Zinc Oxide Nanoparticles and Their Biosynthesis: Overview
Abstract
:1. Introduction
1.1. Zinc
1.2. Plant Absorption of Zn
1.3. Effect of Zn on Plant Growth
1.4. Protective Role of Zn in Plants
1.5. Proteins with Zn Fingers
2. Role of Zn in Plant Nutrition
2.1. Interactions of Zn with Other Nutrients
2.2. Interactions between P and Zn
2.3. N-Zn Interactions
2.4. Interaction between Macronutrients
3. Role of Zn in Metal-Contaminated Soil
4. Nanoparticles
4.1. Methods for Synthesis of Nanoparticles
4.2. Zn-NPs
4.3. NPs of ZnO
4.4. Plants and Modified ZnO-NPs
4.5. ZnO Nanostructures Synthesis
5. ZnO-NPs Synthesis by Chemical Methods
5.1. Benefits of Chemical Methods
5.2. Reaction of Zn and Alcohol
5.3. Vapor Transport Synthesis
5.4. Hydrothermal Methodology
5.5. ZnO-NPs Green Synthesis
5.6. Bacterial-Based Green Synthesis of ZnO-NPs
5.7. Microalgae and Macroalgae Are Used in the Green Method of ZnO-NPs
5.8. Benefits of Green Synthesis of NPs
5.9. Nano Agrochemicals
5.10. Nano Fertilizers
5.11. Micronutrient Nano-Fertilizers
5.12. Nano Pesticides
5.13. Nano Biosensors
6. Applications of ZnO
6.1. Cancer Treatment Using ZnO-NPs
6.2. Applications in Biomedicine
6.3. Antimicrobial Properties (Anti-Fungal and Anti-Bacterial)
6.4. The Function of ZnO-NPs in the Agriculture
6.5. Use in Water Treatment
6.6. Effects of ZnO-NPs on the Plant Growth
6.7. ZnO-NPs Have Negative or Toxic Effects
7. Application of ZnO Reduce the Heavy Metal Stress
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zaheer, I.E.; Ali, S.; Rizwan, M.; Abbas, Z.; Bukhari, S.A.H.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Zinc-lysine prevents chromium-induced morphological, photosynthetic, and oxidative alterations in spinach irrigated with tannery wastewater. Environ. Sci. Pollut. Res. 2019, 26, 28951–28961. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, X.; Liu, Y.; Wei, J.; Shen, W.; Shen, Z.; Cui, J. Hemin-mediated alleviation of zinc, lead and chromium toxicity is associated with elevated photosynthesis, antioxidative capacity; suppressed metal uptake and oxidative stress in rice seedlings. Plant Growth Regul. 2017, 81, 253–264. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Yousaf, H.S.; Malik, A.; Abbas, Z.; Rizwan, M.; Abualreesh, M.H.; Alatawi, A.; Wang, X. Combined application of zinc and iron-lysine and its effects on morpho-physiological traits, antioxidant capacity and chromium uptake in rapeseed (Brassica napus L.). PLoS ONE 2022, 17, e0262140. [Google Scholar] [CrossRef]
- Javed, H.; Naeem, A.; Rengel, Z.; Dahlawi, S. Timing of foliar Zn application plays a vital role in minimizing Cd accumulation in wheat. Environ. Sci. Pollut. Res. 2016, 23, 16432–16439. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; ur Rehman, M.Z.; Maqbool, A. A critical review on the effects of zinc at toxic levels of cadmium in plants. Environ. Sci. Pollut. Res. 2019, 26, 6279–6289. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Hussain, A.; Ali, Q.; Shakoor, M.B.; Zia-ur-Rehman, M.; Farid, M.; Asma, M. Effect of zinc-lysine on growth, yield and cadmium uptake in wheat (Triticum aestivum L.) and health risk assessment. Chemosphere 2017, 187, 35–42. [Google Scholar] [CrossRef]
- Feller, U.; Anders, I.; Wei, S. Distribution and Redistribution of 109Cd and 65Zn in the Heavy Metal Hyperaccumulator Solanum nigrum L.: Influence of Cadmium and Zinc Concentrations in the Root Medium. Plants 2019, 8, 340. [Google Scholar] [CrossRef] [Green Version]
- Jian, L.; Bai, X.; Zhang, H.; Song, X.; Li, Z. Promotion of growth and metal accumulation of alfalfa by coinoculation with Sinorhizobium and Agrobacterium under copper and zinc stress. PeerJ 2019, 7, e6875. [Google Scholar] [CrossRef] [Green Version]
- Bhantana, P.; Rana, M.S.; Sun, X.-c.; Moussa, M.G.; Saleem, M.H.; Syaifudin, M.; Shah, A.; Poudel, A.; Pun, A.B.; Bhat, M.A. Arbuscular mycorrhizal fungi and its major role in plant growth, zinc nutrition, phosphorous regulation and phytoremediation. Symbiosis 2021, 84, 19–37. [Google Scholar] [CrossRef]
- Yang, W.-D.; Wang, Y.-Y.; Zhao, F.-L.; Ding, Z.-L.; Zhang, X.-C.; Zhu, Z.-Q.; Yang, X.-E. Variation in copper and zinc tolerance and accumulation in 12 willow clones: Implications for phytoextraction. J. Zhejiang Univ-Sci. B 2014, 15, 788–800. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, R.; Ishaque, W.; Khan, M.; Ashraf, U.; Riaz, M.A.; Ghulam, S.; Ahmad, A.; Rizwan, M.; Ali, S.; Alkahtani, S. Relief role of lysine chelated zinc (Zn) on 6-week-old maize plants under tannery wastewater irrigation stress. Int. J. Environ. Res. Public Health 2020, 17, 5161. [Google Scholar] [CrossRef]
- Ugwu, E.I.; Agunwamba, J.C. A review on the applicability of activated carbon derived from plant biomass in adsorption of chromium, copper, and zinc from industrial wastewater. Environ. Monit. Assess. 2020, 192, 240. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Aarts, M.G. The molecular mechanism of zinc and cadmium stress response in plants. Cell. Mol. Life Sci. 2012, 69, 3187–3206. [Google Scholar] [CrossRef]
- Houben, D.; Evrard, L.; Sonnet, P. Mobility, bioavailability and pH-dependent leaching of cadmium, zinc and lead in a contaminated soil amended with biochar. Chemosphere 2013, 92, 1450–1457. [Google Scholar] [CrossRef]
- Shemi, R.; Wang, R.; Gheith, E.-S.M.; Hussain, H.A.; Hussain, S.; Irfan, M.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Effects of salicylic acid, zinc and glycine betaine on morpho-physiological growth and yield of maize under drought stress. Sci. Rep. 2021, 11, 3195. [Google Scholar] [CrossRef]
- Assareh, M. Seedling response of three Eucalyptus species to copper and zinc toxic concentrations. Casp. J. Environ. Sci. 2008, 6, 97–103. [Google Scholar]
- Jiao, Y.; Grant, C.; Bailey, L. Effects of phosphorus and zinc fertilizer on cadmium uptake and distribution in flax and durum wheat. J. Sci. Food Agric. 2004, 84, 777–785. [Google Scholar] [CrossRef]
- Anwaar, S.A.; Ali, S.; Ali, S.; Ishaque, W.; Farid, M.; Farooq, M.A.; Najeeb, U.; Abbas, F.; Sharif, M. Silicon (Si) alleviates cotton (Gossypium hirsutum L.) from zinc (Zn) toxicity stress by limiting Zn uptake and oxidative damage. Environ. Sci. Pollut. Res. 2015, 22, 3441–3450. [Google Scholar] [CrossRef]
- Murakami, M.; Ae, N. Potential for phytoextraction of copper, lead, and zinc by rice (Oryza sativa L.), soybean (Glycine max [L.] Merr.), and maize (Zea mays L.). J. Hazard. Mater. 2009, 162, 1185–1192. [Google Scholar] [CrossRef]
- Race, M.; Marotta, R.; Fabbricino, M.; Pirozzi, F.; Andreozzi, R.; Cortese, L.; Giudicianni, P. Copper and zinc removal from contaminated soils through soil washing process using ethylenediaminedisuccinic acid as a chelating agent: A modeling investigation. J. Environ. Chem. Eng. 2016, 4, 2878–2891. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.; El-Mageed, A.; Taia, A.; El-Mageed, A.; Shimaa, A.; Rady, M.M.; Ali, E.F. Foliar application of zinc oxide nanoparticles promotes drought stress tolerance in eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar] [CrossRef]
- Shi, W.G.; Li, H.; Liu, T.X.; Polle, A.; Peng, C.H.; Luo, Z.B. Exogenous abscisic acid alleviates zinc uptake and accumulation in P opulus× canescens exposed to excess zinc. Plant Cell Environ. 2015, 38, 207–223. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Song, S.; Xu, F.; Pan, Y.; Wang, H. Transcriptomic and proteomic analyses reveal new insight into chlorophyll synthesis and chloroplast structure of maize leaves under zinc deficiency stress. J. Proteom. 2019, 199, 123–134. [Google Scholar] [CrossRef]
- Shnain, R.; Prasad, V.; Saravanan, S. Effect of zinc and boron on growth, yield and quality of tomato (Lycopersicon esculentum.Mill) cv. Heem Sohna, under protected cultivation. Eur. Acad. Res. 2014, 2, 78–79. [Google Scholar]
- Khan, M.I.R.; Jahan, B.; Alajmi, M.F.; Rehman, M.T.; Khan, N.A. Exogenously-sourced ethylene modulates defense mechanisms and promotes tolerance to zinc stress in mustard (Brassica juncea L.). Plants 2019, 8, 540. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, M.; Ali, S.; Ali, B.; Adrees, M.; Arshad, M.; Hussain, A.; ur Rehman, M.Z.; Waris, A.A. Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere 2019, 214, 269–277. [Google Scholar] [CrossRef]
- Paunov, M.; Koleva, L.; Vassilev, A.; Vangronsveld, J.; Goltsev, V. Effects of different metals on photosynthesis: Cadmium and zinc affect chlorophyll fluorescence in durum wheat. Int. J. Mol. Sci. 2018, 19, 787. [Google Scholar] [CrossRef] [Green Version]
- Saleem, M.H.; Fahad, S.; Adnan, M.; Ali, M.; Rana, M.S.; Kamran, M.; Ali, Q.; Hashem, I.A.; Bhantana, P.; Ali, M.; et al. Foliar application of gibberellic acid endorsed phytoextraction of copper and alleviates oxidative stress in jute (Corchorus capsularis L.) plant grown in highly copper-contaminated soil of China. Environ. Sci. Pollut. Res. 2020, 27, 37121–37133. [Google Scholar] [CrossRef]
- Saleem, M.H.; Wang, X.; Ali, S.; Zafar, S.; Nawaz, M.; Adnan, M.; Fahad, S.; Shah, A.; Alyemeni, M.N.; Hefft, D.I.; et al. Interactive effects of gibberellic acid and NPK on morpho-physio-biochemical traits and organic acid exudation pattern in coriander (Coriandrum sativum L.) grown in soil artificially spiked with boron. Plant Physiol. Biochem. 2021, 167, 884–900. [Google Scholar] [CrossRef]
- Afzal, J.; Wang, X.; Saleem, M.-H.; Sun, X.; Hussain, S.; Khan, I.; Rana, M.-S.; Ahmed, S.; Awan, S.-A.; Fiaz, S.; et al. Application of ferrous sulfate alleviates negative impact of cadmium in rice (Oryza sativa L.). Biocell 2021, 45, 1631–1649. [Google Scholar] [CrossRef]
- Afzal, J.; Saleem, M.H.; Batool, F.; Elyamine, A.M.; Rana, M.S.; Shaheen, A.; El-Esawi, M.A.; Tariq Javed, M.; Ali, Q.; Arslan Ashraf, M.; et al. Role of Ferrous Sulfate (FeSO4) in Resistance to Cadmium Stress in Two Rice (Oryza sativa L.) Genotypes. Biomolecules 2020, 10, 1693. [Google Scholar] [CrossRef]
- Reichman, S. The Responses of Plants to Metal Toxicity: A Review Forusing on Copper, Manganese & Zinc; Australian Minerals & Energy Environment Foundation: Melbourne, Australia, 2002. [Google Scholar]
- Sofy, M.R.; Elhindi, K.M.; Farouk, S.; Alotaibi, M.A. Zinc and paclobutrazol mediated regulation of growth, upregulating antioxidant aptitude and plant productivity of pea plants under salinity. Plants 2020, 9, 1197. [Google Scholar] [CrossRef]
- Mahmoud, A.W.M.; Abdeldaym, E.A.; Abdelaziz, S.M.; El-Sawy, M.B.; Mottaleb, S.A. Synergetic effects of zinc, boron, silicon, and zeolite nanoparticles on confer tolerance in potato plants subjected to salinity. Agronomy 2019, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Lenka, B.; Das, S.K. Effect of boron and zinc application on growth and productivity of potato (Solanum tuberosum) at alluvial soil (Entisols) of India. Indian J. Agron. 2019, 64, 129–137. [Google Scholar]
- Rajput, V.D.; Minkina, T.M.; Behal, A.; Sushkova, S.N.; Mandzhieva, S.; Singh, R.; Gorovtsov, A.; Tsitsuashvili, V.S.; Purvis, W.O.; Ghazaryan, K.A. Effects of zinc-oxide nanoparticles on soil, plants, animals and soil organisms: A review. Environ. Nanotechnol. Monit. Manag. 2018, 9, 76–84. [Google Scholar] [CrossRef]
- Qin, F.; Liu, G.; Huang, G.; Dong, T.; Liao, Y.; Xu, X. Zinc application alleviates the adverse effects of lead stress more in female Morus alba than in males. Environ. Exp. Bot. 2018, 146, 68–76. [Google Scholar] [CrossRef]
- ul Hassan, Z.; Ali, S.; Rizwan, M.; Hussain, A.; Akbar, Z.; Rasool, N.; Abbas, F. Role of zinc in alleviating heavy metal stress. In Essential Plant Nutrients; Springer: Berlin/Heidelberg, Germany, 2017; pp. 351–366. [Google Scholar]
- Farooq, M.; Ullah, A.; Usman, M.; Siddique, K.H. Application of zinc and biochar help to mitigate cadmium stress in bread wheat raised from seeds with high intrinsic zinc. Chemosphere 2020, 260, 127652. [Google Scholar] [CrossRef]
- Ullah, A.; Farooq, M.; Rehman, A.; Hussain, M.; Siddique, K.H. Zinc nutrition in chickpea (Cicer arietinum): A review. Crop Pasture Sci. 2020, 71, 199–218. [Google Scholar] [CrossRef]
- Prasad, T.; Sudhakar, P.; Sreenivasulu, Y.; Latha, P.; Munaswamy, V.; Reddy, K.R.; Sreeprasad, T.; Sajanlal, P.; Pradeep, T. Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J. Plant Nutr. 2012, 35, 905–927. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, S.; Rizwan, M.; ur Rehman, M.Z.; Hameed, A.; Hafeez, F.; Alamri, S.A.; Alyemeni, M.N.; Wijaya, L. Role of zinc–lysine on growth and chromium uptake in rice plants under Cr stress. J. Plant Growth Regul. 2018, 37, 1413–1422. [Google Scholar] [CrossRef]
- Tang, L.; Hamid, Y.; Liu, D.; Shohag, M.J.I.; Zehra, A.; He, Z.; Feng, Y.; Yang, X. Foliar application of zinc and selenium alleviates cadmium and lead toxicity of water spinach–Bioavailability/cytotoxicity study with human cell lines. Environ. Int. 2020, 145, 106122. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Saleem, M.H.; Fahad, S.; Bashir, S.; Peng, D.; Deng, G.; Alamri, S.; Siddiqui, M.H.; Khan, S.M.; Shah, R.A. Effects of rice straw biochar and nitrogen fertilizer on ramie (Boehmeria nivea L.) morpho-physiological traits, copper uptake and post-harvest soil characteristics, grown in an aged-copper contaminated soil. J. Plant Nutr. 2021, 45, 11–24. [Google Scholar] [CrossRef]
- Heile, A.O.; Zaman, Q.U.; Aslam, Z.; Hussain, A.; Aslam, M.; Saleem, M.H.; Abualreesh, M.H.; Alatawi, A.; Ali, S. Alleviation of Cadmium Phytotoxicity Using Silicon Fertilization in Wheat by Altering Antioxidant Metabolism and Osmotic Adjustment. Sustainability 2021, 13, 11317. [Google Scholar] [CrossRef]
- Santos, G.C.G.d.; Rodella, A.A.; Abreu, C.A.d.; Coscione, A.R. Vegetable species for phytoextraction of boron, copper, lead, manganese and zinc from contaminated soil. Sci. Agric. 2010, 67, 713–719. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Tawwab, M.; Sharafeldin, K.M.; Ismaiel, N. Interactive effects of coffee bean supplementation and waterborne zinc toxicity on growth performance, biochemical variables, antioxidant activity and zinc bioaccumulation in whole body of common carp, Cyprinus carpio L. Aquac. Nutr. 2018, 24, 123–130. [Google Scholar] [CrossRef]
- MacFarlane, G. Chlorophyll a fluorescence as a potential biomarker of zinc stress in the grey mangrove, Avicennia marina (Forsk.) Vierh. Bull. Environ. Contam. Toxicol. 2003, 70, 90–96. [Google Scholar] [CrossRef]
- García-López, J.I.; Niño-Medina, G.; Olivares-Sáenz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Vázquez-Alvarado, R.; Rodríguez-Salinas, P.A.; Zavala-García, F. Foliar application of zinc oxide nanoparticles and zinc sulfate boosts the content of bioactive compounds in habanero peppers. Plants 2019, 8, 254. [Google Scholar] [CrossRef] [Green Version]
- Taspinar, M.S.; Agar, G.; Alpsoy, L.; Yildirim, N.; Bozari, S.; Sevsay, S. The protective role of zinc and calcium in Vicia faba seedlings subjected to cadmium stress. Toxicol. Ind. Health 2011, 27, 73–80. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; Dacrory, S.; Hashem, A.H.; Attia, M.S.; Hasanin, M.; Fouda, H.M.; Kamel, S.; ElSaied, H. Protective role of zinc oxide nanoparticles based hydrogel against wilt disease of pepper plant. Biocatal. Agric. Biotechnol. 2021, 35, 102083. [Google Scholar] [CrossRef]
- Saleem, M.H.; Parveen, A.; Khan, S.U.; Hussain, I.; Wang, X.; Alshaya, H.; El-Sheikh, M.A.; Ali, S. Silicon fertigation regimes attenuates cadmium toxicity and phytoremediation potential in two maize (Zea mays L.) cultivars by minimizing its uptake and oxidative stress. Sustainability 2022, 14, 1462. [Google Scholar] [CrossRef]
- Cardini, A.; Pellegrino, E.; White, P.J.; Mazzolai, B.; Mascherpa, M.C.; Ercoli, L. Transcriptional regulation of genes involved in zinc uptake, sequestration and redistribution following foliar zinc application to Medicago sativa. Plants 2021, 10, 476. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Bai, X.; Zhu, D.; Li, Y.; Ji, W.; Cai, H.; Wu, J.; Liu, B.; Zhu, Y. GsZFP1, a new Cys2/His2-type zinc-finger protein, is a positive regulator of plant tolerance to cold and drought stress. Planta 2012, 235, 1141–1155. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-T.; He, M.; Wang, J.; Wang, Y.-P. Zinc finger protein (ZFP) in plants-A review. Plant Omics 2013, 6, 474–480. [Google Scholar]
- Hall, T.M.T. Multiple modes of RNA recognition by zinc finger proteins. Curr. Opin. Struct. Biol. 2005, 15, 367–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.K.; Rai, A.K.; Kanwar, S.S.; Sharma, T.R. Comparative analysis of zinc finger proteins involved in plant disease resistance. PLoS ONE 2012, 7, e42578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cassandri, M.; Smirnov, A.; Novelli, F.; Pitolli, C.; Agostini, M.; Malewicz, M.; Melino, G.; Raschellà, G. Zinc-finger proteins in health and disease. Cell Death Discov. 2017, 3, 17071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stangoulis, J.C.; Knez, M. Biofortification of major crop plants with iron and zinc-achievements and future directions. Plant Soil 2022, 1–20. Available online: https://link.springer.com/article/10.1007/s11104-022-05330-7 (accessed on 15 March 2022). [CrossRef]
- Zafar, S.; Ashraf, M.Y.; Saleem, M. Shift in physiological and biochemical processes in wheat supplied with zinc and potassium under saline condition. J. Plant Nutr. 2018, 41, 19–28. [Google Scholar] [CrossRef]
- Zvezdanović, J.; Marković, D.; Nikolić, G. Different possibilities for the formation of complexes of copper and zinc with chlorophyll inside photosynthetic organelles: Chloroplasts and thylakoids. J. Serb. Chem. Soc. 2007, 72, 1053–1062. [Google Scholar] [CrossRef]
- Tirani, M.M.; Haghjou, M.M.; Ismaili, A. Hydroponic grown tobacco plants respond to zinc oxide nanoparticles and bulk exposures by morphological, physiological and anatomical adjustments. Funct. Plant Biol. 2019, 46, 360–375. [Google Scholar] [CrossRef]
- Said-Al Ahl, H.; Omer, E. Effect of spraying with zinc and/or iron on growth and chemical composition of coriander (Coriandrum sativum L.) harvested at three stages of development. J. Med. Food Plants 2009, 1, 30–46. [Google Scholar]
- Saad, R.B.; Zouari, N.; Ramdhan, W.B.; Azaza, J.; Meynard, D.; Guiderdoni, E.; Hassairi, A. Improved drought and salt stress tolerance in transgenic tobacco overexpressing a novel A20/AN1 zinc-finger “AlSAP” gene isolated from the halophyte grass Aeluropus littoralis. Plant Mol. Biol. 2010, 72, 171. [Google Scholar] [CrossRef] [PubMed]
- Albornoz, C.B.; Larsen, K.; Landa, R.; Quiroga, M.A.; Najle, R.; Marcovecchio, J. Lead and zinc determinations in Festuca arundinacea and Cynodon dactylon collected from contaminated soils in Tandil (Buenos Aires Province, Argentina). Environ. Earth Sci. 2016, 75, 742. [Google Scholar] [CrossRef]
- Cherif, J.; Mediouni, C.; Ammar, W.B.; Jemal, F. Interactions of zinc and cadmium toxicity in their effects on growth and in antioxidative systems in tomato plants (Solarium lycopersicum). J. Environ. Sci. 2011, 23, 837–844. [Google Scholar] [CrossRef]
- Valivand, M.; Amooaghaie, R. Foliar spray with sodium hydrosulfide and calcium chloride advances dynamic of critical elements and efficiency of nitrogen metabolism in Cucurbita pepo L. under nickel stress. Sci. Hortic. 2021, 283, 110052. [Google Scholar] [CrossRef]
- Irshad, S.; Xie, Z.; Kamran, M.; Nawaz, A.; Mehmood, S.; Gulzar, H.; Saleem, M.H.; Rizwan, M.; Malik, Z.; Parveen, A.; et al. Biochar composite with microbes enhanced arsenic biosorption and phytoextraction by Typha latifolia in hybrid vertical subsurface flow constructed wetland. Environ. Pollut. 2021, 291, 118269. [Google Scholar] [CrossRef]
- Zhang, M.; Ran, R.; Nao, W.S.; Feng, Y.; Jia, L.; Sun, K.; Wang, R.; Feng, H. Physiological effects of short-term copper stress on rape (Brassica napus L.) seedlings and the alleviation of copper stress by attapulgite clay in growth medium. Ecotoxicol. Environ. Saf. 2019, 171, 878–886. [Google Scholar] [CrossRef]
- Khalaj, K.; Ahmadi, N.; Souri, M.K. Improvement of postharvest quality of Asian pear fruits by foliar application of boron and calcium. Horticulturae 2017, 3, 15. [Google Scholar] [CrossRef]
- Upadhyay, R.; Panda, S.K. Zinc reduces copper toxicity induced oxidative stress by promoting antioxidant defense in freshly grown aquatic duckweed Spirodela polyrhiza L. J. Hazard. Mater. 2010, 175, 1081–1084. [Google Scholar] [CrossRef]
- Ilyas, F.; Ali, M.A.; Modhish, A.; Ahmed, N.; Hussain, S.; Bilal, M.; Arshad, M.; Danish, S.; Ghoneim, A.M.; Ilyas, A. Synchronisation of zinc application rates with arbuscular mycorrhizal fungi and phosphorus to maximise wheat growth and yield in zinc-deficient soil. Crop Pasture Sci. 2022. [Google Scholar] [CrossRef]
- Pedrosa, T.D.; Ozima, H.T.; Schneider, R.M.; de Souza, A.P.; de Andrade, E.A.; Mattos, L.V. Phosphorus, copper and zinc leached in lysimeters with red-yellow latosol subjected to different rates of reused swine water and irrigation water. Afr. J. Agric. Res. 2017, 12, 2902–2909. [Google Scholar]
- Saeed, M.; Fox, R.L. Influence of phosphate fertilization on zinc adsorption by tropical soils. Soil Sci. Soc. Am. J. 1979, 43, 683–686. [Google Scholar] [CrossRef]
- Recena, R.; García-López, A.M.; Delgado, A. Zinc uptake by plants as affected by fertilization with Zn sulfate, phosphorus availability, and soil properties. Agronomy 2021, 11, 390. [Google Scholar] [CrossRef]
- Arnamwong, S.; Wu, L.; Hu, P.; Yuan, C.; Thiravetyan, P.; Luo, Y.; Christie, P. Phytoextraction of cadmium and zinc by Sedum plumbizincicola using different nitrogen fertilizers, a nitrification inhibitor and a urease inhibitor. Int. J. Phytoremediation 2015, 17, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Shivay, Y.S.; Prasad, R.; Pal, M. Effect of variety and zinc application on yield, profitability, protein content and zinc and nitrogen uptake by chickpea (Cicer arietinum). Indian J. Agron. 2014, 59, 317–321. [Google Scholar]
- Kutman, U.B.; Yildiz, B.; Ozturk, L.; Cakmak, I. Biofortification of durum wheat with zinc through soil and foliar applications of nitrogen. Cereal Chem. 2010, 87, 1–9. [Google Scholar] [CrossRef]
- Hakoomat, A.; Hasnain, Z.; Shahzad, A.N.; Sarwar, N.; Qureshi, M.K.; Khaliq, S.; Qayyum, M.F. Nitrogen and zinc interaction improves yield and quality of submerged basmati rice (Oryza sativa L.). Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 372–379. [Google Scholar]
- Zhang, Y.Y.; Stockmann, R.; Ng, K.; Ajlouni, S. Revisiting phytate-element interactions: Implications for iron, zinc and calcium bioavailability, with emphasis on legumes. Crit. Rev. Food Sci. Nutr. 2020, 62, 1696–1712. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Stockmann, R.; Ng, K.; Ajlouni, S. Opportunities for plant-derived enhancers for iron, zinc, and calcium bioavailability: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 652–685. [Google Scholar] [CrossRef]
- Sawan, Z.M.; Mahmoud, M.H.; El-Guibali, A.H. Influence of potassium fertilization and foliar application of zinc and phosphorus on growth, yield components, yield and fiber properties of Egyptian cotton (Gossypium barbadense L.). J. Plant Ecol. 2008, 1, 259–270. [Google Scholar] [CrossRef] [Green Version]
- Marschner, H.; Cakmak, I. High light intensity enhances chlorosis and necrosis in leaves of zinc, potassium, and magnesium deficient bean (Phaseolus vulgaris) plants. J. Plant Physiol. 1989, 134, 308–315. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Noor, I.; El-Esawi, M.A.; Hayat, K.; Rizwan, M.; Abbas, Z.; El-Sheikh, M.A.; Alyemeni, M.N. Iron–Lysine Mediated Alleviation of Chromium Toxicity in Spinach (Spinacia oleracea L.) Plants in Relation to Morpho-Physiological Traits and Iron Uptake When Irrigated with Tannery Wastewater. Sustainability 2020, 12, 6690. [Google Scholar] [CrossRef]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Imran, M.; Alnusairi, G.S.H.; Alharbi, B.M.; Riaz, M.; Abbas, Z.; Rizwan, M.; Soliman, M.H. Role of iron–lysine on morpho-physiological traits and combating chromium toxicity in rapeseed (Brassica napus L.) plants irrigated with different levels of tannery wastewater. Plant Physiol. Biochem. 2020, 155, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Javed, M.T.; Tanwir, K.; Akram, M.S.; Tazeen, S.K.; Saleem, M.H.; Masood, S.; Mujtaba, S.; Chaudhary, H.J. Plant growth-promoting Bacillus sp. strain SDA-4 confers Cd tolerance by physio-biochemical improvements, better nutrient acquisition and diminished Cd uptake in Spinacia oleracea L. Physiol. Mol. Biol. Plants 2020, 26, 417–2433. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Hussain, S.; El-Esawi, M.A.; Rana, M.S.; Saleem, M.H.; Riaz, M.; Ashraf, U.; Potcho, M.P.; Duan, M.; Rajput, I.A. Molybdenum Supply Alleviates the Cadmium Toxicity in Fragrant Rice by Modulating Oxidative Stress and Antioxidant Gene Expression. Biomolecules 2020, 10, 1582. [Google Scholar] [CrossRef]
- Imran, M.; Sun, X.; Hussain, S.; Rana, M.S.; Saleem, M.H.; Riaz, M.; Tang, X.; Khan, I.; Hu, C. Molybdenum supply increases root system growth of winter wheat by enhancing nitric oxide accumulation and expression of NRT genes. Plant Soil 2020, 459, 235–248. [Google Scholar] [CrossRef]
- Kamran, M.; Danish, M.; Saleem, M.H.; Malik, Z.; Parveen, A.; Abbasi, G.H.; Jamil, M.; Ali, S.; Afzal, S.; Riaz, M. Application of abscisic acid and 6-benzylaminopurine modulated morpho-physiological and antioxidative defense responses of tomato (Solanum lycopersicum L.) by minimizing cobalt uptake. Chemosphere 2020, 263, 128169. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Rehman, M.; Rana, M.S.; Rizwan, M.; Kamran, M.; Imran, M.; Riaz, M.; Soliman, M.H.; Elkelish, A. Influence of phosphorus on copper phytoextraction via modulating cellular organelles in two jute (Corchorus capsularis L.) varieties grown in a copper mining soil of Hubei Province, China. Chemosphere 2020, 248, 126032. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Hussain, S.; Kamran, M.; Chattha, M.S.; Ahmad, S.; Aqeel, M.; Rizwan, M.; Aljarba, N.H.; Alkahtani, S. Flax (Linum usitatissimum L.): A Potential Candidate for Phytoremediation? Biological and Economical Points of View. Plants 2020, 9, 496. [Google Scholar] [CrossRef] [Green Version]
- Saleem, M.H.; Ali, S.; Rehman, M.; Hasanuzzaman, M.; Rizwan, M.; Irshad, S.; Shafiq, F.; Iqbal, M.; Alharbi, B.M.; Alnusaire, T.S. Jute: A Potential Candidate for Phytoremediation of Metals—A Review. Plants 2020, 9, 258. [Google Scholar] [CrossRef] [Green Version]
- Rehman, M.; Yang, M.; Fahad, S.; Saleem, M.H.; Liu, L.; Liu, F.; Deng, G. Morpho-physiological traits, antioxidant capacity, and nitrogen metabolism in ramie under nitrogen fertilizer. Agron. J. 2020, 112, 2988–2997. [Google Scholar] [CrossRef]
- Saleem, M.H.; Kamran, M.; Zhou, Y.; Parveen, A.; Rehman, M.; Ahmar, S.; Malik, Z.; Mustafa, A.; Anjum, R.M.A.; Wang, B. Appraising growth, oxidative stress and copper phytoextraction potential of flax (Linum usitatissimum L.) grown in soil differentially spiked with copper. J. Environ. Manag. 2020, 257, 109994. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.H.; Fahad, S.; Khan, S.U.; Din, M.; Ullah, A.; Sabagh, A.E.L.; Hossain, A.; Llanes, A.; Liu, L. Copper-induced oxidative stress, initiation of antioxidants and phytoremediation potential of flax (Linum usitatissimum L.) seedlings grown under the mixing of two different soils of China. Environ. Sci. Pollut. Res. 2020, 27, 5211–5221. [Google Scholar] [CrossRef] [PubMed]
- Zaheer, I.E.; Ali, S.; Saleem, M.H.; Ali, M.; Riaz, M.; Javed, S.; Sehar, A.; Abbas, Z.; Rizwan, M.; El-Sheikh, M.A.; et al. Interactive role of zinc and iron lysine on Spinacia oleracea L. growth, photosynthesis and antioxidant capacity irrigated with tannery wastewater. Physiol. Mol. Biol. Plants 2020, 26, 2435–2452. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, N.; Bibi, S.; Ahmad, M.; Ok, Y.S. Effectiveness of zinc application to minimize cadmium toxicity and accumulation in wheat (Triticum aestivum L.). Environ. Earth Sci. 2014, 71, 1663–1672. [Google Scholar]
- Saleem, M.H.; Fahad, S.; Khan, S.U.; Ahmar, S.; Khan, M.H.U.; Rehman, M.; Maqbool, Z.; Liu, L. Morpho-physiological traits, gaseous exchange attributes, and phytoremediation potential of jute (Corchorus capsularis L.) grown in different concentrations of copper-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 189, 109915. [Google Scholar] [CrossRef]
- Hashem, I.A.; Abbas, A.Y.; Abd El-Hamed, A.E.-N.H.; Salem, H.M.S.; El-hosseiny, O.E.M.; Abdel-Salam, M.A.; Saleem, M.H.; Zhou, W.; Hu, R. Potential of rice straw biochar, sulfur and ryegrass (Lolium perenne L.) in remediating soil contaminated with nickel through irrigation with untreated wastewater. PeerJ 2020, 8, e9267. [Google Scholar] [CrossRef]
- Perveen, R.; Wang, X.; Jamil, Y.; Ali, Q.; Ali, S.; Zakaria, M.Q.; Afzaal, M.; Kasana, R.A.; Saleem, M.H.; Fiaz, S. Quantitative Determination of the Effects of He–Ne Laser Irradiation on Seed Thermodynamics, Germination Attributes and Metabolites of Safflower (Carthamus tinctorius L.) in Relation with the Activities of Germination Enzymes. Agronomy 2021, 11, 1411. [Google Scholar] [CrossRef]
- Tariq, F.; Wang, X.; Saleem, M.H.; Khan, Z.I.; Ahmad, K.; Saleem Malik, I.; Munir, M.; Mahpara, S.; Mehmood, N.; Ahmad, T.; et al. Risk Assessment of Heavy Metals in Basmati Rice: Implications for Public Health. Sustainability 2021, 13, 8513. [Google Scholar] [CrossRef]
- Ghani, M.A.; Abbas, M.M.; Ali, B.; Aziz, R.; Qadri, R.W.K.; Noor, A.; Azam, M.; Bahzad, S.; Saleem, M.H.; Abualreesh, M.H.; et al. Alleviating Role of Gibberellic Acid in Enhancing Plant Growth and Stimulating Phenolic Compounds in Carrot (Daucus carota L.) under Lead Stress. Sustainability 2021, 13, 12329. [Google Scholar] [CrossRef]
- Yasmin, H.; Bano, A.; Wilson, N.L.; Nosheen, A.; Naz, R.; Hassan, M.N.; Illyas, N.; Saleem, M.H.; Noureldeen, A.; Ahmad, P. Drought tolerant Pseudomonas sp. showed differential expression of stress-responsive genes and induced drought tolerance in Arabidopsis thaliana. Physiol. Plant. 2021, 174, e13497. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Saleem, M.H.; Mumtaz, S.; Rasheed, R.; Ashraf, M.A.; Maqsood, F.; Rehman, M.; Yasmin, H.; Ahmed, S.; Ishtiaq, M. Choline Chloride Mediates Chromium Tolerance in Spinach (Spinacia oleracea L.) by Restricting its Uptake in Relation to Morpho-physio-biochemical Attributes. J. Plant Growth Regul. 2021, 1–21. Available online: https://link.springer.com/article/10.1007/s00344-021-10401-7 (accessed on 15 March 2022). [CrossRef]
- Brennan, R.; Bolland, M. Application of increasing levels of zinc to soil reduced accumulation of cadmium in lupin grain. J. Plant Nutr. 2014, 37, 147–160. [Google Scholar] [CrossRef]
- Benáková, M.; Ahmadi, H.; Dučaiová, Z.; Tylová, E.; Clemens, S.; Tůma, J. Effects of Cd and Zn on physiological and anatomical properties of hydroponically grown Brassica napus plants. Environ. Sci. Pollut. Res. 2017, 24, 20705–20716. [Google Scholar] [CrossRef]
- Ahmad, S.; Mfarrej, M.F.B.; El-Esawi, M.A.; Waseem, M.; Alatawi, A.; Nafees, M.; Saleem, M.H.; Rizwan, M.; Yasmeen, T.; Anayat, A.; et al. Chromium-resistant Staphylococcus aureus alleviates chromium toxicity by developing synergistic relationships with zinc oxide nanoparticles in wheat. Ecotoxicol. Environ. Saf. 2022, 230, 113142. [Google Scholar] [CrossRef]
- Faizan, M.; Sehar, S.; Rajput, V.D.; Faraz, A.; Afzal, S.; Minkina, T.; Sushkova, S.; Adil, M.F.; Yu, F.; Alatar, A.A.; et al. Modulation of Cellular Redox Status and Antioxidant Defense System after Synergistic Application of Zinc Oxide Nanoparticles and Salicylic Acid in Rice (Oryza sativa) Plant under Arsenic Stress. Plants 2021, 10, 2254. [Google Scholar] [CrossRef]
- Bhat, J.A.; Faizan, M.; Bhat, M.A.; Huang, F.; Yu, D.; Ahmad, A.; Bajguz, A.; Ahmad, P. Defense interplay of the zinc-oxide nanoparticles and melatonin in alleviating the arsenic stress in soybean (Glycine max L.). Chemosphere 2022, 288, 132471. [Google Scholar] [CrossRef]
- Ahmad, P.; Alyemeni, M.N.; Al-Huqail, A.A.; Alqahtani, M.A.; Wijaya, L.; Ashraf, M.; Kaya, C.; Bajguz, A. Zinc oxide nanoparticles application alleviates arsenic (As) toxicity in soybean plants by restricting the uptake of as and modulating key biochemical attributes, antioxidant enzymes, ascorbate-glutathione cycle and glyoxalase system. Plants 2020, 9, 825. [Google Scholar] [CrossRef]
- Siddiqui, H.; Ahmed, K.B.M.; Sami, F.; Hayat, S. Silicon nanoparticles and plants: Current knowledge and future perspectives. Sustain. Agric. Rev. 2020, 41, 129–142. [Google Scholar]
- Welch, C.M.; Compton, R.G. The use of nanoparticles in electroanalysis: A review. Anal. Bioanal. Chem. 2006, 384, 601–619. [Google Scholar] [CrossRef]
- Muszynska, E.; Hanus-Fajerska, E. Why are heavy metal hyperaccumulating plants so amazing? BioTechnologia J. Biotechnol. Comput. Biol. Bionanotechnol. 2015, 96, 265–271. [Google Scholar] [CrossRef]
- Faizan, M.; Bhat, J.A.; Hessini, K.; Yu, F.; Ahmad, P. Zinc oxide nanoparticles alleviates the adverse effects of cadmium stress on Oryza sativa via modulation of the photosynthesis and antioxidant defense system. Ecotoxicol. Environ. Saf. 2021, 220, 112401. [Google Scholar] [CrossRef] [PubMed]
- Zarschler, K.; Rocks, L.; Licciardello, N.; Boselli, L.; Polo, E.; Garcia, K.P.; De Cola, L.; Stephan, H.; Dawson, K.A. Ultrasmall inorganic nanoparticles: State-of-the-art and perspectives for biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1663–1701. [Google Scholar] [CrossRef] [PubMed]
- Hannah, W.; Thompson, P.B. Nanotechnology, risk and the environment: A review. J. Environ. Monit. 2008, 10, 291–300. [Google Scholar] [CrossRef]
- McNeil, S.E. Nanotechnology for the biologist. J. Leukoc. Biol. 2005, 78, 585–594. [Google Scholar] [CrossRef]
- Manjunatha, S.; Biradar, D.; Aladakatti, Y.R. Nanotechnology and its applications in agriculture: A review. J. Farm. Sci. 2016, 29, 1–13. [Google Scholar]
- Ali, M.; Wang, X.; Haroon, U.; Chaudhary, H.J.; Kamal, A.; Ali, Q.; Saleem, M.H.; Usman, K.; Alatawi, A.; Ali, S.; et al. Antifungal activity of Zinc nitrate derived nano Zno fungicide synthesized from Trachyspermum ammi to control fruit rot disease of grapefruit. Ecotoxicol. Environ. Saf. 2022, 233, 113311. [Google Scholar] [CrossRef]
- Suriyaprabha, R.; Karunakaran, G.; Yuvakkumar, R.; Prabu, P.; Rajendran, V.; Kannan, N. Growth and physiological responses of maize (Zea mays L.) to porous silica nanoparticles in soil. J. Nanoparticle Res. 2012, 14, 1294. [Google Scholar] [CrossRef]
- Fu, X.; Cai, J.; Zhang, X.; Li, W.-D.; Ge, H.; Hu, Y. Top-down fabrication of shape-controlled, monodisperse nanoparticles for biomedical applications. Adv. Drug Deliv. Rev. 2018, 132, 169–187. [Google Scholar] [CrossRef]
- Cao, Z.; Rossi, L.; Stowers, C.; Zhang, W.; Lombardini, L.; Ma, X. The impact of cerium oxide nanoparticles on the physiology of soybean (Glycine max (L.) Merr.) under different soil moisture conditions. Environ. Sci. Pollut. Res. 2018, 25, 930–939. [Google Scholar] [CrossRef]
- Rossi, L.; Fedenia, L.N.; Sharifan, H.; Ma, X.; Lombardini, L. Effects of foliar application of zinc sulfate and zinc nanoparticles in coffee (Coffea arabica L.) plants. Plant Physiol. Biochem. 2019, 135, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Faraz, A.; Mir, A.R.; Hayat, S. Role of zinc oxide nanoparticles in countering negative effects generated by cadmium in Lycopersicon esculentum. J. Plant Growth Regul. 2021, 40, 101–115. [Google Scholar] [CrossRef]
- Yuvaraj, M.; Subramanian, K. Controlled-release fertilizer of zinc encapsulated by a manganese hollow core shell. Soil Sci. Plant Nutr. 2015, 61, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, A.; Pokhrel, S.; Bandyopadhyay, S.; Mädler, L.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. A soil mediated phyto-toxicological study of iron doped zinc oxide nanoparticles (Fe@ ZnO) in green peas (Pisum sativum L.). Chem. Eng. J. 2014, 258, 394–401. [Google Scholar] [CrossRef]
- Moghaddasi, S.; Fotovat, A.; Khoshgoftarmanesh, A.H.; Karimzadeh, F.; Khazaei, H.R.; Khorassani, R. Bioavailability of coated and uncoated ZnO nanoparticles to cucumber in soil with or without organic matter. Ecotoxicol. Environ. Saf. 2017, 144, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Hudlikar, M.; Joglekar, S.; Dhaygude, M.; Kodam, K. Latex-mediated synthesis of ZnS nanoparticles: Green synthesis approach. J. Nanoparticle Res. 2012, 14, 865. [Google Scholar] [CrossRef]
- Kong, X.Y.; Wang, Z.L. Spontaneous polarization-induced nanohelixes, nanosprings, and nanorings of piezoelectric nanobelts. Nano Lett. 2003, 3, 1625–1631. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.Y.; Tseng, T.Y.; Li, S.Y.; Lin, P. Effect of phosphorus dopant on photoluminescence and field-emission characteristics of Mg0.1 Zn0.9 O nanowires. J. Appl. Phys. 2006, 99, 024303. [Google Scholar] [CrossRef] [Green Version]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.-C.; Kahru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C. ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in Clusterbean (Cyamopsis tetragonoloba L.). Agric. Res. 2013, 2, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Ruchika, A.K. Performance analysis of Zinc oxide based alcohol sensors. Int. J. Appl. Sci. Eng. Res. 2015, 4, 428–436. [Google Scholar]
- Ambika, S.; Sundrarajan, M. Antibacterial behaviour of Vitex negundo extract assisted ZnO nanoparticles against pathogenic bacteria. J. Photochem. Photobiol. B Biol. 2015, 146, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Kittelson, D.B. Engines and nanoparticles: A review. J. Aerosol Sci. 1998, 29, 575–588. [Google Scholar] [CrossRef]
- Thema, F.; Manikandan, E.; Dhlamini, M.; Maaza, M. Green synthesis of ZnO nanoparticles via Agathosma betulina natural extract. Mater. Lett. 2015, 161, 124–127. [Google Scholar] [CrossRef]
- Adrees, M.; Khan, Z.S.; Ali, S.; Hafeez, M.; Khalid, S.; ur Rehman, M.Z.; Hussain, A.; Hussain, K.; Chatha, S.A.S.; Rizwan, M. Simultaneous mitigation of cadmium and drought stress in wheat by soil application of iron nanoparticles. Chemosphere 2020, 238, 124681. [Google Scholar] [CrossRef]
- Mohanraj, V.; Chen, Y. Nanoparticles-a review. Trop. J. Pharm. Res. 2006, 5, 561–573. [Google Scholar] [CrossRef] [Green Version]
- Mandal, S.M.; Bhattacharyya, R.N. Heavy metal toxicity on seed germination of four pulses. Int. J. Plant Sci. 2007, 2, 124–127. [Google Scholar]
- Hussain, A.; Rizwan, M.; Ali, Q.; Ali, S. Seed priming with silicon nanoparticles improved the biomass and yield while reduced the oxidative stress and cadmium concentration in wheat grains. Environ. Sci. Pollut. Res. 2019, 26, 7579–7588. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Study on the correlation between copper oxide nanoparticles induced growth suppression and enhanced lignification in Indian mustard (Brassica juncea L.). Ecotoxicol. Environ. Saf. 2015, 113, 302–313. [Google Scholar] [CrossRef]
- Kamal, A.; Saleem, M.H.; Alshaya, H.; Okla, M.K.; Chaudhary, H.J.; Munis, M.F.H. Ball-milled synthesis of maize biochar-ZnO nanocomposite (MB-ZnO) and estimation of its photocatalyticability against different organic and inorganic pollutants. J. Saudi Chem. Soc. 2022, 26, 101445. [Google Scholar] [CrossRef]
- Raj, S.N.; Anooj, E.; Rajendran, K.; Vallinayagam, S. A comprehensive review on regulatory invention of nano pesticides in Agricultural nano formulation and food system. J. Mol. Struct. 2021, 1239, 130517. [Google Scholar]
- Benelli, G.; Maggi, F.; Pavela, R.; Murugan, K.; Govindarajan, M.; Vaseeharan, B.; Petrelli, R.; Cappellacci, L.; Kumar, S.; Hofer, A. Mosquito control with green nanopesticides: Towards the One Health approach? A review of non-target effects. Environ. Sci. Pollut. Res. 2018, 25, 10184–10206. [Google Scholar] [CrossRef] [PubMed]
- Chhipa, H. Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 2017, 15, 15–22. [Google Scholar] [CrossRef]
- Hayles, J.; Johnson, L.; Worthley, C.; Losic, D. Nanopesticides: A review of current research and perspectives. New Pestic. Soil Sens. 2017, 193–225. [Google Scholar] [CrossRef]
- Kah, M.; Hofmann, T. Nanopesticide research: Current trends and future priorities. Environ. Int. 2014, 63, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.L.; Formisano, N.; Estrela, P.; Carrara, S.; Tkac, J. Electrochemical biosensors and nanobiosensors. Essays Biochem. 2016, 60, 69–80. [Google Scholar] [PubMed] [Green Version]
- Razavi, H.; Janfaza, S. Medical nanobiosensors: A tutorial review. Nanomed. J. 2015, 2, 74–87. [Google Scholar]
- Chen, J.; Miao, Y.; He, N.; Wu, X.; Li, S. Nanotechnology and biosensors. Biotechnol. Adv. 2004, 22, 505–518. [Google Scholar]
- Seow, Z.; Wong, A.; Thavasi, V.; Jose, R.; Ramakrishna, S.; Ho, G. Controlled synthesis and application of ZnO nanoparticles, nanorods and nanospheres in dye-sensitized solar cells. Nanotechnology 2008, 20, 045604. [Google Scholar] [CrossRef]
- Pandey, A.C.; Sanjay, S.S.; Yadav, R. Application of ZnO nanoparticles in influencing the growth rate of Cicer arietinum. J. Exp. Nanosci. 2010, 5, 488–497. [Google Scholar] [CrossRef]
- Salah, S.M.; Yajing, G.; Dongdong, C.; Jie, L.; Aamir, N.; Qijuan, H.; Weimin, H.; Mingyu, N.; Jin, H. Seed priming with polyethylene glycol regulating the physiological and molecular mechanism in rice (Oryza sativa L.) under nano-ZnO stress. Sci. Rep. 2015, 5, 14278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.; Jang, N.-Y.; Lee, J.-W.; Park, B.C.; Kim, Y.K.; Cho, N.-H. Application of ZnO-based nanocomposites for vaccines and cancer immunotherapy. Pharmaceutics 2019, 11, 493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotloff, K.L.; Winickoff, J.P.; Ivanoff, B.; Clemens, J.D.; Swerdlow, D.L.; Sansonetti, P.J.; Adak, G.; Levine, M. Global burden of Shigella infections: Implications for vaccine development and implementation of control strategies. Bull. World Health Organ. 1999, 77, 651. [Google Scholar] [PubMed]
- Cheng, B.; Chen, F.; Wang, C.; Liu, X.; Yue, L.; Cao, X.; Wang, Z.; Xing, B. The molecular mechanisms of silica nanomaterials enhancing the rice (Oryza sativa L.) resistance to planthoppers (Nilaparvata lugens Stal). Sci. Total Environ. 2021, 767, 144967. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, M.; Vishwakarma, K.; Kumar, V.; Arif, N.; Das, S.; Johnson, R.; Janeeshma, E.; Puthur, J.T.; Aliniaeifard, S.; Chauhan, D.K.; et al. Metal/Metalloid-Based Nanomaterials for Plant Abiotic Stress Tolerance: An Overview of the Mechanisms. Plants 2022, 11, 316. [Google Scholar] [CrossRef] [PubMed]
- Pavić, V.; Flačer, D.; Jakovljević, M.; Molnar, M.; Jokić, S. Assessment of total phenolic content, in vitro antioxidant and antibacterial activity of Ruta graveolens L. extracts obtained by choline chloride based natural deep eutectic solvents. Plants 2019, 8, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dominic, S.; Hussain, A.I.; Saleem, M.H.; Alshaya, H.; Jan, B.L.; Ali, S.; Wang, X. Variation in the Primary and Secondary Metabolites, Antioxidant and Antibacterial Potentials of Tomatoes, Grown in Soil Blended with Different Concentration of Fly Ash. Plants 2022, 11, 551. [Google Scholar] [CrossRef]
- Hasnidawani, J.N.; Azlina, H.N.; Norita, H.; Bonnia, N.N.; Ratim, S.; Ali, E.S. Synthesis of ZnO Nanostructures Using Sol-Gel Method. Procedia Chem. 2016, 19, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Bogue, R. Nanosensors: A review of recent progress. Sens. Rev. 2008, 28, 12–17. [Google Scholar] [CrossRef]
- Ahmed Rather, G.; Nanda, A.; Ahmad Pandit, M.; Yahya, S.; Sofi, M.A.; Barabadi, H.; Saravanan, M. Biosynthesis of Zinc oxide nanoparticles using Bergenia ciliate aqueous extract and evaluation of their photocatalytic and antioxidant potential. Inorg. Chem. Commun. 2021, 134, 109020. [Google Scholar] [CrossRef]
- Polishchuk, S.; Nazarova, A.; Kutskir, M.; Churilov, D.; Ivanycheva, Y.; Kiryshin, V.; Churilov, G. Ecologic-biological effects of cobalt, cuprum, copper oxide nano-powders and humic acids on wheat seeds. Mod. Appl. Sci. 2015, 9, 354. [Google Scholar]
- van Ommen, J.R.; Valverde, J.M.; Pfeffer, R. Fluidization of nanopowders: A review. J. Nanoparticle Res. 2012, 14, 737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hameed, A.; Akram, N.A.; Saleem, M.H.; Ashraf, M.; Ahmed, S.; Ali, S.; Abdullah Alsahli, A.; Alyemeni, M.N. Seed Treatment with α-Tocopherol Regulates Growth and Key Physio-Biochemical Attributes in Carrot (Daucus carota L.) Plants under Water Limited Regimes. Agronomy 2021, 11, 469. [Google Scholar] [CrossRef]
- Gehrke, I.; Geiser, A.; Somborn-Schulz, A. Innovations in nanotechnology for water treatment. Nanotechnol. Sci. Appl. 2015, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.; Zheng, Y.-M.; Zhang, B.-G.; Dai, Y.-R. A critical review on the electrospun nanofibrous membranes for the adsorption of heavy metals in water treatment. J. Hazard. Mater. 2020, 401, 123608. [Google Scholar] [CrossRef]
- Theron, J.; Walker, J.A.; Cloete, T.E. Nanotechnology and water treatment: Applications and emerging opportunities. Crit. Rev. Microbiol. 2008, 34, 43–69. [Google Scholar] [CrossRef]
- Al-Qurainy, F.; Nadeem, M.; Khan, S.; Siddiqui, M.R.; Husain, F.M.; Gaafar, A.R.Z.; Alansi, S.; Alshameri, A.; Tarroum, M.; Alenezi, N.A. Phytosynthesis and assessment of silver nano particles from in vitro developed Ochradenus arabicus (Resedaceae) and evaluation of antibacterial potential. Biotechnol. Biotechnol. Equip. 2021, 35, 1238–1246. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, S.; Rizwan, M.; ur Rehman, M.Z.; Qayyum, M.F.; Wang, H.; Rinklebe, J. Responses of wheat (Triticum aestivum) plants grown in a Cd contaminated soil to the application of iron oxide nanoparticles. Ecotoxicol. Environ. Saf. 2019, 173, 156–164. [Google Scholar] [CrossRef]
- Mohan, S.; Vellakkat, M.; Aravind, A.U.R. Hydrothermal synthesis and characterization of Zinc Oxide nanoparticles of various shapes under different reaction conditions. Nano Express 2020, 1, 030028. [Google Scholar] [CrossRef]
- Vicario-Parés, U.; Castañaga, L.; Lacave, J.M.; Oron, M.; Reip, P.; Berhanu, D.; Valsami-Jones, E.; Cajaraville, M.P.; Orbea, A. Comparative toxicity of metal oxide nanoparticles (CuO, ZnO and TiO2) to developing zebrafish embryos. J. Nanoparticle Res. 2014, 16, 1–16. [Google Scholar] [CrossRef]
- Jeyabharathi, S.; Kalishwaralal, K.; Sundar, K.; Muthukumaran, A. Synthesis of zinc oxide nanoparticles (ZnONPs) by aqueous extract of Amaranthus caudatus and evaluation of their toxicity and antimicrobial activity. Mater. Lett. 2017, 209, 295–298. [Google Scholar] [CrossRef]
- Konate, A.; He, X.; Zhang, Z.; Ma, Y.; Zhang, P.; Alugongo, G.M.; Rui, Y. Magnetic (Fe3O4) nanoparticles reduce heavy metals uptake and mitigate their toxicity in wheat seedling. Sustainability 2017, 9, 790. [Google Scholar] [CrossRef] [Green Version]
- Saleem, M.H.; Ali, S.; Irshad, S.; Hussaan, M.; Rizwan, M.; Rana, M.S.; Hashem, A.; Abd_Allah, E.F.; Ahmad, P. Copper Uptake and Accumulation, Ultra-Structural Alteration, and Bast Fibre Yield and Quality of Fibrous Jute (Corchorus capsularis L.) Plants Grown Under Two Different Soils of China. Plants 2020, 9, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleem, M.H.; Ali, S.; Kamran, M.; Iqbal, N.; Azeem, M.; Tariq Javed, M.; Ali, Q.; Zulqurnain Haider, M.; Irshad, S.; Rizwan, M. Ethylenediaminetetraacetic Acid (EDTA) Mitigates the Toxic Effect of Excessive Copper Concentrations on Growth, Gaseous Exchange and Chloroplast Ultrastructure of Corchorus capsularis L. and Improves Copper Accumulation Capabilities. Plants 2020, 9, 756. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Pandey, S.C.; Joshi, S.; Chaudhary, P.; Pathak, V.M.; Huang, Y.; Wu, X.; Zhou, Z.; Chen, S. Nanobioremediation: A sustainable approach for the removal of toxic pollutants from the environment. J. Hazard. Mater. 2022, 427, 128033. [Google Scholar] [CrossRef]
- Khaleghi, S.; Khayatzadeh, J.; Neamati, A. Biosynthesis of Zinc Oxide nanoparticles using Origanum majorana L. leaf extract, its antioxidant and cytotoxic activities. Mater. Technol. 2022, 1–10. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Rehman, M.; Rizwan, M.; Kamran, M.; Mohamed, I.A.; Bamagoos, A.A.; Alharby, H.F.; Hakeem, K.R.; Liu, L. Individual and combined application of EDTA and citric acid assisted phytoextraction of copper using jute (Corchorus capsularis L.) seedlings. Environ. Technol. Innov. 2020, 19, 100895. [Google Scholar] [CrossRef]
- Saleem, M.H.; Rehman, M.; Kamran, M.; Afzal, J.; Noushahi, H.A.; Liu, L. Investigating the potential of different jute varieties for phytoremediation of copper-contaminated soil. Environ. Sci. Pollut. Res. 2020, 27, 30367–30377. [Google Scholar] [CrossRef]
- Kareem, H.A.; Hassan, M.U.; Zain, M.; Irshad, A.; Shakoor, N.; Saleem, S.; Niu, J.; Skalicky, M.; Chen, Z.; Guo, Z.; et al. Nanosized zinc oxide (n-ZnO) particles pretreatment to alfalfa seedlings alleviate heat-induced morpho-physiological and ultrastructural damages. Environ. Pollut. 2022, 303, 119069. [Google Scholar] [CrossRef]
- Sayadi, M.H.; Ghollasimood, S.; Ahmadpour, N.; Homaeigohar, S. Biosynthesis of the ZnO/SnO2 nanoparticles and characterization of their photocatalytic potential for removal of organic water pollutants. J. Photochem. Photobiol. A Chem. 2022, 425, 113662. [Google Scholar] [CrossRef]
- Saleem, M.H.; Ali, S.; Seleiman, M.F.; Rizwan, M.; Rehman, M.; Akram, N.A.; Liu, L.; Alotaibi, M.; Al-Ashkar, I.; Mubushar, M. Assessing the Correlations between Different Traits in Copper-Sensitive and Copper-Resistant Varieties of Jute (Corchorus capsularis L.). Plants 2019, 8, 545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suhag, R.; Kumar, R.; Dhiman, A.; Sharma, A.; Prabhakar, P.K.; Gopalakrishnan, K.; Kumar, R.; Singh, A. Fruit peel bioactives, valorisation into nanoparticles and potential applications: A review. Crit. Rev. Food Sci. Nutr. 2022, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Liu, L.; Bashir, S.; Saleem, M.H.; Chen, C.; Peng, D.; Siddique, K.H. Influence of rice straw biochar on growth, antioxidant capacity and copper uptake in ramie (Boehmeria nivea L.) grown as forage in aged copper-contaminated soil. Plant Physiol. Biochem. 2019, 138, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.T.; Tanwir, K.; Abbas, S.; Saleem, M.H.; Iqbal, R.; Chaudhary, H.J. Chromium retention potential of two contrasting Solanum lycopersicum Mill. cultivars as deciphered by altered pH dynamics, growth, and organic acid exudation under Cr stress. Environ. Sci. Pollut. Res. 2021, 28, 27542–27554. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.T.; Saleem, M.H.; Aslam, S.; Rehman, M.; Iqbal, N.; Begum, R.; Ali, S.; Alsahli, A.A.; Alyemeni, M.N.; Wijaya, L. Elucidating silicon-mediated distinct morpho-physio-biochemical attributes and organic acid exudation patterns of cadmium stressed Ajwain (Trachyspermum ammi L.). Plant Physiol. Biochem. 2020, 157, 23–37. [Google Scholar] [CrossRef]
- Aziz, H.; Murtaza, G.; Saleem, M.H.; Ali, S.; Rizwan, M.; Riaz, U.; Niaz, A.; Abualreesh, M.H.; Alatawi, A. Alleviation of Chlorpyrifos Toxicity in Maize (Zea mays L.) by Reducing Its Uptake and Oxidative Stress in Response to Soil-Applied Compost and Biochar Amendments. Plants 2021, 10, 2170. [Google Scholar] [CrossRef]
- Imran, M.; Hussain, S.; Rana, M.S.; Saleem, M.H.; Rasul, F.; Ali, K.H.; Potcho, M.P.; Pan, S.; Duan, M.; Tang, X. Molybdenum improves 2-acetyl-1-pyrroline, grain quality traits and yield attributes in fragrant rice through efficient nitrogen assimilation under cadmium toxicity. Ecotoxicol. Environ. Saf. 2021, 211, 111911. [Google Scholar] [CrossRef]
- Kour, J.; Kohli, S.K.; Khanna, K.; Bakshi, P.; Sharma, P.; Singh, A.D.; Ibrahim, M.; Devi, K.; Sharma, N.; Ohri, P.; et al. Brassinosteroid Signaling, Crosstalk and, Physiological Functions in Plants Under Heavy Metal Stress. Front. Plant Sci. 2021, 12, 608061. [Google Scholar] [CrossRef]
- Cao, Y.; Dhahad, H.A.; El-Shorbagy, M.A.; Alijani, H.Q.; Zakeri, M.; Heydari, A.; Bahonar, E.; Slouf, M.; Khatami, M.; Naderifar, M.; et al. Green synthesis of bimetallic ZnO–CuO nanoparticles and their cytotoxicity properties. Sci. Rep. 2021, 11, 23479. [Google Scholar] [CrossRef]
- Mfarrej, M.; Wang, X.; Hamzah Saleem, M.; Hussain, I.; Rasheed, R.; Arslan Ashraf, M.; Iqbal, M.; Sohaib Chattha, M.; Nasser Alyemeni, M. Hydrogen sulphide and nitric oxide mitigate the negative impacts of waterlogging stress on wheat (Triticum aestivum L.). Plant Biol. 2021. [Google Scholar] [CrossRef]
- Hashmat, S.; Shahid, M.; Tanwir, K.; Abbas, S.; Ali, Q.; Niazi, N.K.; Akram, M.S.; Saleem, M.H.; Javed, M.T. Elucidating distinct oxidative stress management, nutrient acquisition and yield responses of Pisum sativum L. fertigated with diluted and treated wastewater. Agric. Water Manag. 2021, 247, 106720. [Google Scholar] [CrossRef]
- Irshad, S.; Xie, Z.; Wang, J.; Nawaz, A.; Luo, Y.; Wang, Y.; Mehmood, S. Indigenous strain Bacillus XZM assisted phytoremediation and detoxification of arsenic in Vallisneria denseserrulata. J. Hazard. Mater. 2020, 381, 120903. [Google Scholar] [CrossRef] [PubMed]
- Mohsenzadeh, F.; Chehregani Rad, A. Bioremediation of petroleum polluted soils using Amaranthus retroflexus L. and its rhizospheral funji. In Phytoremediation for Green Energy; Springer: Berlin/Heidelberg, Germany, 2015; pp. 131–139. [Google Scholar]
- Ma, C.; White, J.C.; Dhankher, O.P.; Xing, B. Metal-based nanotoxicity and detoxification pathways in higher plants. Environ. Sci. Technol. 2015, 49, 7109–7122. [Google Scholar] [CrossRef]
- Subbaiah, L.V.; Prasad, T.N.V.K.V.; Krishna, T.G.; Sudhakar, P.; Reddy, B.R.; Pradeep, T. Novel effects of nanoparticulate delivery of zinc on growth, productivity, and zinc biofortification in maize (Zea mays L.). J. Agric. Food Chem. 2016, 64, 3778–3788. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, J.; Dou, R.; Gao, X.; Mao, C.; Wang, L. Assessment of the phytotoxicity of metal oxide nanoparticles on two crop plants, maize (Zea mays L.) and rice (Oryza sativa L.). Int. J. Environ. Res. Public Health 2015, 12, 15100–15109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López, J.; Martínez, J.; Abundiz, N.; Domínguez, D.; Murillo, E.; Castillón, F.; Machorro, R.; Farías, M.; Tiznado, H. Thickness effect on the optical and morphological properties in Al2O3/ZnO nanolaminate thin films prepared by atomic layer deposition. Superlattices Microstruct. 2016, 90, 265–273. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N. Cold, salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]



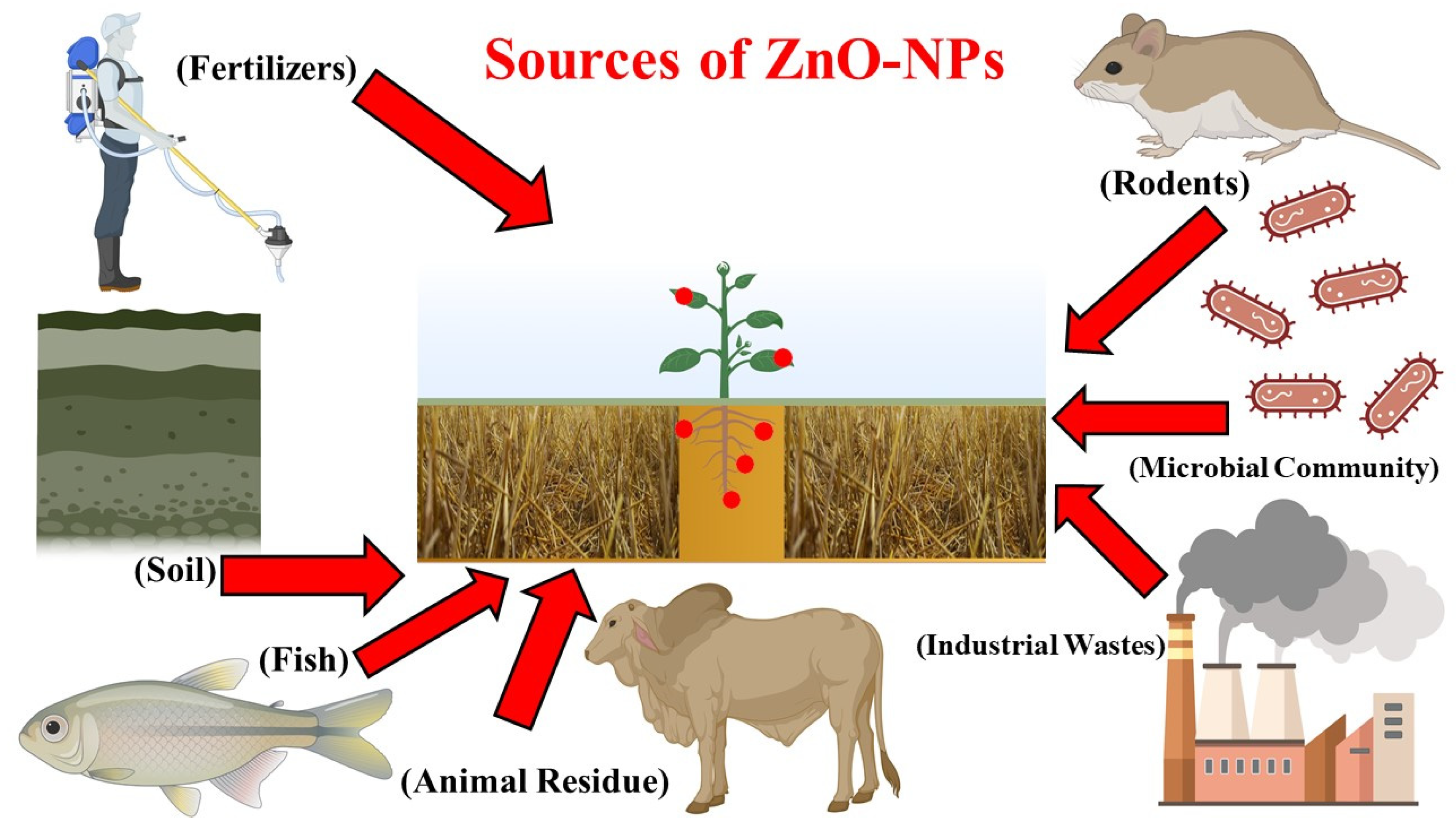
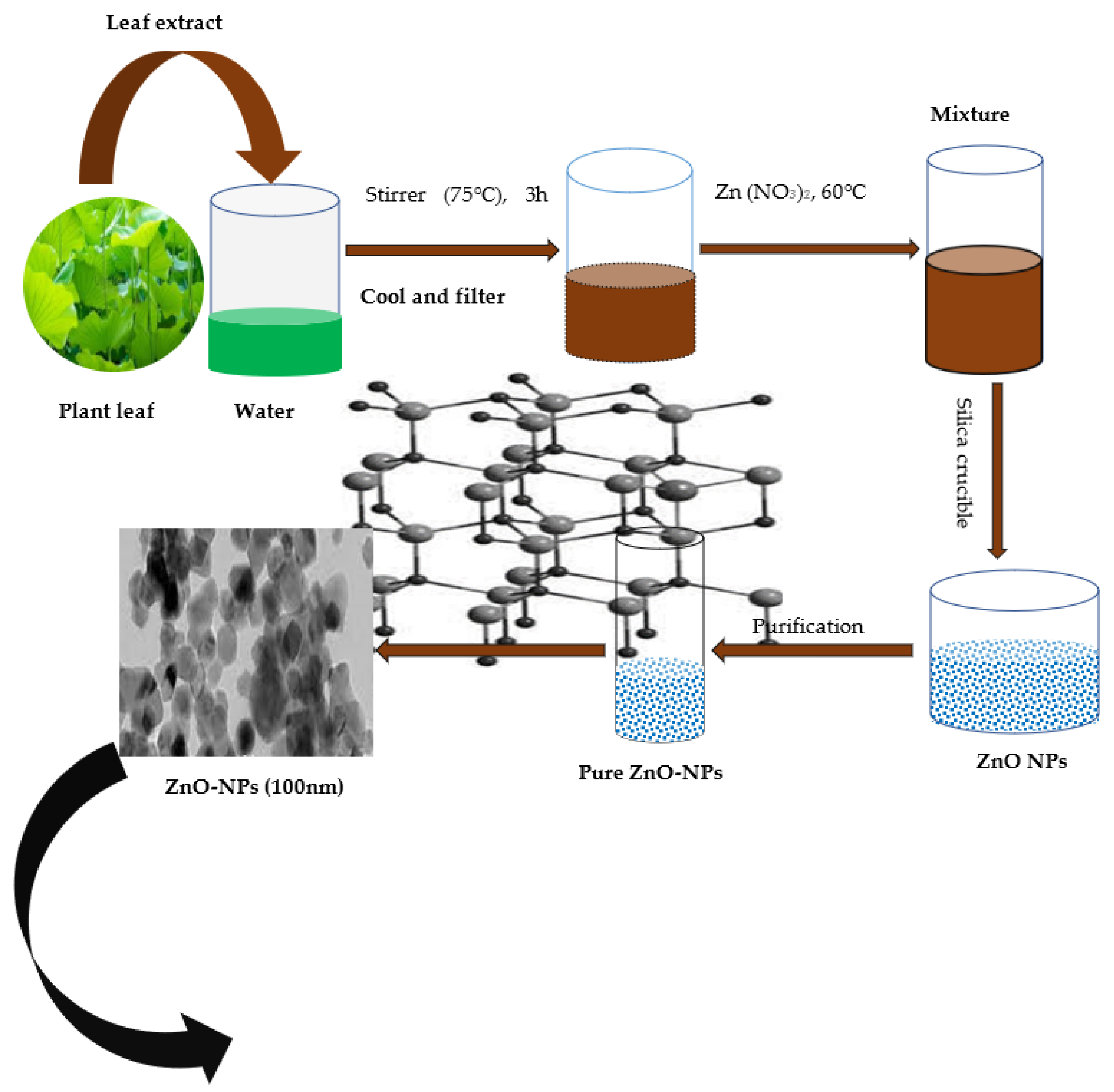

| Plant Species | Metal Type | Culture | Metal Duration (Days) | Comments | References |
|---|---|---|---|---|---|
| Yellow Lupine | Cd | Soil | Full maturity | Zn application enhanced plant yield under metal stress | [105] |
| Brassica napus | Cd | Hydroponic | 14 | Depending upon the different cultivars, the shoots Cd was decreased | [106] |
| Triticum aestivum | Cd | Soil | 125 | Application of Zn enhanced eco-physiology of the plant | [6] |
| Oryza sativa | Cr | Soil | 70 | Application of Zn enhanced growth and decreased Cr contents | [42] |
| Triticum aestivum | Cr | Soil | 120 | Zn application decreased oxidative damaged in the membrane bounded organelles | [107] |
| Oryza sativa | As | Soil | 50 | ZnO regulated various transcriptional pathways participated in oxidative stress tolerance | [108] |
| Glycine max | As | Soil | Maturity | As stress inhibited growth and photosynthesis, but regulated by the application of ZnO | [109] |
| Glycine max | As | Soil | 60 | ZnO application decreased As concentration in the roots and shoots of the plants | [110] |
| Morus alba | Pb | Soil | 90 | Zn improved gas exchange capacity, increasing growth and biomass, and improved redox imbalance in the plants | [37] |
| Methods | Process | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Chemical synthesis | Spray pyrolysis, thermal breakdown, molecular beam epitaxy, chemical vapor deposition. | It is the most significant proces, and it is performed with a variety of precursors and under a variety of variables. The size and geometries of NPs are morphologically changed | Hazardous compounds adsorbed on the surface, which could have negative consequences. | [128] |
| Vapor transport synthesis | Zinc and oxygen vapors react with each other | It is the most prevalent method and growth temperature is relatively moderate. | Imbalance vapor pressure ratio may affect the ZnO nanostructure. | [129] |
| Hydrothermal synthesis | Low temperature process | The use of simple equipment, catalyst-free growth, low cost, homogeneous production, Eco friendliness, and being less toxic. | May require high temperature to initiate. | [130] |
| Green synthesis | plant components such as the leaf, and other parts | This is a very environment friendly, low-cost method that does not require the use of intermediate base groups. | [131] | |
| Bacterial based synthesis | Green synthesis | Increased photocatalytic activity when compared to other substances, which destroys organic waste and can, thus, be utilized as a bioremediation method. | Time-consuming microbe screening, careful monitoring to avoid contamination. | [132] |
| Plant Species | Application of Nanoparticles | Effects | References |
|---|---|---|---|
| Zea mays | Foliar spray | Grain yield increased and zinc content of grain also increased | [197] |
| Oryza sativa | Plant agar | Growth increased | [198] |
| Glycin max | Paper (petri dishes) | Seedling growth inhibited | [199] |
| Phaseolous vulgaris | Foliar spray | All the growth parameters prompted and increased the content of guar gun | [132] |
| Solanum lycopersicum | substrate | It reduced the chlorophyll and the activity of antioxidants increased | [115] |
| Pisum sativum | substrate | sucrose, carotenoids and chlorophyll content increased | [126] |
| Arabidopsis thaliana | Plant agar | Germination and growth of seedling inhibited | [130] |
| Vigna radiate | Plant agar | Seedling growth promoted at <20 mg/L concentration | [200] |
| Arachis hypogea | Foliar spray | Promote early flowering, increase the chlorophyll content, better sapling viability, germination also promoted | [41] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Jabri, H.; Saleem, M.H.; Rizwan, M.; Hussain, I.; Usman, K.; Alsafran, M. Zinc Oxide Nanoparticles and Their Biosynthesis: Overview. Life 2022, 12, 594. https://doi.org/10.3390/life12040594
Al Jabri H, Saleem MH, Rizwan M, Hussain I, Usman K, Alsafran M. Zinc Oxide Nanoparticles and Their Biosynthesis: Overview. Life. 2022; 12(4):594. https://doi.org/10.3390/life12040594
Chicago/Turabian StyleAl Jabri, Hareb, Muhammad Hamzah Saleem, Muhammad Rizwan, Iqbal Hussain, Kamal Usman, and Mohammed Alsafran. 2022. "Zinc Oxide Nanoparticles and Their Biosynthesis: Overview" Life 12, no. 4: 594. https://doi.org/10.3390/life12040594
APA StyleAl Jabri, H., Saleem, M. H., Rizwan, M., Hussain, I., Usman, K., & Alsafran, M. (2022). Zinc Oxide Nanoparticles and Their Biosynthesis: Overview. Life, 12(4), 594. https://doi.org/10.3390/life12040594








