Thyroxine and Thyroid-Stimulating Hormone in Own Mother’s Milk, Donor Milk, and Infant Formula
Abstract
1. Introduction
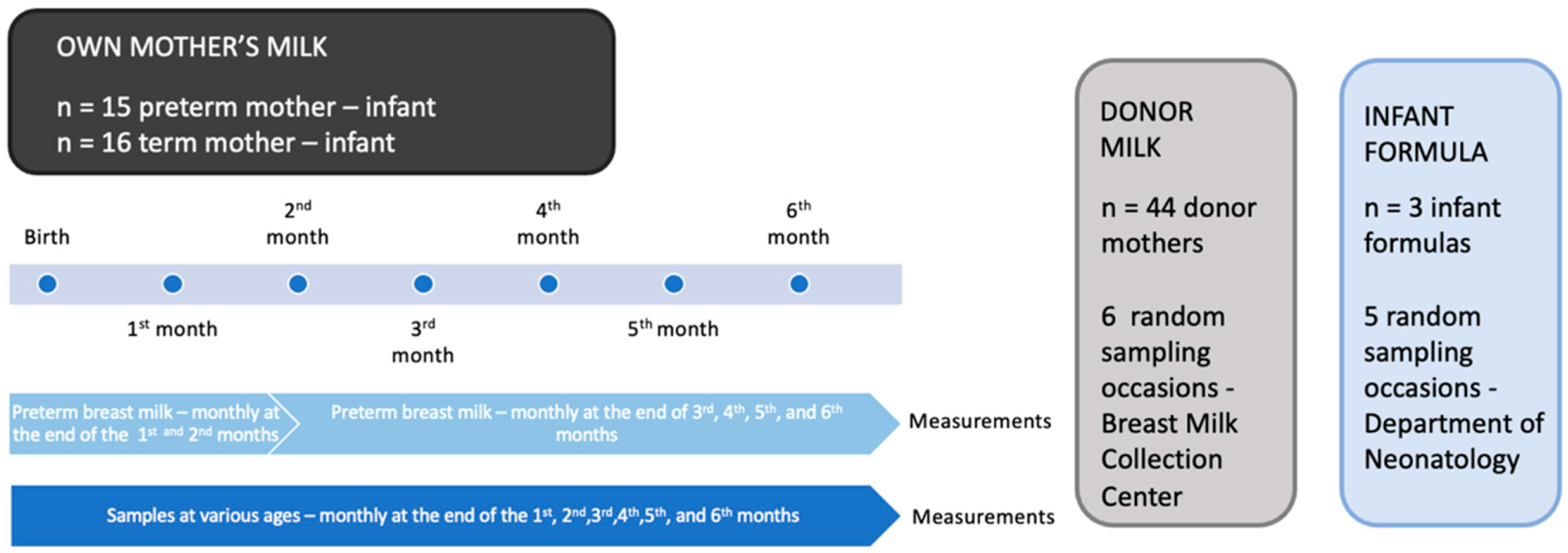
2. Materials and Methods
3. Results
3.1. Maternal Characteristics
3.2. Preterm Breast Milk
3.3. Comparison of Preterm and Term Breast Milk
3.4. Effect of Holder Pasteurization on Breast Milk
3.5. Infant Formula Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatric Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Vass, R.A.; Kemeny, A.; Dergez, T.; Ertl, T.; Reglodi, D.; Jungling, A.; Tamas, A. Distribution of bioactive factors in human milk samples. Int. Breastfeed. J. 2019, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Pediatrics Committee on Nutrition, Section on Breastfeeding, and Committee on Fetus and Newborn. Donor human milk for the high-risk infant: Preparation, safety, and usage options in the United States. Pediatrics 2017, 139, e20163440. [Google Scholar] [CrossRef] [PubMed]
- Escuder-Vieco, D.; Espinosa-Martos, I.; Rodríguez, J.M.; Fernández, L.; Pallás-Alonso, C.R. Effect of HTST and Holder Pasteurization on the concentration of immunoglobulins, growth factors, and hormones in donor human milk. Front. Immunol. 2018, 9, 2222. [Google Scholar] [CrossRef] [PubMed]
- Vass, R.A.; Kiss, G.; Bell, E.F.; Roghair, R.D.; Miseta, A.; Bódis, J.; Funke, S.; Ertl, T. Breast milk for term and preterm infants-own mother’s milk or donor milk? Nutrients 2021, 13, 424. [Google Scholar] [CrossRef]
- de Zegher, F.; Pernasetti, F.; Vanhole, C.; Devlieger, H.; Van den Berghe, G.; Martial, J.A. The prenatal role of thyroid hormone evidenced by fetomaternal Pit-1 deficiency. J. Clin. Endocrinol. Metab. 1995, 80, 3127–3130. [Google Scholar]
- Cao, X.Y.; Jiang, X.M.; Dou, Z.H.; Rakeman, M.A.; Zhang, M.L.; O’Donnell, K.; Ma, T.; Amette, K.; DeLong, N.; DeLong, G.R. Timing of vulnerability of the brain to iodine deficiency in endemic cretinism. N. Engl. J. Med. 1994, 331, 1739–1744. [Google Scholar] [CrossRef]
- Haddow, J.E.; Palomaki, G.E.; Allan, W.C.; Williams, J.R.; Knight, G.J.; Gagnon, J.; O’Heir, C.E.; Mitchell, M.L.; Hermos, R.J.; Waisbren, S.E.; et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N. Engl. J. Med. 1999, 341, 549–555. [Google Scholar] [CrossRef]
- Richard, K.; Hume, R.; Kaptein, E.; Sanders, J.P.; van Toor, H.; De Herder, W.W.; den Hollander, J.C.; Krenning, E.P.; Visser, T.J. Ontogeny of iodothyronine deiodinases in human liver. J. Clin. Endocrinol. Metab. 1998, 83, 2868–2874. [Google Scholar] [CrossRef][Green Version]
- Kaluarachchi, D.C.; Colaizy, T.T.; Pesce, L.M.; Tansey, M.; Klein, J.M. Congenital hypothyroidism with delayed thyroid-stimulating hormone elevation in premature infants born at less than 30 weeks gestation. J. Perinatol. 2017, 37, 277–282. [Google Scholar] [CrossRef]
- Laukkarinen, J.; Sand, J.; Saaristo, R.; Salmi, J.; Turjanmaa, V.; Vehkalahti, P.; Nordback, I. Is bile flow reduced in patients with hypothyroidism? Surgery 2003, 133, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Cosmi, E.V. Prenatal prevention of respiratory distress syndrome: New pharmacologic approaches. Early Hum. Dev. 1992, 29, 283–286. [Google Scholar] [CrossRef]
- Chopra, I.J.; Crandall, B.F. Thyroid hormones and thyrotropin in amniotic fluid. N. Engl. J. Med. 1975, 293, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Sugio, S.; Kashima, A.; Mochizuki, S.; Noda, M.; Kobayashi, K. Crystal structure of human serum albumin at 2.5 A resolution. Protein Eng. 1999, 12, 439–446. [Google Scholar] [CrossRef]
- Vass, R.A.; Bell, E.F.; Colaizy, T.T.; Schmelzel, M.L.; Johnson, K.J.; Walker, J.R.; Ertl, T.; Roghair, R.D. Hormone levels in pre-term and donor human milk before and after Holder pasteurization. Pediatr. Res. 2020, 88, 612–617. [Google Scholar] [CrossRef]
- Vass, R.A.; Roghair, R.D.; Bell, E.F.; Colaizy, T.T.; Schmelzel, M.L.; Johnson, K.J.; Walker, J.R.; Ertl, T. Pituitary glycoprotein hormones in human milk before and after pasteurization or refrigeration. Nutrients 2020, 12, 687. [Google Scholar] [CrossRef]
- Gregory, K.E.; Walker, W.A. Immunologic factors in human milk and disease prevention in the preterm infant. Curr. Pediatrics Rep. 2013, 1, 222–228. [Google Scholar] [CrossRef]
- Fisher, D.A. Thyroid function in premature infants. The hypothyroxinemia of prematurity. Clin. Perinatol. 1998, 25, 999–1014. [Google Scholar] [CrossRef]
- Lanciotti, L.; Cofini, M.; Leonardi, A.; Penta, L.; Esposito, S. Up-to-date review about minipuberty and overview on hypothalamic-pituitary-gonadal axis activation in fetal and neonatal life. Front Endocrinol. 2018, 9, 410. [Google Scholar] [CrossRef]
- Reuss, M.L.; Levinton, A.; Paneth, N.; Susser, M. Thyroxine values from newborn screening of 919 infants born before 29 week’s gestation. Am. J. Publ. Health 1997, 87, 1693–1697. [Google Scholar] [CrossRef]
- Ares, S.; Escobar-Morreale, H.F.; Quero, J.; Durán, S.; Presas, M.J.; Herruzo, R.; Morreale de Escobar, G. Neonatal hypothyroxinemia: Effects of iodine intake and premature birth. J. Clin. Endocrinol. Metab. 1997, 82, 1704–1712. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hays, M.T. Thyroid hormone and the gut. Endocr. Res. 1988, 14, 203–224. [Google Scholar] [CrossRef] [PubMed]
- Sack, J.; Amado, O.; Lunenfeld, B. Thyroxine concentration in human milk. J. Clin. Endocrinol. Metab. 1977, 45, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Vulsma, T.; Gons, M.H.; de Vijlder, J.J. Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect or thyroid agenesis. N. Engl. J. Med. 1989, 321, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Gushurst, C.A.; Mueller, J.A.; Green, J.A.; Sedor, F. Breast milk iodide: Reassessment in the 1980s. Pediatrics 1984, 73, 354–357. [Google Scholar] [CrossRef]
- Wirth, E.K.; Schweizer, U.; Köhrle, J. Transport of thyroid hormone in brain. Front. Endocrinol. 2014, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- van Wassenaer, A.G.; Kok, J.H.; Dekker, F.W.; de Vijlder, J.J. Thyroid function in very preterm infants: Influences of gestational age and disease. Pediatr. Res. 1997, 42, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Suzuki, Y. Presence of triiodothyronine, no detectable thyroxine and reverse triiodothyronine in human milk. Endocrinol. Jpn. 1979, 26, 507–513. [Google Scholar] [CrossRef]
- Mizuta, H.; Amino, N.; Ichihara, K.; Harada, T.; Nose, O.; Tanizawa, O.; Miyai, K. Thyroid hormones in human milk and their influence on thyroid function of breast-fed babies. Pediatric Res. 1983, 17, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.; Pasquier, S.; Carasso, A.; Von Muralt, G. Postnatal thyroxine administration for idiopathic respiratory distress syndrome in preterm infants. Horm. Res. 1988, 29, 27–30. [Google Scholar] [CrossRef]
- Ng, S.M.; Turner, M.A.; Weindling, A.M. Neurodevelopmental outcomes at 42 months after thyroxine supplementation in infants below 28 weeks’ gestation: A randomized controlled trial. Thyroid 2020, 30, 948–954. [Google Scholar] [CrossRef]
- Schömig, C.S.; Robinson, M.È.; von Oettingen, J.E. Treatment of congenital hypothyroidism in a newborn with malabsorption after subtotal ileum resection. Endocrinol. Diabetes Metab. Case Rep. 2018, 17, 0156. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yu, Z.; Li, Q.; Zhou, J.; Yin, X.; Ma, Y.; Yin, Y.; Jiang, S.; Zhu, R.; Wu, Y.; et al. Dose-dependent effect of human milk on bronchopulmonary dysplasia in very low birth weight infants. BMC Pediatrics 2020, 20, 522. [Google Scholar] [CrossRef] [PubMed]
- Bertino, E.; Peila, C.; Cresi, F.; Maggiora, E.; Sottemano, S.; Gazzolo, D.; Arslanoglu, S.; Coscia, A. Donor human milk: Effects of storage and heat treatment on oxidative stress markers. Front. Pediatrics 2018, 6, 253. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, T.; Kiyosawa, I.; Fukuwatari, Y.; Kitayama, T.; Uechi, M. Alpha-lactalbumin and serum albumin in human milk. J. Dairy Sci. 1973, 56, 177–180. [Google Scholar] [CrossRef]
| Preterm Maternal | Term Maternal | Donor | |
|---|---|---|---|
| Number | 15 | 16 | 44 |
| Maternal age (years) | 31.7 ± 1.1 | 32.1 ± 2.7 | 32.4 ± 0.5 |
| Gestational age (weeks) | 31.4 ± 2.1 | 39.6 ± 0.5 | 39.5 ± 0.2 |
| Maternal BMI | 27.8 ± 0.2 | 26.9 ± 0.5 | 26.3 ± 1.9 |
| Gender of newborn | |||
| Female | 7 | 9 | 19 |
| Male | 8 | 7 | 25 |
| Delivery | |||
| Natural | 4 | 11 | 27 |
| Cesarean section | 11 | 5 | 17 |
| Analyte | 1st and 2nd Months | 3rd–6th Months | p-Value |
|---|---|---|---|
| TSH, nU/L | 23.2 ± 2.2 | 16.2 ± 1.8 | 0.0335 |
| Thyroxine, nmol/L | 842.2 ± 158.8 | 595.7 ± 49.2 | 0.0486 |
| Albumin, mg/L | 349.9 ± 34.1 | 318.3 ± 19.4 | 0.3919 |
| Analyte | Preterm (n = 90) | Term (n = 96) | p-Value |
|---|---|---|---|
| TSH, nU/L | 18.4 ± 1.4 | 24.7 ± 2.8 | 0.0959 |
| Thyroxine, nmol/L | 671.6 ± 61.2 | 11,245.5 ± 73.8 | <0.0001 |
| Albumin, mg/L | 328.6 ± 17.1 | 264.2 ± 6.8 | 0.0041 |
| Analyte | Raw | HoP | p-Value |
|---|---|---|---|
| TSH, nU/L | 20.6 ± 3.3 | 5.4 ± 0.6 | <0.0001 |
| Thyroxine, nmol/L | 640.1 ± 32.4 | 506.1 ± 11.2 | 0.0072 |
| Albumin, mg/L | 289.1 ± 4.6 | 224.1 ± 5.1 | 0.0028 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vass, R.A.; Kiss, G.; Bell, E.F.; Miseta, A.; Bódis, J.; Funke, S.; Bokor, S.; Molnár, D.; Kósa, B.; Kiss, A.A.; et al. Thyroxine and Thyroid-Stimulating Hormone in Own Mother’s Milk, Donor Milk, and Infant Formula. Life 2022, 12, 584. https://doi.org/10.3390/life12040584
Vass RA, Kiss G, Bell EF, Miseta A, Bódis J, Funke S, Bokor S, Molnár D, Kósa B, Kiss AA, et al. Thyroxine and Thyroid-Stimulating Hormone in Own Mother’s Milk, Donor Milk, and Infant Formula. Life. 2022; 12(4):584. https://doi.org/10.3390/life12040584
Chicago/Turabian StyleVass, Réka A., Gabriella Kiss, Edward F. Bell, Attila Miseta, József Bódis, Simone Funke, Szilvia Bokor, Dénes Molnár, Balázs Kósa, Anna A. Kiss, and et al. 2022. "Thyroxine and Thyroid-Stimulating Hormone in Own Mother’s Milk, Donor Milk, and Infant Formula" Life 12, no. 4: 584. https://doi.org/10.3390/life12040584
APA StyleVass, R. A., Kiss, G., Bell, E. F., Miseta, A., Bódis, J., Funke, S., Bokor, S., Molnár, D., Kósa, B., Kiss, A. A., Takács, T., Dombai, F., & Ertl, T. (2022). Thyroxine and Thyroid-Stimulating Hormone in Own Mother’s Milk, Donor Milk, and Infant Formula. Life, 12(4), 584. https://doi.org/10.3390/life12040584