Serum C-X-C Chemokine Ligand 1 Levels in Patients with Systemic Sclerosis: Relationship of Clinical and Laboratory Observations to Anti-CD20 Monoclonal Antibody Administration
Abstract
:1. Introduction
2. Experimental Section
2.1. Serum Sample from SSc Patient and Healthy Controls
2.2. Measurement of Serum CXCL1 Levels
2.3. Clinical Assessment of Patient
2.4. Statistical Analysis
3. Result
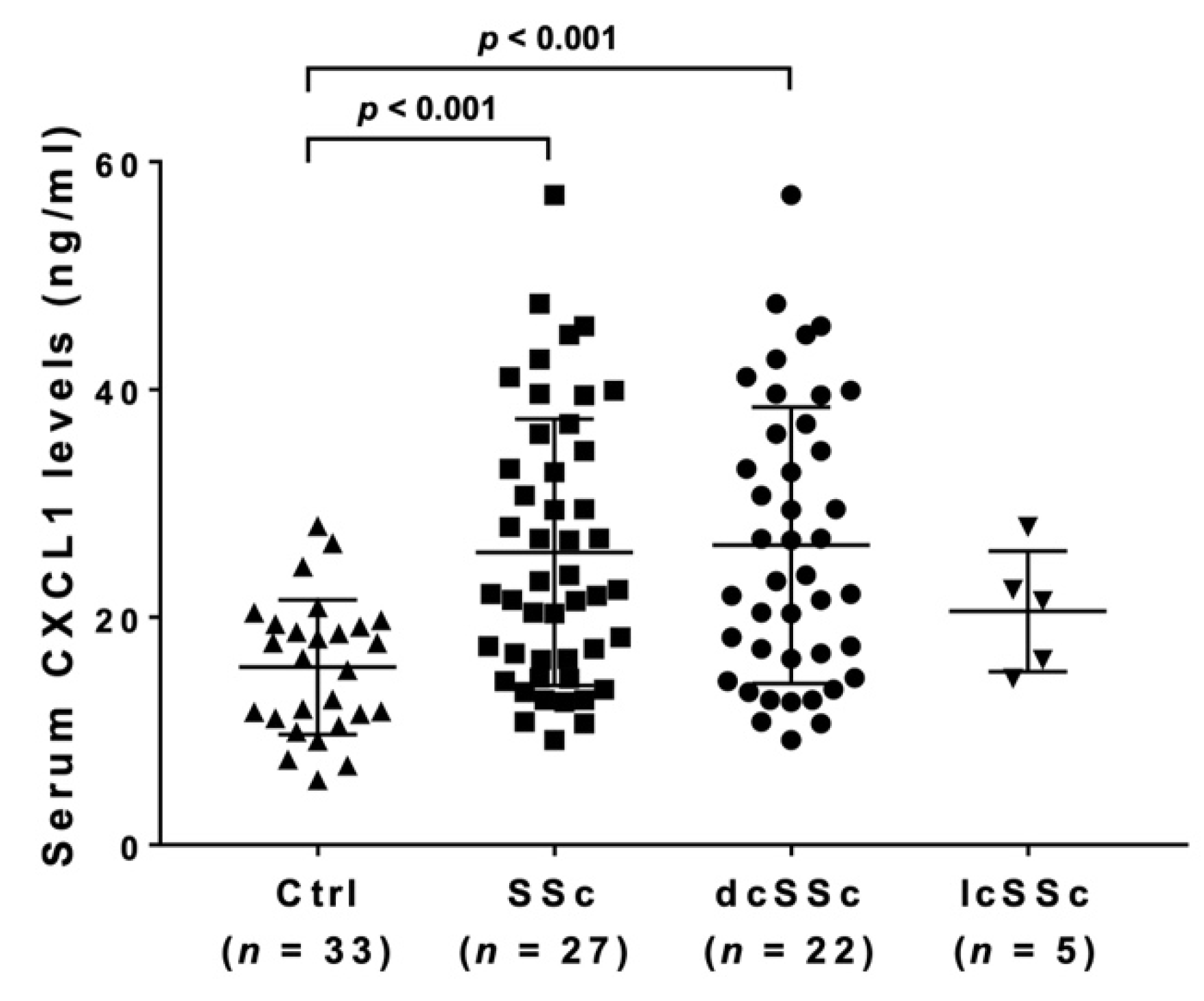
3.1. Serum CXCL1 Levels in SSc Patients and Healthy Controls
3.2. Clinical and Laboratory Features of SSc Patients with Elevated Serum CXCL1 Levels
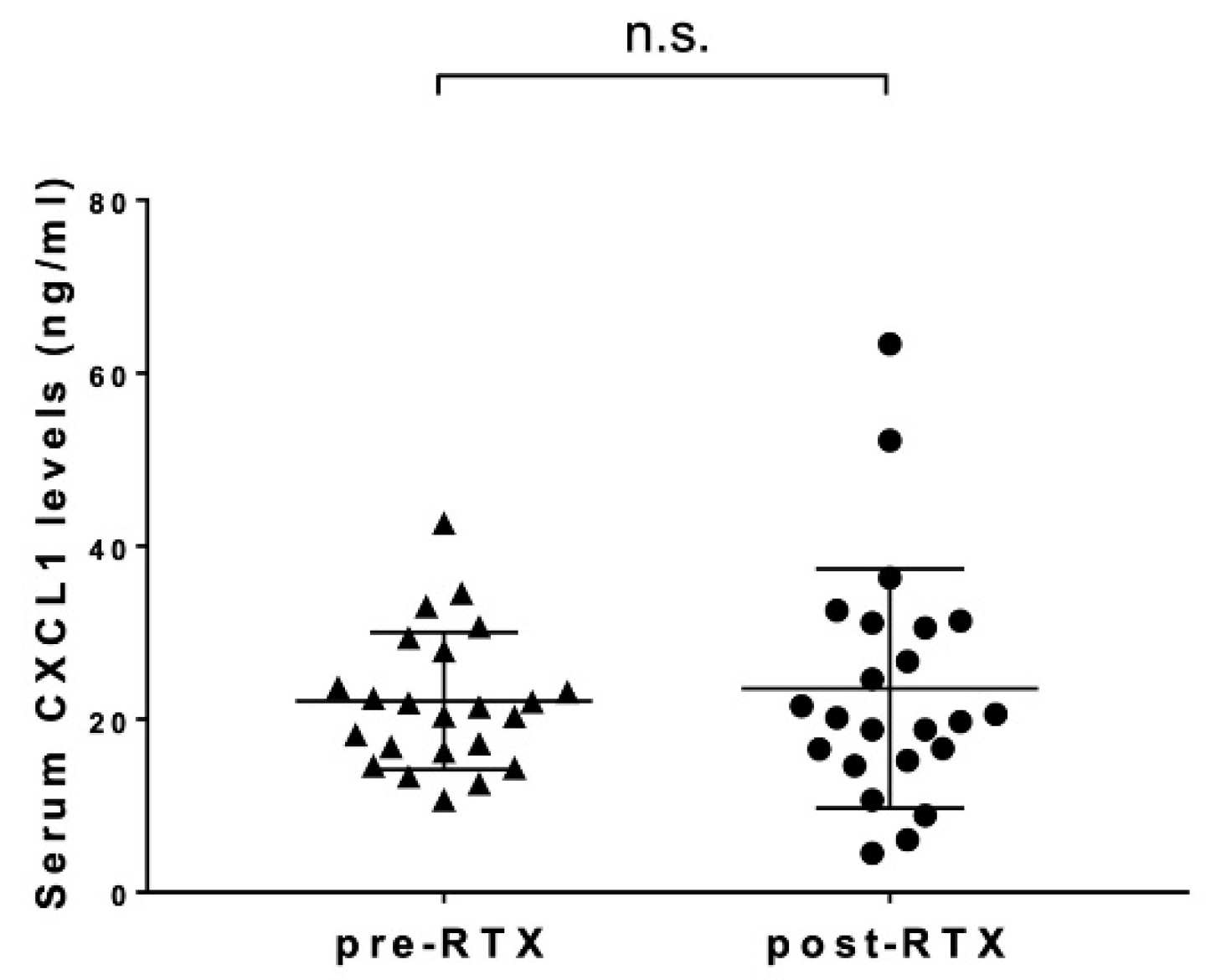
3.3. Changes in Serum CXCL1 Levels after Rituximab Administration
3.4. The Correlations between Serum CXCL1 Levels and Clinical Symptoms and Laboratory Findings during Rituximab Treatment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- LeRoy, E.C.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; Medsger, T.A., Jr.; Rowell, N.; Wollheim, F. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar] [PubMed]
- Furst, D.E.; Clements, P.J. Hypothesis for the pathogenesis of systemic sclerosis. J. Rheumatol. Suppl. 1997, 48, 53–57. [Google Scholar] [PubMed]
- Yoshizaki, A.; Yanaba, K.; Iwata, Y.; Komura, K.; Ogawa, A.; Muroi, E.; Ogawa, F.; Takenaka, M.; Shimizu, K.; Hasegawa, M.; et al. Elevated serum interleukin-27 levels in patients with systemic sclerosis: Association with T cell, B cell, and fibroblast activation. Ann. Rheum. Dis. 2011, 70, 194–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshizaki, A.; Komura, K.; Iwata, Y.; Ogawa, F.; Hara, T.; Muroi, E.; Takenaka, M.; Shimizu, K.; Hasegawa, M.; Fujimoto, M.; et al. Clinical significance of serum HMGB-1 and sRAGE levels in systemic sclerosis: Association with disease severity. J. Clin. Immunol. 2009, 29, 180–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergamasco, A.; Hartmann, N.; Wallace, L.; Verpillat, P. Epidemiology of systemic sclerosis and systemic sclerosis-associated interstitial lung disease. Clin. Epidemiol. 2019, 11, 257–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, S.; Fujimoto, M.; Hasegawa, M.; Takehara, K.; Tedder, T.F. Altered B lymphocyte function induces systemic autoimmunity in systemic sclerosis. Mol. Immunol. 2004, 41, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Rivas, M.; Royo, C.; Simeón, C.P.; Corbella, X.; Fonollosa, V. Mortality and survival in systemic sclerosis: Systematic review and meta-analysis. Semin. Arthritis Rheum. 2014, 44, 208–219. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Varga, J. Emerging targets of disease-modifying therapy for systemic sclerosis. Nat. Rev. Rheumatol. 2019, 15, 208–224. [Google Scholar] [CrossRef]
- Chung, M.P.; Chung, L. Drugs in phase I and phase II clinical trials for systemic sclerosis. Expert Opin. Investig. Drugs 2020, 29, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Ebata, S.; Yoshizaki, A.; Oba, K.; Kashiwabara, K.; Ueda, K.; Uemura, Y.; Watadani, T.; Fukasawa, T.; Miura, S.; Yoshizaki-Ogawa, A.; et al. Safety and efficacy of rituximab in systemic sclerosis (DESIRES): A double-blind, investigator-initiated, randomized, placebo-controlled trial. Lancet Rheum. 2021, 3, e489–e497. [Google Scholar] [CrossRef]
- Goswami, R.P.; Ray, A.; Chatterjee, M.; Mukherjee, A.; Sircar, G.; Ghosh, P. Rituximab in the treatment of systemic sclerosis-related interstitial lung disease: A systematic review and meta-analysis. Rheumatology 2021, 60, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Yoshizaki, A.; Takahashi, T.; Saigusa, R.; Taniguchi, T.; Asano, Y.; Gonoi, W.; Hinata, M.; Shinozaki-Ushiku, A.; Sato, S. The first case report of fatal acute pulmonary dysfunction in a systemic sclerosis patient treated with rituximab. Scand. J. Rheumatol. 2016, 45, 249–250. [Google Scholar] [CrossRef]
- Richmond, A.; Thomas, H.G. Purification of melanoma growth stimulatory activity. J. Cell. Physiol. 1986, 129, 375–384. [Google Scholar] [CrossRef]
- Bechara, C.; Chai, H.; Lin, P.H.; Yao, Q.; Chen, C. Growth-related oncogene-alpha (GRO-alpha): Roles in atherosclerosis, angiogenesis, and other inflammatory conditions. Med. Sci. Monit. 2007, 13, RA87–RA90. [Google Scholar] [PubMed]
- Zeng, Y.; Lin, Q.; Yu, L.; Wang, X.; Lin, Y.; Zhang, Y.; Yan, S.; Lu, X.; Li, Y.; Li, W.; et al. Chemokine CXCL1 as a potential marker of disease activity in systemic lupus erythematosus. BMC Immunol. 2021, 21, 611–619. [Google Scholar] [CrossRef]
- Furuse, S.; Fujii, H.; Kaburagi, Y.; Fujimoto, M.; Hasegawa, M.; Takehara, K.; Sato, S. Serum concentrations of the CXC chemokines interleukin 8 and growth-regulated oncogene-alpha are elevated in patients with systemic sclerosis. J. Rheumatol. 2003, 30, 1524–1528. [Google Scholar]
- Van Den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Arthritis Rheumatol. 2013, 72, 1747–1755. [Google Scholar] [CrossRef] [Green Version]
- Khanna, D.; Furst, D.E.; Clements, P.J.; Allanore, Y.; Baron, M.; Czirjak, L.; Distler, O.; Foeldvari, I.; Kuwana, M.; Matucci-Cerinic, M.; et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J. Scleroderma Relat. Disord. 2017, 2, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muangchan, C.; Harding, S.; Khimdas, S.; Bonner, A.; Canadian Scleroderma Research Group; Baron, M.; Pope, J. Association of C-reactive protein with high disease activity in systemic sclerosis: Results from the Canadian Scleroderma Research Group. Arthritis Care Res. 2012, 64, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Korbecki, J.; Barczak, K.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. CXCL1: Gene, Promoter, Regulation of Expression, mRNA Stability, Regulation of Activity in the Intercellular Space. Int. J. Mol. Sci. 2022, 23, 792. [Google Scholar] [CrossRef]
- Taniuchi, N.; Ghazizadeh, M.; Enomoto, T.; Matsuda, K.; Sato, M.; Takizawa, Y.; Jin, E.; Egawa, S.; Azuma, A.; Gemma, A.; et al. Evaluation of fractional analysis of bronchoalveolar lavage combined with cellular morphological features. Int. J. Med. Sci. 2009, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kase, K.; Watanabe, S.; Saeki, K.; Waseda, Y.; Takato, H.; Ichikawa, Y.; Murata, A.; Yasui, M.; Noriyuki, O.; Hara, J.; et al. Fractional analysis of bronchoalveolar lavage in systemic sclerosis-associated interstitial lung disease. J. Thorac. Dis. 2021, 13, 4146–4155. [Google Scholar] [CrossRef]
- Liang, M.; Jiang, Z.; Huang, Q.; Liu, L.; Xue, Y.; Zhu, X.; Yu, Y.; Wan, W.; Yang, H.; Zou, H. Clinical Association of Chemokine (C-X-C motif) Ligand 1 (CXCL1) with Interstitial Pneumonia with Autoimmune Features (IPAF). Sci. Rep. 2016, 6, 38949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shattuck, R.L.; Wood, L.D.; Jaffe, G.J.; Richmond, A. MGSA/GRO transcription is differentially regulated in normal retinal pigment epithelial and melanoma cells. Mol. Cell. Biol. 1994, 14, 791–802. [Google Scholar]
- Cuenca, R.E.; Azizkhan, R.G.; Haskill, S. Characterization of GRO alpha, beta and gamma expression in human colonic tumors: Potential significance of cytokine involvement. Surg. Oncol. 1992, 1, 323–329. [Google Scholar] [CrossRef]
- Huang, F.; Kao, C.Y.; Wachi, S.; Thai, P.; Ryu, J.; Wu, R. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-kappaB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J. Immunol. 2007, 179, 6504–6513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, Z.; Ge, D.; Liu, S.; Zhang, Q.; Borowsky, A.D.; Melamed, J. Interleukin-17 Induces Expression of Chemokines and Cytokines in Prostatic Epithelial Cells but Does Not Stimulate Cell Growth In Vitro. Int. J. Med. Biol. Front. 2012, 18, 629–644. [Google Scholar] [PubMed]
- Wu, H.H.; Hwang-Verslues, W.W.; Lee, W.H.; Huang, C.K.; Wei, P.C.; Chen, C.L.; Shew, J.Y.; Lee, E.Y.; Jeng, Y.M.; Tien, Y.W.; et al. Targeting IL-17B-IL-17RB signaling with an anti-IL-17RB antibody blocks pancreatic cancer metastasis by silencing multiple chemokines. J. Exp. Med. 2015, 212, 333–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisi, S.; Sisto, M.; Lofrumento, D.D.; D’Amore, M.; De Lucro, R.; Ribatti, D. A potential role of the GRO-α/CXCR2 system in Sjögren’s syndrome: Regulatory effects of pro-inflammatory cytokines. Histochem. Cell Biol. 2013, 139, 371–379. [Google Scholar] [CrossRef]
- Scala, E.; Pallotta, S.; Frezzolini, A.; Abeni, D.; Barbieri, C.; Sampogna, F.; De Pita, O.; Puddu, P.; Paganelli, R.; Russo, G. Cytokine and chemokine levels in systemic sclerosis: Relationship with cutaneous and internal organ involvement. Clin. Exp. Immunol. 2004, 138, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Sato, S.; Fujimoto, M.; Ihn, H.; Kikuchi, K.; Takehara, K. Serum levels of Interleukin 6 (IL-6), oncostatin M, soluble IL-6 receptor, and soluble gp130 in patients with systemic sclerosis. J. Rheumatol. 1998, 25, 308–316. [Google Scholar]
- De Santis, M.; Bosello, S.; La Torre, G.; Capuano, A.; Tolusso, B.; Pagliari, G.; Pistelli, R.; Maria Danza, F.; Zoli, A.; Ferraccioli, G. Functional, radiological and biological markers of alveolitis and infections of the lower respiratory tract in patients with systemic sclerosis. Respir. Res. 2005, 6, 96–106. [Google Scholar] [CrossRef] [Green Version]
- Koch, A.E.; Kronfeld-Harrington, L.B.; Szekanecz, Z.; Cho, M.M.; Haines, K.; Harlow, L.A.; Strieter, R.M.; Kunkel, S.L.; Massa, M.C.; Barr, W.G.; et al. In situ expression of cytokines and cellular adhesion molecules in the skin of patients with systemic sclerosis. Their role in early and late disease. Pathobiology 1993, 61, 239–246. [Google Scholar] [CrossRef]
- Kadono, T.; Kikuchi, K.; Ihn, H.; Takehara, K.; Tamaki, K. Increased production of interleukin 6 and interleukin 8 in scleroderma fibroblasts. J. Rheumatol. 1998, 25, 296–301. [Google Scholar] [CrossRef]
- Kondo, K.; Okada, T.; Matsui, T.; Kato, S.; Date, K.; Yoshihara, M.; Nagata, Y.; Takagi, H.; Yoneda, M.; Sugie, I. Establishment and characterization of a human B cell line from the lung tissue of a patient with scleroderma; extraordinary high level of IL-6 secretion by stimulated fibroblasts. Cytokine 2001, 13, 220–226. [Google Scholar] [CrossRef]
- Matsushita, T.; Hasegawa, M.; Yanaba, K.; Kodera, M.; Takehara, K.; Sato, S. Elevated serum BAFF levels in patients with systemic sclerosis: Enhanced BAFF signaling in systemic sclerosis B lymphocytes. Arthritis Rheum. 2006, 54, 192–201. [Google Scholar] [CrossRef]
- Matsushita, T.; Fujimoto, M.; Hasegawa, M.; Matsushita, Y.; Komura, K.; Ogawa, F.; Watanabe, R.; Takehara, K.; Sato, S. BAFF antagonist attenuates the development of skin fibrosis in tight-skin mice. J. Investig. Dermatol. 2007, 127, 2772–2780. [Google Scholar] [CrossRef] [Green Version]
- Bosello, S.; De Santis, M.; Lama, G.; Spanò, C.; Angelucci, C.; Tolusso, B.; Sica, G.; Ferraccioli, G. B cell depletion in diffuse progressive systemic sclerosis: Safety, skin score modification and IL-6 modulation in an up to thirty-six months follow-up open-label trial. Arthritis Res. Ther. 2010, 12, R54. [Google Scholar] [CrossRef] [Green Version]
- Tanasescu, C.; Balanescu, E.; Balanescu, P.; Olteanu, R.; Badea, C.; Grancea, C.; Vagu, C.; Bleotu, C.; Ardeleanu, C.; Georgescu, A. IL-17 in cutaneous lupus erythematosus. Eur. J. Intern. Med. 2010, 21, 202–207. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.G.; Li, Y.H.; Qi, L.; Liu, X.G.; Yuan, C.Z.; Hu, N.W.; Ma, D.X.; Li, Z.F.; Yang, Q.; et al. Increased frequencies of Th22 cells as well as Th17 cells in the peripheral blood of patients with ankylosing spondylitis and rheumatoid arthritis. PLoS ONE 2012, 7, e31000. [Google Scholar] [CrossRef]
- Blauvelt, A. T-helper 17 cells in psoriatic plaques and additional genetic links between IL-23 and psoriasis. J. Investig. Dermatol. 2008, 128, 1064–1067. [Google Scholar] [CrossRef] [Green Version]
- Murata, M.; Fujimoto, M.; Matsushita, T.; Hamaguchi, Y.; Hasegawa, M.; Takehara, K.; Komura, K.; Sato, S. Clinical association of serum interleukin-17 levels in systemic sclerosis: Is systemic sclerosis a Th17 disease? J. Dermatol. Sci. 2008, 50, 240–242. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, Y.; Hasegawa, M.; Matsushita, T.; Hamaguchi, Y.; Huu, D.L.; Iwakura, Y.; Fujimoto, M.; Takehara, K. Potential roles of interleukin-17A in the development of skin fibrosis in mice. Arthritis Rheum. 2012, 64, 3726–3735. [Google Scholar] [CrossRef]


| Elevated CXCL1 Levels (n = 27) | Normal CXCL1 Levels (n = 20) | |
|---|---|---|
| Age, years | 47 (27–71) | 50 (27–78) |
| Sex (male/female) | 3/24 | 0/20 |
| Clinical features | ||
| dcSSc | 25 (92.6%) | 17 (85.0%) |
| lcSSc | 2 (7.4%) | 3 (15%) |
| mRSS | 13 (10–28) | 14 (10–31) |
| Raynauds phenomenon, % | 25 (92.6%) | 19 (95.0%) |
| Nail fold breeding, % | 19 (70.4%) | 12 (60.0%) |
| Telangiectasia, % | 11 (40.7%) | 6 (30.0%) |
| Pitting scars, % | 10 (37.0%) | 10 (50.0%) |
| Skin ulcer, % | 12 (44.4%) | 10 (50.0%) |
| Reflex oesophagitis, % | 7 (25.9%) | 5 (23.52%) |
| Autoantibodies | ||
| Anti-topoisomerase I Ab, % | 17 (63%) | 10 (50%) |
| Anti-centromere Ab, % | 5 (18.5%) | 3 (15%) |
| Anti-RNA polymerase III Ab, % | 2 (7.40%) | 3 (15%) |
| Area occupied with interstitial shadows, % of lung fields. | 13 * (1–52%) | 6.5 (1–29%) |
| Laboratory findings | ||
| %FVC, % | 83.6 (72.2–108) | 88.3 (63.2–124) |
| %DLco, % | 78.8 (63.1–125) | 88.75 (60.8–122) |
| SP-A, ng/mL | 40.3 * (9.2–149) | 29.8 (13.3–71.6) |
| SP-D, ng/mL | 153 * (26.8–363) | 120 (25.5–318) |
| KL-6, ng/mL | 488 ** (84–3370) | 392 (141–2534) |
| CRP, mg/dL | 0.16 * (0.02–1.17) | 0.03 (0.02–0.67) |
| IgG, mg/dL | 1400 (766–2909) | 1251 (703–1692) |
| BNP, pg/mL | 25.7 (4–275) | 15.3 (4–41.5) |
| Creatinin, mg/dL | 0.61 (0.45–0.73) | 0.62 (0.46–0.87) |
| Present medications | ||
| Systemic corticosteroid use, % | 17 (63%) | 9 (45%) |
| Dose of systemic corticosteroid, mg/day | 3.87 (3.52) | 3.40 (4.03) |
| ERA and/or PDE-5 inhibitor use | 18 (66.7%) | 9 (45%) |
| Proton pump inhibitor use | 24 (88.9%) | 16 (45%) |
| Autoantibody | n | Serum CXCL1 Levels |
|---|---|---|
| Anti-topoisomerase I Ab | 27 | 23.2 (24.5–26.0) |
| Anti-centromere Ab | 8 | 27.4 (26.2–31.6) |
| Anti-RNA polymerase III Ab | 5 | 20.3 (16.9–21.5) |
| Correlation | Strength of Correlation (r) |
|---|---|
| Pre-CXCL1 levels vs. Δclinical/laboratory data | |
| Pre-CXCL1 levels vs. ΔmRSS | 0.163 |
| Pre-CXCL1 levels vs. Δ%FVC | 0.260 |
| Pre-CXCL1 levels vs. Δ%DLco | 0.531 ** |
| Pre-CXCL1 levels vs. ΔSP-A | 0.130 |
| Pre-CXCL1 levels vs. ΔSP-D | 0.018 |
| Pre-CXCL1 levels vs. ΔKL-6 | −0.177 |
| Pre-CXCL1 levels vs ΔArea occupied with interstitial shadows | −0.305 |
| ΔCXCL1 vs. Δclinical/laboratory data | |
| ΔCXCL1 vs. ΔmRSS | −0.222 |
| ΔCXCL1 vs. Δ%FVC | −0.275 |
| ΔCXCL1 vs. Δ%DLco | −0.122 |
| ΔCXCL1 vs. ΔSP-A | −0.500 * |
| ΔCXCL1 vs. ΔSP-D | −0.116 |
| ΔCXCL1 vs. ΔKL-6 | 0.211 |
| ΔCXCL1 vs ΔArea occupied with interstitial shadows | −0.098 |
| Post-CXCL1 levels post vs. post-clinical/laboratory data | |
| Post-CXCL1 levels vs. post-mRSS | 0.145 |
| Post-CXCL1 levels vs. post-%FVC | −0.511 * |
| Post-CXCL1 levels vs. post-%DLco | −0.379 |
| Post-CXCL1 levels vs. post-SP-A levels | 0.232 |
| Post-CXCL1 levels vs. post-SP-D levels | 0.498 * |
| Post-CXCL1 levels vs. post-KL-6 levels | 0.178 |
| Post-CXCL1 levels vs post-Area occupied with interstitial shadows | 0.115 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawanabe, R.; Yoshizaki, A.; Matsuda, K.M.; Kotani, H.; Hisamoto, T.; Norimatsu, Y.; Kuzumi, A.; Fukasawa, T.; Ebata, S.; Yoshizaki-Ogawa, A.; et al. Serum C-X-C Chemokine Ligand 1 Levels in Patients with Systemic Sclerosis: Relationship of Clinical and Laboratory Observations to Anti-CD20 Monoclonal Antibody Administration. Life 2022, 12, 646. https://doi.org/10.3390/life12050646
Kawanabe R, Yoshizaki A, Matsuda KM, Kotani H, Hisamoto T, Norimatsu Y, Kuzumi A, Fukasawa T, Ebata S, Yoshizaki-Ogawa A, et al. Serum C-X-C Chemokine Ligand 1 Levels in Patients with Systemic Sclerosis: Relationship of Clinical and Laboratory Observations to Anti-CD20 Monoclonal Antibody Administration. Life. 2022; 12(5):646. https://doi.org/10.3390/life12050646
Chicago/Turabian StyleKawanabe, Ruriko, Ayumi Yoshizaki, Kazuki M. Matsuda, Hirohito Kotani, Teruyoshi Hisamoto, Yuta Norimatsu, Ai Kuzumi, Takemichi Fukasawa, Satoshi Ebata, Asako Yoshizaki-Ogawa, and et al. 2022. "Serum C-X-C Chemokine Ligand 1 Levels in Patients with Systemic Sclerosis: Relationship of Clinical and Laboratory Observations to Anti-CD20 Monoclonal Antibody Administration" Life 12, no. 5: 646. https://doi.org/10.3390/life12050646
APA StyleKawanabe, R., Yoshizaki, A., Matsuda, K. M., Kotani, H., Hisamoto, T., Norimatsu, Y., Kuzumi, A., Fukasawa, T., Ebata, S., Yoshizaki-Ogawa, A., & Sato, S. (2022). Serum C-X-C Chemokine Ligand 1 Levels in Patients with Systemic Sclerosis: Relationship of Clinical and Laboratory Observations to Anti-CD20 Monoclonal Antibody Administration. Life, 12(5), 646. https://doi.org/10.3390/life12050646






