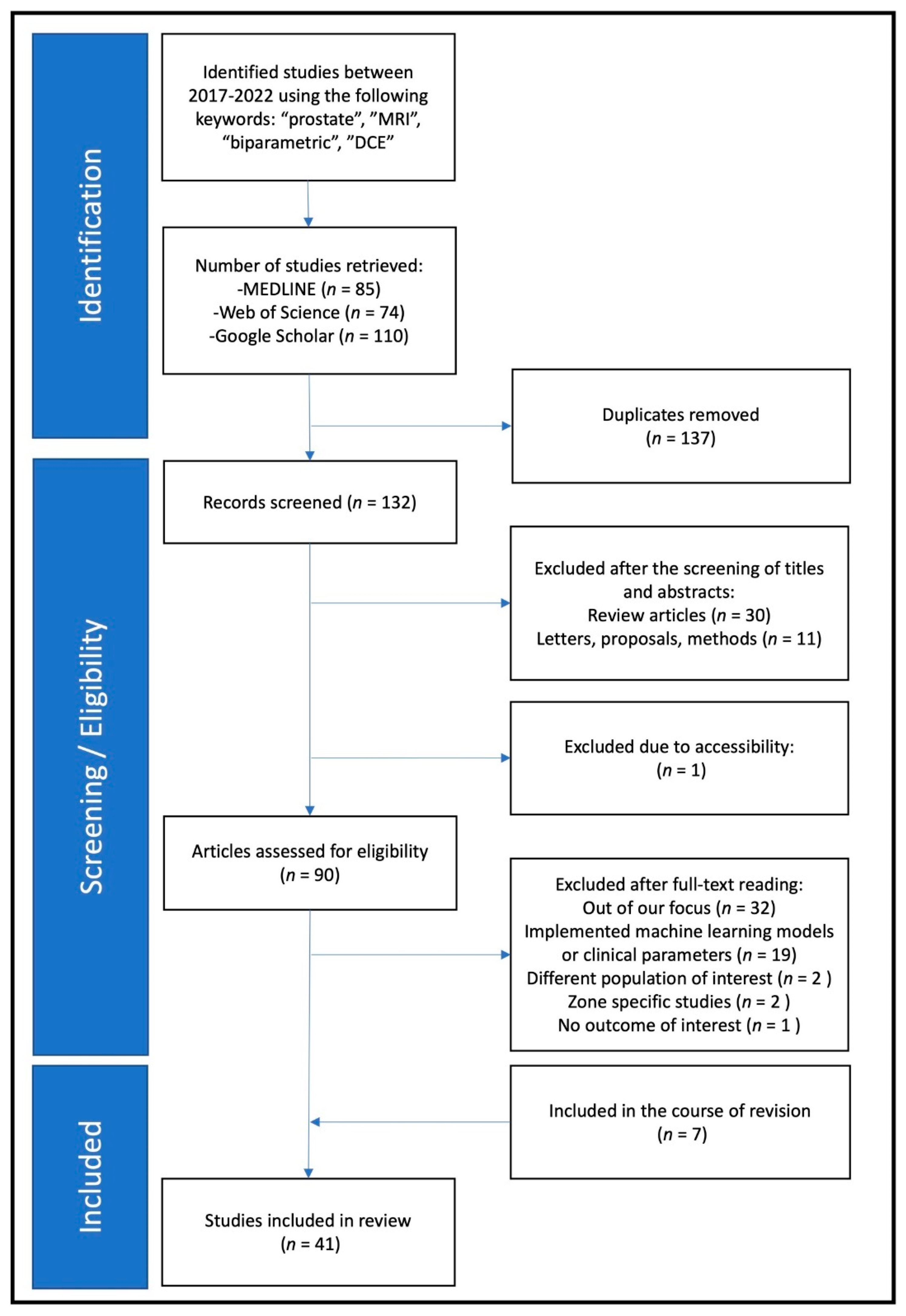
2. Methods
2.1. Paper Eligibility and Selection
2.2. Data Collection
3. Results
3.1. Clinical Characteristics
3.2. Technical Characteristics
4. Discussion
4.1. Studies Meeting the Majority of the Defined DCE Indications
4.2. DCE Indications and Frequency of Reporting
4.3. Study Heterogeneity
4.4. Limitations
4.5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.; Suh, C.H.; Kim, S.Y.; Cho, J.Y.; Kim, S.H.; Moon, M.H. Head-to-Head Comparison between Biparametric and Multiparametric MRI for the Diagnosis of Prostate Cancer: A Systematic Review and Meta-Analysis. Am. J. Roentgenol. 2018, 211, W226–W241. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.; Reis, I.M.; Breto, A.L.; Tschudi, Y.; Gautney, N.; Zavala-Romero, O.; Lopez, C.; Ford, J.C.; Punnen, S.; Pollack, A.; et al. Classification of suspicious lesions on prostate multiparametric MRI using machine learning. J. Med. Imaging 2018, 5, 034502. [Google Scholar] [CrossRef]
- Min, X.; Li, M.; Dong, D.; Feng, Z.; Zhang, P.; Ke, Z.; You, H.; Han, F.; Ma, H.; Tian, J.; et al. Multi-parametric MRI-based radiomics signature for discriminating between clinically significant and insignificant prostate cancer: Cross-validation of a machine learning method. Eur. J. Radiol. 2019, 115, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Gaur, S.; Lay, N.; Harmon, S.A.; Doddakashi, S.; Mehralivand, S.; Argun, B.; Barrett, T.; Bednarova, S.; Girometti, R.; Karaarslan, E.; et al. Can computer-aided diagnosis assist in the identification of prostate cancer on prostate MRI? A multi-center, multi-reader investigation. Oncotarget 2018, 9, 33804–33817. [Google Scholar] [CrossRef] [Green Version]
- Obmann, V.C.; Pahwa, S.; Tabayayong, W.; Jiang, Y.; O’Connor, G.; Dastmalchian, S.; Lu, J.; Shah, S.; Herrmann, K.A.; Paspulati, R.; et al. Diagnostic Accuracy of a Rapid Biparametric MRI Protocol for Detection of Histologically Proven Prostate Cancer. Urology 2018, 122, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Russo, F.; Mazzetti, S.; Regge, D.; Ambrosini, I.; Giannini, V.; Manfredi, M.; De Luca, S.; Bollito, E.; Porpiglia, F. Diagnostic Accuracy of Single-plane Biparametric and Multiparametric Magnetic Resonance Imaging in Prostate Cancer: A Randomized Noninferiority Trial in Biopsy-naïve Men. Eur. Urol. Oncol. 2021, 4, 855–862. Available online: https://www.sciencedirect.com/science/article/pii/S2588931121000742 (accessed on 11 November 2021). [CrossRef]
- Barentsz, J.O.; Richenberg, J.; Clements, R.; Choyke, P.; Verma, S.; Villeirs, G.; Rouviere, O.; Logager, V.; Fütterer, J.J. European Society of Urogenital Radiology ESUR prostate MR guidelines 2012. Eur. Radiol. 2012, 22, 746–757. [Google Scholar] [CrossRef] [Green Version]
- Barrett, T.; Rajesh, A.; Rosenkrantz, A.B.; Choyke, P.L.; Turkbey, B. PI-RADS version 2.1: One small step for prostate MRI. Clin. Radiol. 2019, 74, 841–852. [Google Scholar] [CrossRef]
- Beyer, T.; Schlemmer, H.-P.; Weber, M.-A.; Thierfelder, K.M. PI-RADS 2.1—Image Interpretation: The Most Important Updates and Their Clinical Implications. Rofo 2021, 193, 787–796. [Google Scholar] [CrossRef]
- Choi, M.H.; Lee, Y.J.; Jung, S.E.; Rha, S.E.; Byun, J.Y. Prebiopsy biparametric MRI: Differences of PI-RADS version 2 in patients with different PSA levels. Clin. Radiol. 2018, 73, 810–817. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, G.; Shi, B.; Liu, Y.; Zou, T.; Yan, W.; Xiao, Y.; Xue, H.; Feng, F.; Lei, J.; et al. Comparison of biparametric and multiparametric MRI in the diagnosis of prostate cancer. Cancer Imaging 2019, 19, 90. [Google Scholar] [CrossRef] [PubMed]
- Scialpi, M.; Scialpi, P.; Martorana, E.; Torre, R.; Improta, A.; Aisa, M.C.; D’Andrea, A.; Blasi, A.D. Simplified PI-RADS (S-PI-RADS) for biparametric MRI to detect and manage prostate cancer: What urologists need to know. Turk. J. Urol. 2021, 47, 175–182. [Google Scholar] [CrossRef]
- Porter, K.K.; King, A.; Galgano, S.J.; Sherrer, R.L.; Gordetsky, J.B.; Rais-Bahrami, S. Financial implications of biparametric prostate MRI. Prostate Cancer Prostatic Dis. 2020, 23, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Girometti, R.; Cereser, L.; Bonato, F.; Zuiani, C. Evolution of prostate MRI: From multiparametric standard to less-is-better and different-is better strategies. Eur. Radiol. Exp. 2019, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Zawaideh, J.P.; Sala, E.; Shaida, N.; Koo, B.; Warren, A.Y.; Carmisciano, L.; Saeb-Parsy, K.; Gnanapragasam, V.J.; Kastner, C.; Barrett, T. Diagnostic accuracy of biparametric versus multiparametric prostate MRI: Assessment of contrast benefit in clinical practice. Eur. Radiol. 2020, 30, 4039–4049. [Google Scholar] [CrossRef]
- Stabile, A.; Giganti, F.; Kasivisvanathan, V.; Giannarini, G.; Moore, C.M.; Padhani, A.R.; Panebianco, V.; Rosenkrantz, A.B.; Salomon, G.; Turkbey, B.; et al. Factors Influencing Variability in the Performance of Multiparametric Magnetic Resonance Imaging in Detecting Clinically Significant Prostate Cancer: A Systematic Literature Review. Eur. Urol. Oncol. 2020, 3, 145–167. [Google Scholar] [CrossRef]
- van der Leest, M.; Israël, B.; Cornel, E.B.; Zámecnik, P.; Schoots, I.G.; van der Lelij, H.; Padhani, A.R.; Rovers, M.; van Oort, I.; Sedelaar, M.; et al. High Diagnostic Performance of Short Magnetic Resonance Imaging Protocols for Prostate Cancer Detection in Biopsy-naïve Men: The Next Step in Magnetic Resonance Imaging Accessibility. Eur. Urol. 2019, 76, 574–581. [Google Scholar] [CrossRef]
- Niu, X.; Chen, X.; Chen, Z.; Chen, L.; Li, J.; Peng, T. Diagnostic Performance of Biparametric MRI for Detection of Prostate Cancer: A Systematic Review and Meta-Analysis. Am. J. Roentgenol. 2018, 211, 369–378. [Google Scholar] [CrossRef]
- Alabousi, M.; Salameh, J.-P.; Gusenbauer, K.; Samoilov, L.; Jafri, A.; Yu, H.; Alabousi, A. Biparametric versus Multiparametric Prostate MRI for the Detection of Prostate Cancer in Treatment-Naive Patients: A Diagnostic Test Accuracy Systematic Review and Meta-Analysis. BJU Int. 2019, 124, 209–220. [Google Scholar] [CrossRef]
- Bass, E.J.; Pantovic, A.; Connor, M.; Gabe, R.; Padhani, A.R.; Rockall, A.; Sokhi, H.; Tam, H.; Winkler, M.; Ahmed, H.U. A systematic review and meta-analysis of the diagnostic accuracy of biparametric prostate MRI for prostate cancer in men at risk. Prostate Cancer Prostatic Dis. 2021, 24, 596–611. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Hu, R.; Yang, Y.J.; An, N.; Duo, X.X.; Liu, Z.; Shi, S.H.; Liu, X.Q. Is dynamic contrast enhancement still necessary in multiparametric magnetic resonance for diagnosis of prostate cancer: A systematic review and meta-analysis. Transl. Urol. 2020, 9, 553–573. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Min, X.; Weinreb, J.; Li, Q.; Feng, Z.; Wang, L. Abbreviated Biparametric Versus Standard Multiparametric MRI for Diagnosis of Prostate Cancer: A Systematic Review and Meta-Analysis. Am. J. Roentgenol. 2019, 212, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Scialpi, M.; Scialpi, P.; D’Andrea, A.; di Blasi, A. Re: Ivo G. Schoots, Jelle O. Barentsz, Leonardo K. Bittencourt; et al. PI-RADS Committee Position on MRI without Contrast Medium in Biopsy-naive Men with Suspected Prostate Cancer: Narrative Review. Am J Roentgenol 2021;216:3-19: PI-RADS v2.1 and Future Direction Towards Prostate Biparametric Magnetic Resonance Imaging. Eur. Urol. 2021, 79, e110–e111. [Google Scholar] [CrossRef] [PubMed]
- Schoots, I.G.; Barentsz, J.O.; Bittencourt, L.K.; Haider, M.A.; Macura, K.J.; Margolis, D.J.A.; Moore, C.M.; Oto, A.; Panebianco, V.; Siddiqui, M.M.; et al. PI-RADS Committee Position on MRI Without Contrast Medium in Biopsy-Naive Men with Suspected Prostate Cancer: Narrative Review. Am. J. Roentgenol. 2021, 216, 3–19. [Google Scholar] [CrossRef]
- Barrett, T.; Ghafoor, S.; Gupta, R.T.; Kim, C.K.; Muglia, V.F.; Macura, K.J.; Purysko, A.S. Prostate MRI Qualification: AJR Expert Panel Narrative Review. Am. J. Roentgenol. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Giganti, F.; Kasivisvanathan, V.; Kirkham, A.; Punwani, S.; Emberton, M.; Moore, C.M.; Allen, C. Prostate MRI quality: A critical review of the last 5 years and the role of the PI-QUAL score. Br. J. Radiol. 2021, 1131, 20210415. [Google Scholar] [CrossRef]
- Giganti, F.; Allen, C.; Emberton, M.; Moore, C.M.; Kasivisvanathan, V. Prostate Imaging Quality (PI-QUAL): A New Quality Control Scoring System for Multiparametric Magnetic Resonance Imaging of the Prostate from the PRECISION trial. Eur. Urol. Oncol. 2020, 3, 615–619. [Google Scholar] [CrossRef]
- Al Salmi, I.; Menezes, T.; El-Khodary, M.; Monteiro, S.; Haider, E.A.; Alabousi, A. Prospective evaluation of the value of dynamic contrast enhanced (DCE) imaging for prostate cancer detection, with pathology correlation. Can. J. Urol. 2020, 27, 10220–10227. [Google Scholar]
- Bao, J.; Zhi, R.; Hou, Y.; Zhang, J.; Wu, C.-J.; Wang, X.-M.; Zhang, Y.-D. Optimized MRI Assessment for Clinically Significant Prostate Cancer: A STARD-Compliant Two-Center Study. J. Magn. Reson. Imaging 2021, 53, 1210–1219. [Google Scholar] [CrossRef]
- Barth, B.K.; De Visschere, P.J.L.; Cornelius, A.; Nicolau, C.; Vargas, H.A.; Eberli, D.; Donati, O.F. Detection of Clinically Significant Prostate Cancer: Short Dual–Pulse Sequence versus Standard Multiparametric MR Imaging—A Multireader Study. Radiology 2017, 284, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Boesen, L.; Nørgaard, N.; Løgager, V.; Balslev, I.; Bisbjerg, R.; Thestrup, K.-C.; Winther, M.D.; Jakobsen, H.; Thomsen, H.S. Assessment of the Diagnostic Accuracy of Biparametric Magnetic Resonance Imaging for Prostate Cancer in Biopsy-Naive Men: The Biparametric MRI for Detection of Prostate Cancer (BIDOC) Study. JAMA Netw. Open 2018, 1, e180219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosaily, A.E.-S.; Frangou, E.; Ahmed, H.U.; Emberton, M.; Punwani, S.; Kaplan, R.; Brown, L.C.; Freeman, A.; Jameson, C.; Hindley, R.; et al. Additional Value of Dynamic Contrast-enhanced Sequences in Multiparametric Prostate Magnetic Resonance Imaging: Data from the PROMIS Study. Eur. Urol. 2020, 78, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Brancato, V.; Di Costanzo, G.; Basso, L.; Tramontano, L.; Puglia, M.; Ragozzino, A.; Cavaliere, C. Assessment of DCE Utility for PCa Diagnosis Using PI-RADS v2.1: Effects on Diagnostic Accuracy and Reproducibility. Diagnostics 2020, 10, 164. [Google Scholar] [CrossRef] [Green Version]
- Brembilla, G.; Giganti, F.; Sidhu, H.; Imbriaco, M.; Mallett, S.; Stabile, A.; Freeman, A.; Ahmed, H.U.; Moore, C.; Emberton, M.; et al. Diagnostic Accuracy of Abbreviated Bi-Parametric MRI (a-bpMRI) for Prostate Cancer Detection and Screening: A Multi-Reader Study. Diagnostics 2022, 12, 231. [Google Scholar] [CrossRef]
- Cai, G.-H.; Yang, Q.-H.; Chen, W.-B.; Liu, Q.-Y.; Zeng, Y.-R.; Zeng, Y.-J. Diagnostic Performance of PI-RADS v2, Proposed Adjusted PI-RADS v2 and Biparametric Magnetic Resonance Imaging for Prostate Cancer Detection: A Preliminary Study. Curr. Oncol. 2021, 28, 1823–1834. [Google Scholar] [CrossRef]
- Cereser, L.; Giannarini, G.; Bonato, F.; Pizzolitto, S.; Como, G.; Valotto, C.; Ficarra, V.; Dal Moro, F.; Zuiani, C.; Girometti, R. Comparison of multiple abbreviated multiparametric MRI-derived protocols for the detection of clinically significant prostate cancer. Minerva Urol. Nephrol. 2022, 74, 29–37. [Google Scholar] [CrossRef]
- Cho, J.; Ahn, H.; Hwang, S.I.; Lee, H.J.; Choe, G.; Byun, S.-S.; Hong, S.K. Biparametric versus multiparametric magnetic resonance imaging of the prostate: Detection of clinically significant cancer in a perfect match group. Prostate Int. 2020, 8, 146–151. [Google Scholar] [CrossRef]
- Choi, M.H.; Kim, C.K.; Lee, Y.J.; Jung, S.E. Prebiopsy Biparametric MRI for Clinically Significant Prostate Cancer Detection With PI-RADS Version 2: A Multicenter Study. Am. J. Roentgenol. 2019, 212, 839–846. [Google Scholar] [CrossRef]
- Christophe, C.; Montagne, S.; Bourrelier, S.; Roupret, M.; Barret, E.; Rozet, F.; Comperat, E.; Coté, J.F.; Lucidarme, O.; Cussenot, O.; et al. Prostate cancer local staging using biparametric MRI: Assessment and comparison with multiparametric MRI. Eur. J. Radiol. 2020, 132, 109350. [Google Scholar] [CrossRef]
- Di Campli, E.; Delli Pizzi, A.; Seccia, B.; Cianci, R.; d’Annibale, M.; Colasante, A.; Cinalli, S.; Castellan, P.; Navarra, R.; Iantorno, R.; et al. Diagnostic accuracy of biparametric vs multiparametric MRI in clinically significant prostate cancer: Comparison between readers with different experience. Eur. J. Radiol. 2018, 101, 17–23. [Google Scholar] [CrossRef]
- EL-Adalany, M.A.; EL-Razek, A.A.E.L.A.; EL-Diasty, T.; EL-Hendy, A.; EL-Metwally, D. Comparison between biparametric and multiparametric MR imaging of Prostate Imaging Reporting and Data System Version 2.1 in detection of prostate cancer. Egypt J. Radiol. Nucl. Med. 2021, 52, 68. [Google Scholar] [CrossRef]
- Eldred-Evans, D.; Neves, J.B.; Simmons, L.A.M.; Kanthabalan, A.; McCartan, N.; Shah, T.T.; Arya, M.; Charman, S.C.; Freeman, A.; Moore, C.M.; et al. Added value of diffusion-weighted images and dynamic contrast enhancement in multiparametric magnetic resonance imaging for the detection of clinically significant prostate cancer in the PICTURE trial. BJU Int. 2020, 125, 391–398. [Google Scholar] [CrossRef]
- Gatti, M.; Faletti, R.; Calleris, G.; Giglio, J.; Berzovini, C.; Gentile, F.; Marra, G.; Misischi, F.; Molinaro, L.; Bergamasco, L.; et al. Prostate cancer detection with biparametric magnetic resonance imaging (bpMRI) by readers with different experience: Performance and comparison with multiparametric (mpMRI). Abdom. Radiol. 2019, 44, 1883–1893. [Google Scholar] [CrossRef]
- Giannarini, G.; Cereser, L.; Como, G.; Bonato, F.; Pizzolitto, S.; Valotto, C.; Ficarra, V.; Dal Moro, F.; Zuiani, C.; Girometti, R. Accuracy of abbreviated multiparametric MRI-derived protocols in predicting local staging of prostate cancer in men undergoing radical prostatectomy. Acta Radiol. 2021, 62, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Liu, S.; Qin, X.B.; Ma, S.; Zhu, L.N.; Wang, X.Y. MRI combined with PSA density in detecting clinically significant prostate cancer in patients with PSA serum levels of 4~10ng/mL: Biparametric versus multiparametric MRI. Diagn. Interv. Imaging 2020, 101, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Jambor, I.; Verho, J.; Ettala, O.; Knaapila, J.; Taimen, P.; Syvanen, K.T.; Kiviniemi, A.; Kahkonen, E.; Perez, I.M.; Seppanen, M.; et al. Validation of IMPROD biparametric MRI in men with clinically suspected prostate cancer: A prospective multi-institutional trial. PLoS Med. 2019, 16, e1002813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Junker, D.; Steinkohl, F.; Fritz, V.; Bektic, J.; Tokas, T.; Aigner, F.; Herrmann, T.R.W.; Rieger, M.; Nagele, U. Comparison of multiparametric and biparametric MRI of the prostate: Are gadolinium-based contrast agents needed for routine examinations? World J. Urol. 2019, 37, 691–699. [Google Scholar] [CrossRef]
- Kim, Y.J.; Huh, J.S.; Park, K.K. Effectiveness of Bi-Parametric MR/US Fusion Biopsy for Detecting Clinically Significant Prostate Cancer in Prostate Biopsy Naïve Men. Yonsei Med. J. 2019, 60, 346. [Google Scholar] [CrossRef]
- Knaapila, J.; Jambor, I.; Ettala, O.; Taimen, P.; Verho, J.; Perez, I.M.; Kiviniemi, A.; Pahikkala, T.; Merisaari, H.; Lamminen, T.; et al. Negative Predictive Value of Biparametric Prostate Magnetic Resonance Imaging in Excluding Significant Prostate Cancer: A Pooled Data Analysis Based on Clinical Data from Four Prospective, Registered Studies. Eur. Urol. Focus 2021, 7, 522–531. [Google Scholar] [CrossRef]
- Kobilnyk, Y.; Mytsyk, Y.; Borzhiyevs’kyy, A.; Stroy, O.; Dutka, I.; Komnatska, I.; Vorobets, D.; Kucher, A.; Dmytriv, V.; Dmytrienko, V.; et al. Efficiency of the biparametric MRI in detection of prostate cancer: Preliminary experience. Eur. J. Med. Technol. 2020, 1, 39–44. [Google Scholar]
- Kuhl, C.K.; Bruhn, R.; Krämer, N.; Nebelung, S.; Heidenreich, A.; Schrading, S. Abbreviated Biparametric Prostate MR Imaging in Men with Elevated Prostate-specific Antigen. Radiology 2017, 285, 493–505. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.H.; Nam, J.K.; Lee, S.S.; Han, J.Y.; Lee, J.W.; Chung, M.K.; Park, S.W. Comparison of Multiparametric and Biparametric MRI in First Round Cognitive Targeted Prostate Biopsy in Patients with PSA Levels under 10 ng/mL. Yonsei Med. J. 2017, 58, 994–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesapane, F.; Acquasanta, M.; Meo, R.D.; Agazzi, G.M.; Tantrige, P.; Codari, M.; Schiaffino, S.; Patella, F.; Esseridou, A.; Sardanelli, F. Comparison of Sensitivity and Specificity of Biparametric versus Multiparametric Prostate MRI in the Detection of Prostate Cancer in 431 Men with Elevated Prostate-Specific Antigen Levels. Diagnostics 2021, 11, 1223. [Google Scholar] [CrossRef]
- Roh, A.T.; Fan, R.E.; Sonn, G.A.; Vasanawala, S.S.; Ghanouni, P.; Loening, A.M. How Often is the Dynamic Contrast Enhanced Score Needed in PI-RADS Version 2? Curr. Probl. Diagn. Radiol. 2020, 49, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Scialpi, M.; Prosperi, E.; D’andrea, A.; Martorana, E.; Malaspina, C.; Palumbo, B.; Orlandi, A.; Falcone, G.; Milizia, M.; Mearini, L.; et al. Biparametric versus Multiparametric MRI with Non-endorectal Coil at 3T in the Detection and Localization of Prostate Cancer. Anticancer Res. 2017, 37, 1263–1271. [Google Scholar] [PubMed] [Green Version]
- Sherrer, R.L.; Glaser, Z.A.; Gordetsky, J.B.; Nix, J.W.; Porter, K.K.; Rais-Bahrami, S. Comparison of biparametric MRI to full multiparametric MRI for detection of clinically significant prostate cancer. Prostate Cancer Prostatic Dis. 2019, 22, 331–336. [Google Scholar] [CrossRef]
- Taghipour, M.; Ziaei, A.; Alessandrino, F.; Hassanzadeh, E.; Harisinghani, M.; Vangel, M.; Tempany, C.M.; Fennessy, F.M. Investigating the role of DCE-MRI, over T2 and DWI, in accurate PI-RADS v2 assessment of clinically significant peripheral zone prostate lesions as defined at radical prostatectomy. Abdom. Radiol. 2019, 44, 1520–1527. [Google Scholar] [CrossRef]
- Tamada, T.; Kido, A.; Yamamoto, A.; Takeuchi, M.; Miyaji, Y.; Moriya, T.; Sone, T. Comparison of Biparametric and Multiparametric MRI for Clinically Significant Prostate Cancer Detection With PI-RADS Version 2.1. J. Magn. Reson. Imaging 2021, 53, 283–291. [Google Scholar] [CrossRef]
- Thestrup, K.C.D.; Logager, V.; Boesen, L.; Thomsen, H.S. Comparison of bi- and multiparametric magnetic resonance imaging to select men for active surveillance. Acta Radiol. Open 2019, 8, 2058460119866352. [Google Scholar] [CrossRef] [Green Version]
- De Visschere, P.; Lumen, N.; Ost, P.; Decaestecker, K.; Pattyn, E.; Villeirs, G. Dynamic contrast-enhanced imaging has limited added value over T2-weighted imaging and diffusion-weighted imaging when using PI-RADSv2 for diagnosis of clinically significant prostate cancer in patients with elevated PSA. Clin. Radiol. 2017, 72, 23–32. [Google Scholar] [CrossRef]
- Wallström, J.; Geterud, K.; Kohestani, K.; Maier, S.E.; Månsson, M.; Pihl, C.-G.; Socratous, A.; Arnsrud Godtman, R.; Hellström, M.; Hugosson, J. Bi- or multiparametric MRI in a sequential screening program for prostate cancer with PSA followed by MRI? Results from the Göteborg prostate cancer screening 2 trial. Eur. Radiol. 2021, 31, 8692–8702. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gao, J.; Zhang, Q.; Zhang, C.; Liu, G.; Wei, W.; Huang, H.; Fu, Y.; Li, D.; Zhang, B.; et al. Investigating the equivalent performance of biparametric compared to multiparametric MRI in detection of clinically significant prostate cancer. Abdom. Radiol. 2020, 45, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Yu, G.; Chen, J.; Yang, G.; Xu, H.; Chen, Z.; Wang, G.; Bai, Z. Can high b-value 3.0 T biparametric MRI with the Simplified Prostate Image Reporting and Data System (S-PI-RADS) be used in biopsy-naïve men? Clin. Imaging 2021. [Google Scholar] [CrossRef]
- Wassberg, C.; Akin, O.; Vargas, H.A.; Shukla-Dave, A.; Zhang, J.; Hricak, H. The Incremental Value of Contrast-Enhanced MRI in the Detection of Biopsy-Proven Local Recurrence of Prostate Cancer After Radical Prostatectomy: Effect of Reader Experience. Am. J. Roentgenol. 2012, 199, 360–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Author (Citation) | Year | Number of Patients | Age in Years (Mean/Median) | PSA in ng/mL (mean/median) | Prostate Volume in mL | PSA Density in ng/mL2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | PCa | csPCa | PZ Lesion | PR-3/LK-3 Lesions | |||||||||
| bpMRI | mpMRI | ||||||||||||
| Overall | csPCa | Overall | csPCa | ||||||||||
| Al Salmi * [29] | 2020 | 100 | 35 | 28 | DNI | 5, 5 | DNI | 6, 1 | DNI | 64 | 10.3 | 69.9 | 0.17 |
| Bao * [30] | 2021 | 638 | 319 | 287 | 338 | 116 | 45 | 72 | 16 | 69 | DNI | DNI | DNI |
| Barth * [31] | 2017 | 63 | 60 | 28 | DNI | DNI | DNI | DNI | DNI | 62.2 | 9.2 | DNI | DNI |
| Boesen * [32] | 2018 | 1020 | 655 | 404 | DNI | 130 | 17 | DNI | DNI | 67 | 8 | 53 | 0.15 |
| Bosaily * [33] | 2020 | 497 | 293 | 61 | DNI | 158 | 37 | 136 | 27 | 64 | 6.5 | DNI | DNI |
| Brancato * [34] | 2020 | 111 | 72 | 38 | 105 | 43, 28, 35 | 12, 8, 8 | 17, 9, 15 | 3, 3, 4 | 69 | DNI | 57.5 | 0.26 |
| Brembilla * [35] | 2022 | 151 | 129 | 76 | DNI | 20 | DNI | 21 | DNI | 62 | 6.8 | DNI | DNI |
| Cai * [36] | 2021 | 224 | 90 | 85 | 77 | 82, 85 | 4 | 53, 58 | 1, 2 | 69 | 14.55 | DNI | DNI |
| Cereser * [37] | 2020 | 108 | 104 | 47 | DNI | 24, 12 | 16, 11 | 13,3 | 10, 3 | 64.8 | 8.4 | DNI | DNI |
| Cho * [38] | 2020 | 41 | DNI | 41 | DNI | DNI | DNI | 1 | DNI | 64.3 | 9.2 | 33.1 | 0.31 |
| Choi * [39] | 2019 | 113 | 113 | 84 | DNI | 23, 35 | 15, 21 | 10, 13 | 6, 7 | 65 | 7.9 | DNI | DNI |
| Christophe * [40] | 2020 | 92 | DNI | DNI | DNI | DNI | DNI | DNI | DNI | 63 | DNI | DNI | 0.24 |
| Di Campli * [41] | 2018 | 85 | 72 | 41 | DNI | DNI | DNI | DNI | DNI | 70,39 | 8.5 | DNI | DNI |
| EL-Adalany * [42] | 2021 | 60 | 35 | 33 | DNI | 17 | 12 | 7 | 2 | 65 | 35 | DNI | DNI |
| Eldred-Evans * [43] | 2020 | 246 | 209 | 103 | DNI | 81 ** | DNI | 83 ** | DNI | 62 | 6.8 | 37 | DNI |
| Gatti * [44] | 2019 | 65 | DNI | 45 | 43 | DNI | DNI | DNI | DNI | 65 (cases), 62 (controls) | 7.5 (cases), 6.35 (controls) | 61.3 (cases), 47.7 (controls) | 0.11(cases), 0.125 (controls) |
| Giannarini * [45] | 2021 | 108 | 34 | 74 | DNI | DNI | DNI | DNI | DNI | 64.8 | 8.4 | DNI | DNI |
| Han * [46] | 2020 | 123 | 50 | 37 | 13 | 18 | 4 | 10 | 3 | 66.3 | 7.227 | DNI | 0.207 |
| Jambor * [47] | 2019 | 338 | 207 | 146 | DNI | 66 ** | 8 ** | DNI | DNI | 64 | 6.9 | 39 | 0.17 |
| Junker * [48] | 2019 | 236 | 135 | DNI | DNI | 69 | 20 | 48 | 12 | 67.6 | 6.4 | 45 | DNI |
| Kim * [49] | 2019 | 140 | 66 | 37 | DNI | 57 | 11 | DNI | DNI | 67.2 | 8.1 | 49 | 0.16 |
| Knaapila * [50] | 2021 | 639 | 410 | 307 | DNI | 110 ** | 13 ** | DNI | DNI | 54 | 8.9 | 43 | 0.23 |
| Kobilnyk * [51] | 2020 | 26 | 14 | DNI | 23 | 8 | 4 | DNI | DNI | 67.6 | DNI | DNI | DNI |
| Kuhl * [52] | 2017 | 542 | 180 | 139 | DNI | DNI | DNI | DNI | DNI | 64.8 | 8.5 | 57.4 | 0.15 |
| Lee * [53] | 2017 | 123 | 35 | 9 | DNI | DNI | DNI | DNI | DNI | 61.8–62 | 6.19–6.7 | 38.6–40.2 | 0.17–0.20 |
| Obmann * [6] | 2018 | 129 | 84 | 45 | DNI | 49 | 11 | DNI | DNI | 61.8 | 8.04 | 53.6 | 0.15 |
| Pesapane * [54] | 2021 | 431 | 195 | 65 | DNI | 119, 132 | DNI | 95, 100 | DNI | 61.5 | 12 | 58 | 0.18 |
| Roh * [55] | 2020 | 594 | DNI | DNI | 332 | 69 | 10 | DNI | DNI | 66 | 7.6 | 60 | 0.17 |
| Russo * [7] | 2021 | 311 | 117 | 94 | DNI | 26 | DNI | 9 | DNI | 66.3 | 5.68 | 49.6 | 0.17 |
| Scialpi * [56] | 2017 | 41 | 41 | 22 | 22 | DNI | DNI | DNI | DNI | 64.5 | 7.8 | DNI | DNI |
| Sherrer * [57] | 2019 | 344 | DNI | DNI | DNI | DNI | DNI | DNI | DNI | 65 | 7.61 | DNI | DNI |
| Taghipour * [58] | 2019 | 271 | 271 | 212 | 209 | 24 | 8 | DNI | DNI | 59 | 6.7 | DNI | DNI |
| Tamada * [59] | 2021 | 103 | DNI | 81 | 78 | 73, 57, 58 | 39, 36, 32 | 34, 21, 26 | DNI | 69.8 | 6.92 | DNI | DNI |
| Thestrup * [60] | 2019 | 101 | 101 | 27 | DNI | 23 | DNI | 21 | DNI | 64 | 6.3 | 49 | 0.13 |
| van der Leest * [18] | 2019 | 626 | 334 | 190 | DNI | 49 | 11 | 40 | 9 | 65 | 6.4 | 56 | 0.11 |
| De Visschere * [61] | 2017 | 245 | DNI | 144 | DNI | 20 | 8 | DNI | DNI | 66 | 9 | 49.3 | DNI |
| Wallstrom * [62] | 2021 | 551 | DNI | DNI | DNI | 59 | DNI | 33 | DNI | 57 | 3.3 | 41 | 0.075 |
| Wang * [63] | 2020 | 109 | 28 | 15 | 109 | DNI | 39 | DNI | DNI | 65–69 | 8.11–15.52 | 35.31–53.48 | 0.20–0.48 |
| Wang * [64] | 2021 | 224 | 79 | 18 | DNI | 86 | DNI | DNI | DNI | 65–71 | 9.84–37.24 | DNI | DNI |
| Xu * [12] | 2019 | 235 | 122 | 99 | DNI | 29 | DNI | 16 | DNI | 66.87 | 4.65 | DNI | DNI |
| Zawaideh et al. [16] | 2020 | 264 | 171 | 93 | DNI | DNI | DNI | DNI | DNI | 65 | 6.08 | 50.55 | 0.11 |
| Total Patients | PCa Patients | csPCa Patients | PZ Patients | PR3 bpMRI | csPCa PR3 bpMRI | PR3 mpMRI | csPCa PR3 mpMRI | |
|---|---|---|---|---|---|---|---|---|
| Max | 1020 | 655 | 404 | 338 | 158 | 45 | 136 | 27 |
| Min | 26 | 14 | 9 | 13 | 8 | 4 | 1 | 2 |
| Median | 151 | 104 | 65 | 78 | 49 | 11 | 21 | 10.5 |
| Total | 10,468 | 4860 | 3255 | 1349 | 1027 | 241 | 414 | 69 |
| Papers Reporting | 41 | 33 | 35 | 11 | 29 | 21 | 20 | 10 |
| Proportion Reporting | 1 | 0.8 | 0.85 | 0.27 | 0.71 | 0.51 | 0.49 | 0.24 |
| Author (Citation) | Year | Study Type | Study Cohort | Excluded Pts. with Bad Quality MRI | Diagnostic Test | Definition of csPCa | MRI Findings Considered Positive/Bx Thresholds | Bx or RP | Field Strength | ERC Usage | No. of Readers | Total Experience (Years) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al Salmi * [29] | 2020 | Pro | DNI Bx status | Y | Both | GS ≥ 7 (3 + 4) | DNI | DNI | 3T | N | 2 | DNI |
| Bao * [30] | 2021 | Ret | DNI Bx status | Y | Both | GS ≥ 7 (3 + 4) | PR ≥ 3 | DNI | 3T | N | 4 | 30 |
| Barth * [31] | 2017 | Pro | Bx-naive | Y | Both | GS ≥ 7 (3 + 4) | All | Bx | 3T | Y | 3 | 29 |
| Boesen * [32] | 2018 | Pro | Bx-naive | N | bpMRI | GS ≥ 7 (4 + 3), CCL > 50% for GS 7 (3 + 4) | PR ≥ 3 + PR_all | Both | 3T | N | 1 | 5 |
| Bosaily * [33] | 2020 | Pro | Bx-naive | N | Both | GS ≥ 7(3 + 4), ≥4 mm CCL | LK ≥ 2 | Bx | 1.5T | N | DNI | DNI |
| Brancato * [34] | 2020 | Ret | Bx-naive | N | mpMRI | GS ≥ 7 (3 + 4) | All | Bx | 1.5T | Y | 3 | 25 |
| Brembilla * [35] | 2022 | Ret | DNI Bx status | N | Both | GS ≥ 7 (3 + 4) | All | Bx | 3T | N | 3 | 28 |
| Cai * [36] | 2021 | Ret | Bx-naive | N | bpMRI | GS ≥ 7 (3 + 4) | DNI | Bx | 1.5T | N | 2 | 5 |
| Cereser * [37] | 2020 | Ret | Proven Ca | Y | Both | GS ≥ 7 (3 + 4) | DNI | RP | 3T | N | 2 | 600 cases/250 cases |
| Cho * [38] | 2020 | Ret | Bx-naive | N | Both | GS ≥ 6, V > 0.5 mL | PR ≥ 4 | RP | 3T | N | 2 | 18 |
| Choi * [39] | 2019 | Ret | Proven Ca | N | Both | GS ≥ 7 (3 + 4), EPE, V > 0.5 mL | PR ≥ 3 | Bx | 3T | N | 2 | 20 |
| Christophe * [40] | 2020 | Ret | DNI Bx status | Y | Both | DNI | All | RP | 3T | N | 4 | 17 |
| Di Campli * [41] | 2018 | Ret | Bx-naive | Y | Both | GS ≥ 7 (3 + 4), EPE | PR ≥ 3 (overall), PR ≥ 4 (csPCa) | Both | 1.5T | N | 3 | 11 |
| EL-Adalany * [42] | 2021 | Pro | Bx-naive | Y | Both | GS ≥ 7 (3 + 4), EPE, V > 0.5 mL | All | Both | 3T | N | 2 | 19 |
| Eldred-Evans * [43] | 2020 | Ret | Repeat Bx | Y | Both | GS ≥ 7 (3 + 4), ≥ 6 mm CCL of any GS | LK ≥ 3 | Bx | 3T | N | 1 | 10 |
| Gatti * [44] | 2019 | Ret | Bx-naive | N | Both | DNI | All | Both | 1.5T | N | 6 | DNI |
| Giannarini * [45] | 2021 | Ret | Proven Ca | Y | Both | ≥pT3 | ≥pT3 | RP | 3T | N | 2 | 12 |
| Han * [46] | 2020 | Ret | Bx-naive | Y | Both | GS ≥ 7 (3 + 4) | All | Bx | 3T | N | 2 | 10 |
| Jambor * [47] | 2019 | Pro | Bx-naive | Y | bpMRI | GS ≥ 7 (3 + 4) | LK ≥ 3 | Bx | 1.5T, 3T | N | 3 | DNI |
| Junker * [48] | 2019 | Ret | Bx-naive | N | Both | GS ≥ 7 (4 + 3) | PR ≥ 3 | Both | 1.5T, 3T | N | 1 | DNI |
| Kim * [49] | 2019 | Ret | Bx-naive | Y | bpMRI | GS ≥ 7 (3 + 4) | PR ≥ 3 | Bx | 3T | N | 2 | 19 |
| Knaapila * [50] | 2021 | Pro | Mixed | N | bpMRI | GS ≥ 7 (3 + 4) | LK ≥ 3, LK ≥ 4 | Both | 1.5T, 3T | N | DNI | DNI |
| Kobilnyk * [51] | 2020 | Ret | Bx-naive | N | bpMRI | GS ≥ 7 (3 + 4) | PR ≥ 4 | Bx | 1.5T | N | DNI | DNI |
| Kuhl * [52] | 2017 | Ret | Repeat Bx | Y | Both | GS ≥ 7, PSA ≥ 20, stage > T2b-T3a | PR ≥ 3 | Bx | 3T | N | 4 | DNI |
| Lee * [53] | 2017 | Ret | Bx-naive | N | Both | GG > 3, >5 mm CCL | DNI | Both | 3T | N | 2 | DNI |
| Obmann * [6] | 2018 | Pro | Bx-naive | Y | bpMRI | GS ≥ 7 (3 + 4) | PR ≥ 3 | Bx | 3T | N | 1 | 14 |
| Pesapane * [54] | 2021 | Ret | DNI Bx status | Y | Both | GS ≥ 7 (3 + 4), EPE, GG ≥ 7 (4 + 3) | PR ≥ 3 | Both | 1.5T | Y | 2 | 8 |
| Roh * [55] | 2019 | Ret | Bx-naive | N | mpMRI | GS ≥ 7 (3 + 4) | PR ≥ 3 | Bx | 3T | DNI | 13 | 6–38 |
| Russo * [7] | 2021 | Pro | Bx-naive | N | Both | GS ≥ 7 (3 + 4) | PR ≥ 3, PSAD ≥ 0.12 | Bx | 1.5T | Y | DNI | DNI |
| Scialpi * [56] | 2017 | Ret | Proven Ca | Y | Both | GS ≥ 7 (3 + 4) | All | RP | 3T | N | 2 | DNI |
| Sherrer * [57] | 2019 | Ret | Mixed | N | Both | DNI | DNI | Bx | DNI | N | DNI | DNI |
| Taghipour * [58] | 2018 | Ret | Proven Ca | N | Both | GS ≥ 7 (3 + 4) | All | RP | 3T | Y | 1 | 14 |
| Tamada * [59] | 2021 | Ret | Bx-naive | N | Both | GS > 6 | All | Both | 3T | N | 3 | 42 |
| Thestrup * [60] | 2019 | Pro | Bx-naive | N | Both | GS ≥ 7 (3 + 4) | PR ≥ 3 | Bx | 3T | N | 1 | 6 |
| van der Leest * [18] | 2019 | Pro | Bx-naive | N | Both | GS ≥ 7 (3 + 4) | PR ≥ 3 | Bx | 3T | N | 2 | 30 |
| De Visschere * [61] | 2017 | Ret | Bx-naive | N | Both | GS ≥ 7 (3 + 4) | All | Bx | 3T | N | DNI | DNI |
| Wallstrom * [62] | 2021 | Pro | Bx-naive | Y | Both | DNI | PR ≥ 4 | Bx | 3T | N | 3 | 22 |
| Wang * [63] | 2020 | Ret | Bx-naive | N | Both | GS ≥ 7 (3 + 4) | PR ≥ 3 | Bx | 3T | N | 2 | 10 |
| Wang * [64] | 2021 | Ret | Bx-naive | Y | Both | GS ≥ 7 (3 + 4) | DNI | Bx | 3T | N | 2 | 16 |
| Xu * [12] | 2019 | Ret | Bx-naive | Y | Both | GS ≥ 7 (3 + 4), EPE, V > 0.5 mL | All | Both | 3T | N | 2 | 18 |
| Zawaideh * [16] | 2020 | Ret | Mixed | N | Both | GS ≥ 7 (3 + 4) | LK ≥ 3, LK ≥ 4 | Bx | 1.5T, 3T | N | 1 | DNI |
| Study Type | Number of Studies | Definition of csPCa | Number of Studies |
| Retrospective | 29 | GS ≥ 7 ± other parameters | 31 |
| Prospective | 12 | DNI | 4 |
| Diagnostic Test | Pathological stage ≥ pT3 | 1 | |
| Both | 32 | GG > 3, >5 mm CCL | 1 |
| bpMRI | 7 | GS ≥ 7 (4 + 3), CCL > 50% for GS 7 (3 + 4) | 1 |
| mpMRI | 2 | GS > 6 | 1 |
| GS > 7 (4 + 3) | 1 | ||
| Study Cohort | GS ≥ 6, V > 0.5 mL | 1 | |
| Bx-naive | 26 | Removed MRI with Artifacts | |
| Mixed | 3 | Yes | 19 |
| Proven Ca | 5 | No | 22 |
| DNI Bx status | 5 | ||
| Repeat Bx | 2 | ||
| Bx Thresholds and Number of Studies | |||
| PIRADS | 17 | Likert | 5 |
| PIRADS ≥ 3 | 11 | Likert ≥ 3 | 2 |
| PIRADS ≥ 4 | 3 | Likert ≥ 3, Likert ≥ 4 | 2 |
| PIRADS ≥ 3, PSAD ≥ 0.12 | 1 | Likert ≥ 2 | 1 |
| PIRADS ≥ 3 (overall), PIRADS ≥ 4 (csPCa) | 1 | ≥pT3 | 1 |
| PIRADS ≥ 3 + PIRADS all | 1 | DNI | 6 |
| All | 12 | ||
| Reference Standard Methods and Number of Studies | |||
| Bx | 23 | RP | 6 |
| Bx and RP | 10 | DNI | 2 |
| Field Strength | Number of Studies | ERC | Number of Studies |
| 3T | 28 | Did not use | 35 |
| 1.5T | 8 | Used | 5 |
| 1.5T, 3T | 4 | DNI | 1 |
| DNI | 1 | ||
| No. of Readers | Number of Studies | Collective Experience of the Readers in Years | Number of Studies |
| Median of 2 (Range 1–13) | 35 | Median of 17 (Range 5–42) | 25 |
| DNI | 6 | DNI | 16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belue, M.J.; Yilmaz, E.C.; Daryanani, A.; Turkbey, B. Current Status of Biparametric MRI in Prostate Cancer Diagnosis: Literature Analysis. Life 2022, 12, 804. https://doi.org/10.3390/life12060804
Belue MJ, Yilmaz EC, Daryanani A, Turkbey B. Current Status of Biparametric MRI in Prostate Cancer Diagnosis: Literature Analysis. Life. 2022; 12(6):804. https://doi.org/10.3390/life12060804
Chicago/Turabian StyleBelue, Mason James, Enis Cagatay Yilmaz, Asha Daryanani, and Baris Turkbey. 2022. "Current Status of Biparametric MRI in Prostate Cancer Diagnosis: Literature Analysis" Life 12, no. 6: 804. https://doi.org/10.3390/life12060804
APA StyleBelue, M. J., Yilmaz, E. C., Daryanani, A., & Turkbey, B. (2022). Current Status of Biparametric MRI in Prostate Cancer Diagnosis: Literature Analysis. Life, 12(6), 804. https://doi.org/10.3390/life12060804






