Quercetin Abrogates Oxidative Neurotoxicity Induced by Silver Nanoparticles in Wistar Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
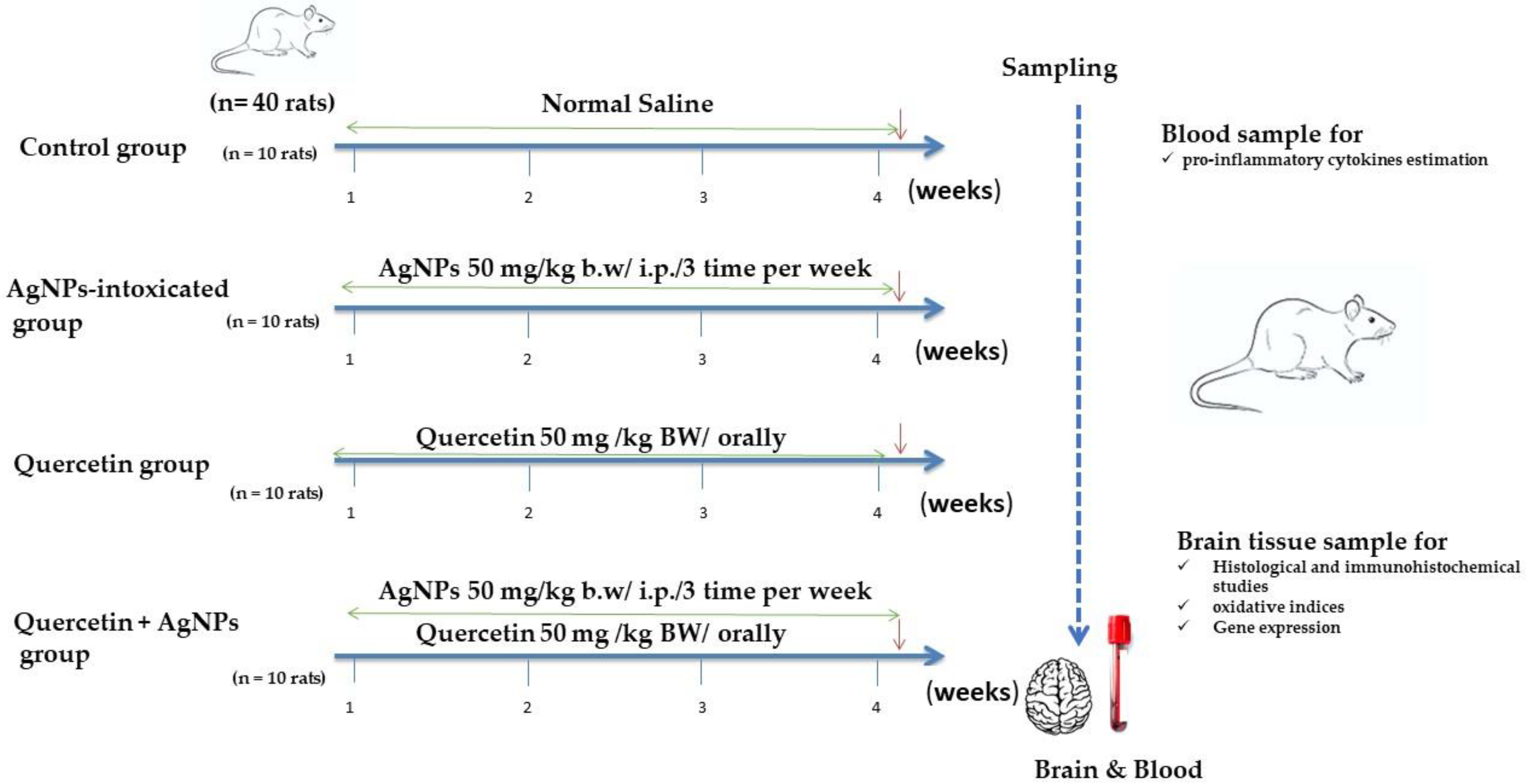
2.2. Experimental Design
2.3. Antioxidant and Lipid Peroxidation Analysis
2.4. Real-Time Polymerase Chain Reaction (RT-PCR)
2.5. Histopathological Examination and Scoring System
2.6. Immunohistochemical Studies
2.7. Morphometric Investigation
2.8. Statistical Analysis
3. Results
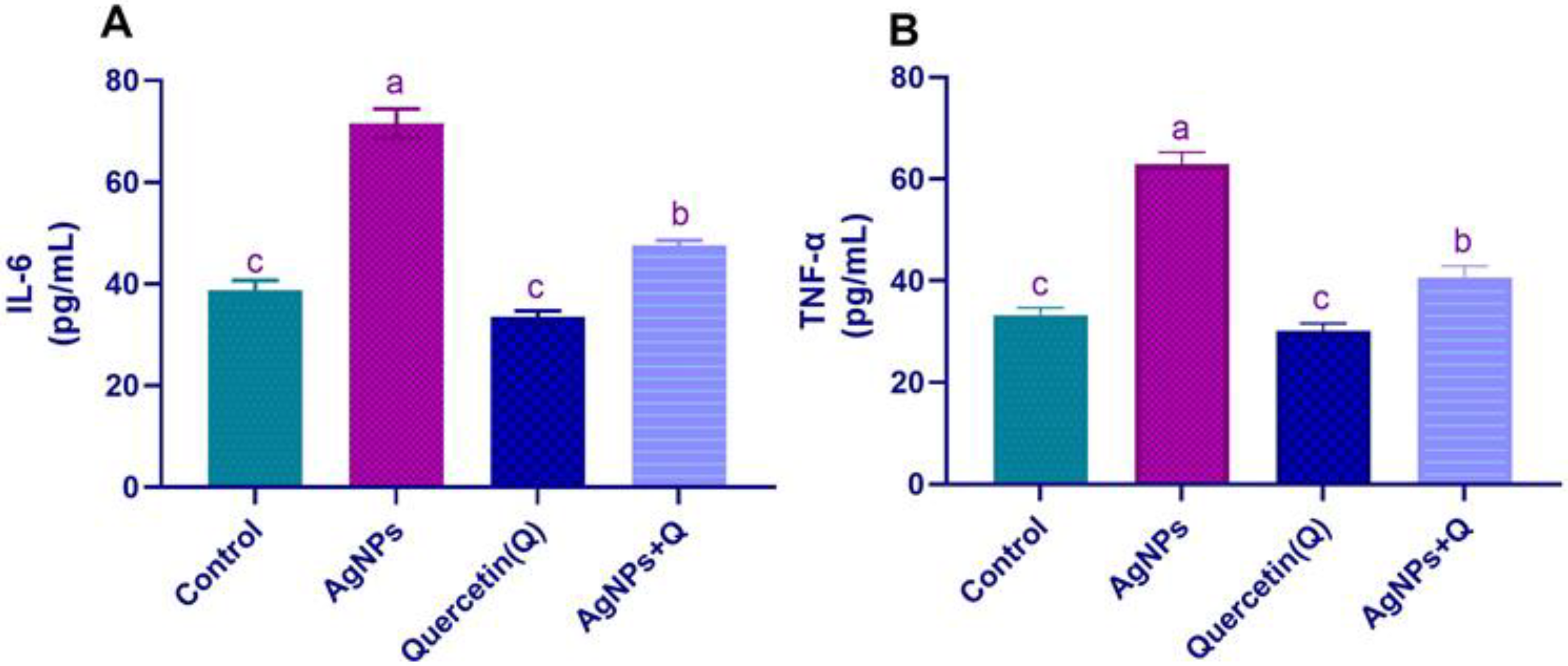
3.1. Serum Pro-inflammatory Cytokines
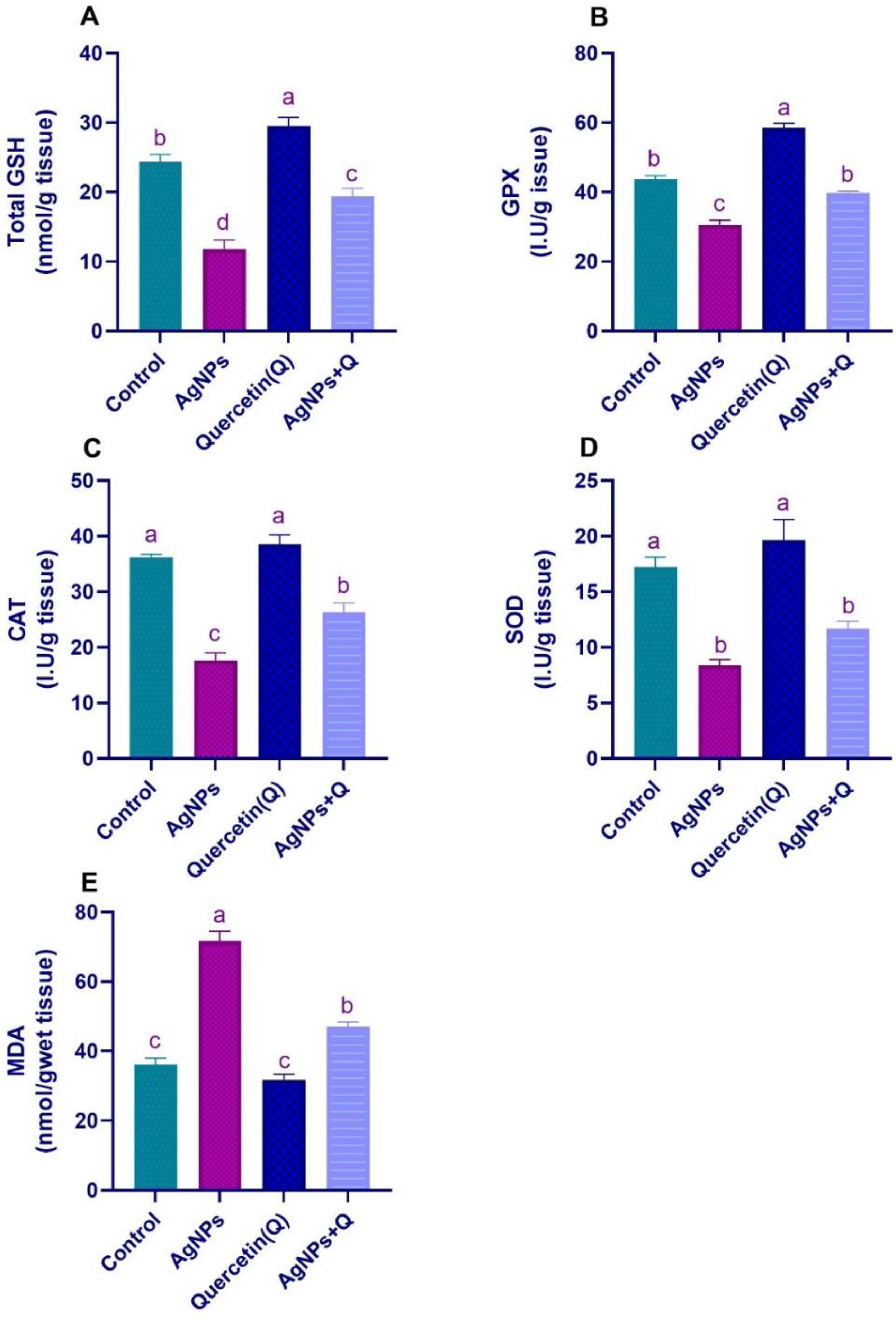
3.2. Brain Oxidative/Antioxidative Indices
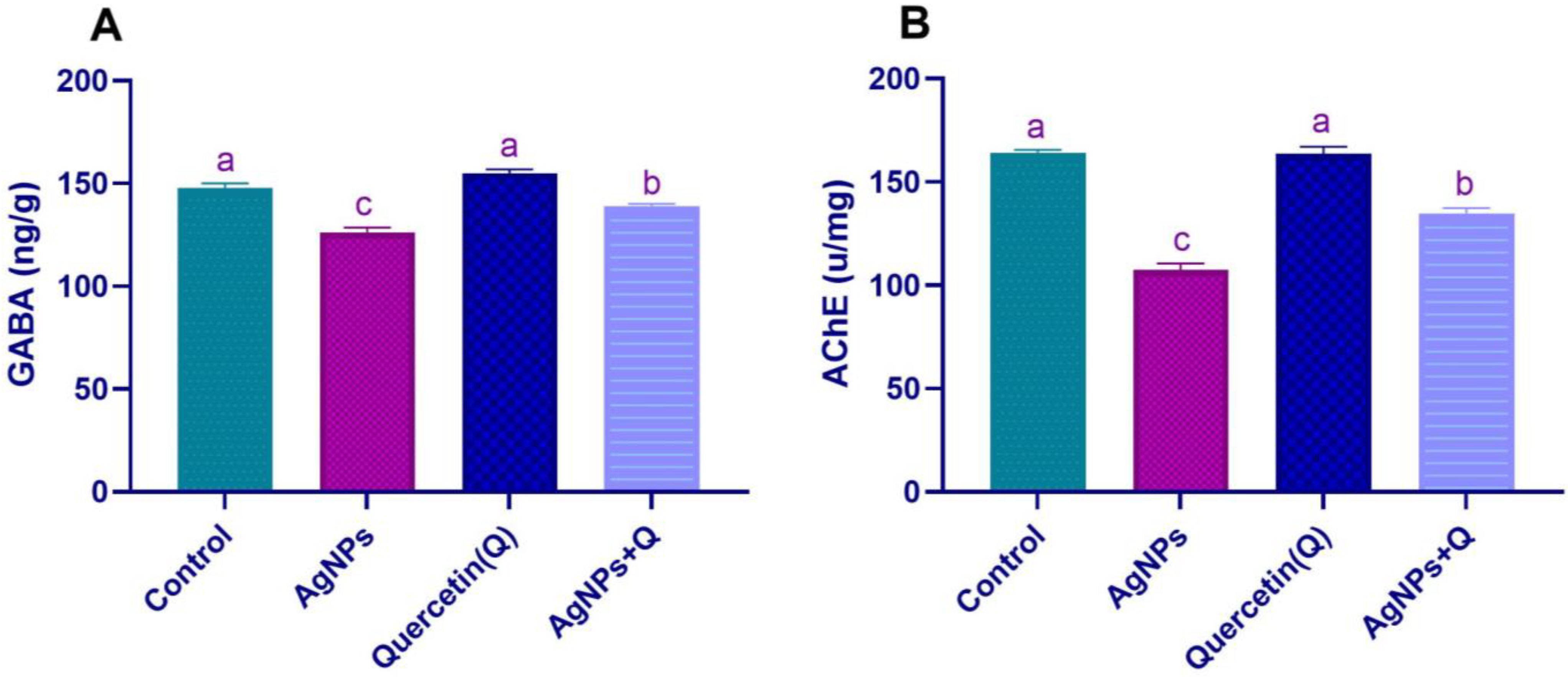
3.3. Brain Neurotransmission
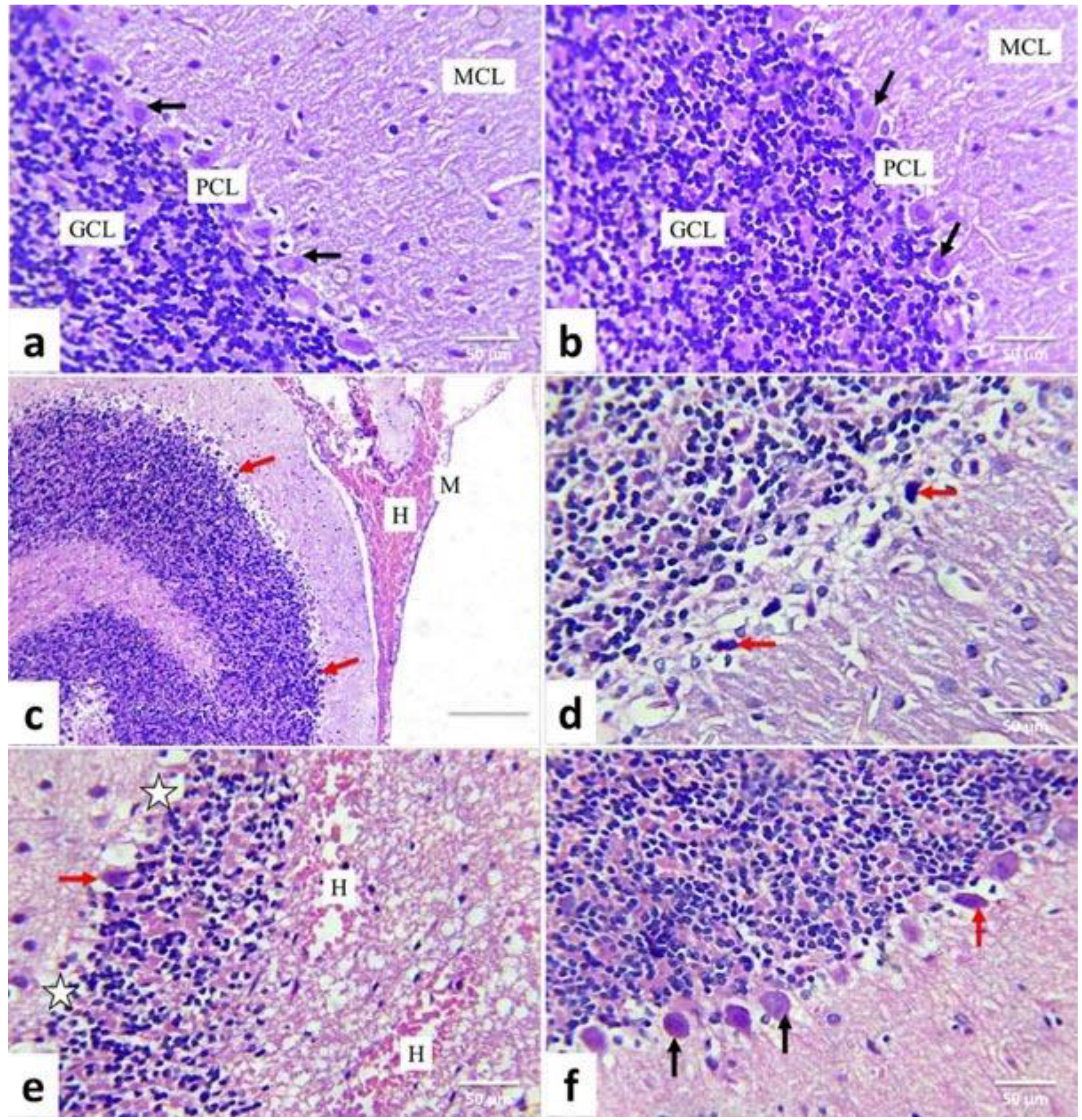
3.4. Lesion Grading and Histopathological Findings
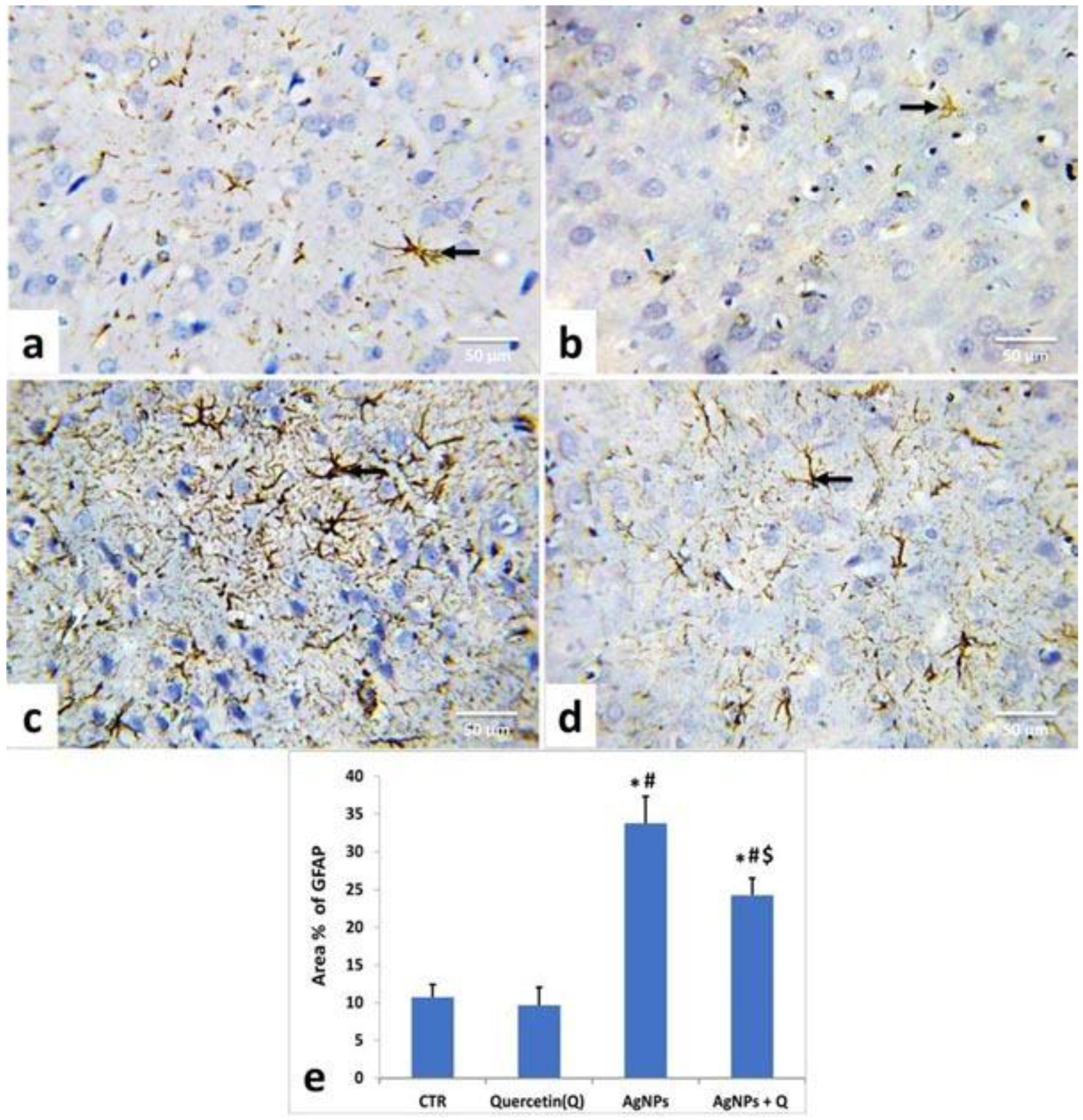
3.5. Immunohistochemical Analysis and Quantitative Assessment
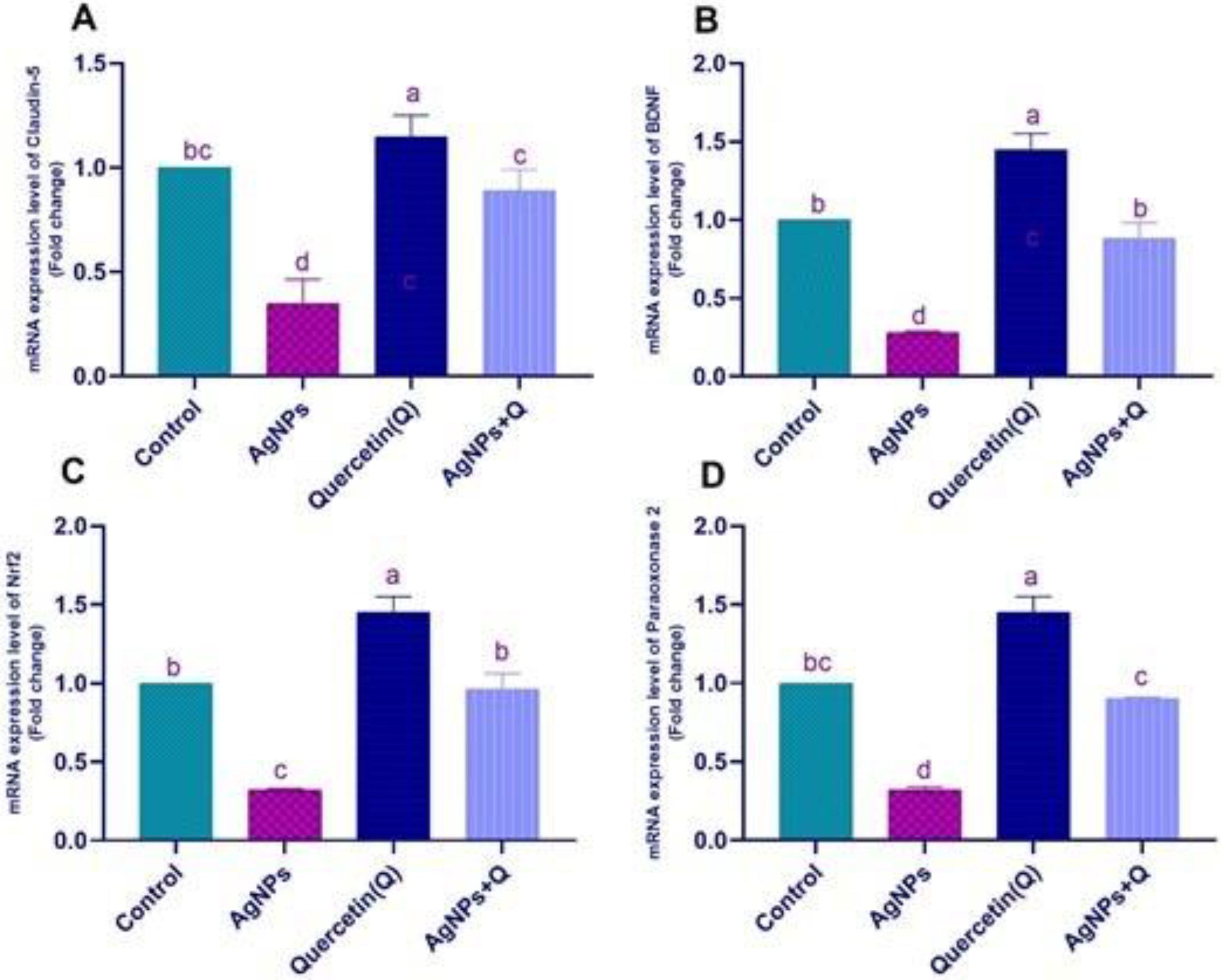
3.6. Effects of Quercetin and/or AgNP on Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, X.; Chen, A.; Zhang, Y.; Wang, J.; Shao, L.; Wei, L. Central nervous system toxicity of metallic nanoparticles. Int. J. Nanomed. 2015, 10, 4321–4340. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, M.; Ferraz, M.P.; Monteiro, F.J.; Fernandes, M.H.; Beppu, M.M.; Mantione, D.; Sardon, H. Antibacterial silk fibroin/nanohydroxyapatite hydrogels with silver and gold nanoparticles for bone regeneration. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Son, M.Y.; Choi, M.S.; Kim, S.; Choi, A.Y.; Lee, H.A.; Kim, K.S.; Kim, J.; Song, C.W.; Yoon, S. Integrative analysis of genes and miRNA alterations in human embryonic stem cells-derived neural cells after exposure to silver nanoparticles. Toxicol. Appl. Pharmacol. 2016, 299, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xiong, L.; Wang, S.; Wang, J.; Liu, L.; Li, J.; Yuan, F.; Xi, T. Distribution, translocation and accumulation of silver nanoparticles in rats. J. Nanosci. Nanotechnol. 2009, 9, 4924–4932. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, S.; Ma, J.; Qu, G.; Wang, X.; Yu, S.; He, J.; Liu, J.; Xia, T.; Jiang, G.B. Silver nanoparticles induced RNA polymerase-silver binding and RNA transcription inhibition in erythroid progenitor cells. ACS Nano 2013, 7, 4171–4186. [Google Scholar] [CrossRef]
- Liu, Z.; Ren, G.; Zhang, T.; Yang, Z. Action potential changes associated with the inhibitory effects on voltage-gated sodium current of hippocampal CA1 neurons by silver nanoparticles. Toxicology 2009, 264, 179–184. [Google Scholar] [CrossRef]
- Johnston, H.J.; Hutchison, G.; Christensen, F.M.; Peters, S.; Hankin, S.; Stone, V. A review of the in vivo and in vitro toxicity of silver and gold particulates: Particle attributes and biological mechanisms responsible for the observed toxicity. Crit. Rev. Toxicol. 2010, 40, 328–346. [Google Scholar] [CrossRef]
- Sharma, H.S.; Ali, S.F.; Tian, Z.R.; Hussain, S.M.; Schlager, J.J.; Sjöquist, P.O.; Sharma, A.; Muresanu, D.F. Chronic treatment with nanoparticles exacerbate hyperthermia induced blood-brain barrier breakdown, cognitive dysfunction and brain pathology in the rat. Neuroprotective effects of nanowired-antioxidant compound H-290/51. J. Nanosci. Nanotechnol. 2009, 9, 5073–5090. [Google Scholar] [CrossRef]
- Xu, L.; Shao, A.; Zhao, Y.; Wang, Z.; Zhang, C.; Sun, Y.; Deng, J.; Chou, L.L. Neurotoxicity of Silver Nanoparticles in Rat Brain After Intragastric Exposure. J. Nanosci. Nanotechnol. 2015, 15, 4215–4223. [Google Scholar] [CrossRef]
- Elblehi, S.S.; El Euony, O.I.; El-Sayed, Y.S. Apoptosis and astrogliosis perturbations and expression of regulatory inflammatory factors and neurotransmitters in acrylamide-induced neurotoxicity under ω3 fatty acids protection in rats. Neurotoxicology 2020, 76, 44–57. [Google Scholar] [CrossRef]
- Bagheri-Abassi, F.; Alavi, H.; Mohammadipour, A.; Motejaded, F.; Ebrahimzadeh-Bideskan, A. The effect of silver nanoparticles on apoptosis and dark neuron production in rat hippocampus. Iran. J. Basic Med. Sci. 2015, 18, 644–648. [Google Scholar] [PubMed]
- Rahman, M.F.; Wang, J.; Patterson, T.A.; Saini, U.T.; Robinson, B.L.; Newport, G.D.; Murdock, R.C.; Schlager, J.J.; Hussain, S.M.; Ali, S.F. Expression of genes related to oxidative stress in the mouse brain after exposure to silver-25 nanoparticles. Toxicol. Lett. 2009, 187, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Xiong, L.; Zhou, G.; Wang, S.; Wang, J.; Liu, L.; Li, J.; Yuan, F.; Lu, S.; Wan, Z.; et al. Silver nanoparticles crossing through and distribution in the blood-brain barrier in vitro. J. Nanosci. Nanotechnol. 2010, 10, 6313–6317. [Google Scholar] [CrossRef] [PubMed]
- Terao, J.; Murota, K.; Kawai, Y. Conjugated quercetin glucuronides as bioactive metabolites and precursors of aglycone in vivo. Food Funct. 2011, 2, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Moghbelinejad, S.; Alizadeh, S.; Mohammadi, G.; Khodabandehloo, F.; Rashvand, Z.; Najafipour, R.; Nassiri-Asl, M. The effects of quercetin on the gene expression of the GABA(A) receptor α5 subunit gene in a mouse model of kainic acid-induced seizure. J. Physiol. Sci. 2017, 67, 339–343. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Hu, J.; Zhong, L.; Wang, N.; Yang, L.; Liu, C.C.; Li, H.; Wang, X.; Zhou, Y.; Zhang, Y.; et al. Quercetin stabilizes apolipoprotein E and reduces brain Aβ levels in amyloid model mice. Neuropharmacology 2016, 108, 179–192. [Google Scholar] [CrossRef]
- Chakraborty, J.; Singh, R.; Dutta, D.; Naskar, A.; Rajamma, U.; Mohanakumar, K.P. Quercetin improves behavioral deficiencies, restores astrocytes and microglia, and reduces serotonin metabolism in 3-nitropropionic acid-induced rat model of Huntington’s Disease. CNS Neurosci. Ther. 2014, 20, 10–19. [Google Scholar] [CrossRef]
- Nassiri-Asl, M.; Hajiali, F.; Taghiloo, M.; Abbasi, E.; Mohseni, F.; Yousefi, F. Comparison between the effects of quercetin on seizure threshold in acute and chronic seizure models. Toxicol. Ind. Health 2016, 32, 936–944. [Google Scholar] [CrossRef]
- Abd El-Maksoud, E.M.; Lebda, M.A.; Hashem, A.E.; Taha, N.M.; Kamel, M.A. Correction to: Ginkgo biloba mitigates silver nanoparticles-induced hepatotoxicity in Wistar rats via improvement of mitochondrial biogenesis and antioxidant status. Environ. Sci. Pollut. Res. Int. 2019, 26, 31552–31553. [Google Scholar] [CrossRef] [Green Version]
- Kale, A.; Piskin, Ö.; Bas, Y.; Aydin, B.G.; Can, M.; Elmas, Ö.; Büyükuysal, Ç. Neuroprotective effects of Quercetin on radiation-induced brain injury in rats. J. Radiat. Res. 2018, 59, 404–410. [Google Scholar] [CrossRef] [Green Version]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Jolitha, A.B.; Subramanyam, M.V.; Asha Devi, S. Modification by vitamin E and exercise of oxidative stress in regions of aging rat brain: Studies on superoxide dismutase isoenzymes and protein oxidation status. Exp. Gerontol. 2006, 41, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Chiang, A.N.; Wu, H.L.; Yeh, H.I.; Chu, C.S.; Lin, H.C.; Lee, W.C. Antioxidant effects of black rice extract through the induction of superoxide dismutase and catalase activities. Lipids 2006, 41, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D.J.m. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxidative Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef] [Green Version]
- Ataie, A.; Shadifar, M.; Ataee, R. Polyphenolic Antioxidants and Neuronal Regeneration. Basic Clin. Neurosci. 2016, 7, 81–90. [Google Scholar] [CrossRef]
- Wang, Z.; Xia, T.; Liu, S. Mechanisms of nanosilver-induced toxicological effects: More attention should be paid to its sublethal effects. Nanoscale 2015, 7, 7470–7481. [Google Scholar] [CrossRef] [Green Version]
- Selvakumar, K.; Bavithra, S.; Krishnamoorthy, G.; Arunakaran, J.J.I.t. Impact of quercetin on tight junctional proteins and BDNF signaling molecules in hippocampus of PCBs-exposed rats. Interdiscip. Toxicol. 2018, 11, 294–305. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Liu, Z.; Allaker, R.; Reip, P.; Oxford, J.; Ahmad, Z.; Ren, G. A review of nanoparticle functionality and toxicity on the central nervous system. J. R. Soc. Interface 2010, 7, S411–S422. [Google Scholar] [CrossRef] [PubMed]
- Enerson, B.E.; Drewes, L.R. The rat blood—brain barrier transcriptome. J. Cereb. Blood Flow Metab. 2006, 26, 959–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martirosyan, A.; Bazes, A.; Schneider, Y.J. In vitro toxicity assessment of silver nanoparticles in the presence of phenolic compounds--preventive agents against the harmful effect? Nanotoxicology 2014, 8, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Gurunathan, S.; Choi, Y.-J.; Han, J.W.; Song, H.; Kim, J.-H. Silver nanoparticles suppresses brain-derived neurotrophic factor-induced cell survival in the human neuroblastoma cell line SH-SY5Y. J. Ind. Eng. Chem. 2017, 47, 62–73. [Google Scholar] [CrossRef]
- Jin, Z.; Ke, J.; Guo, P.; Wang, Y.; Wu, H. Quercetin improves blood-brain barrier dysfunction in rats with cerebral ischemia reperfusion via Wnt signaling pathway. Am. J. Transl. Res. 2019, 11, 4683. [Google Scholar]
- Hu, R.; Xu, C.; Shen, G.; Jain, M.R.; Khor, T.O.; Gopalkrishnan, A.; Lin, W.; Reddy, B.; Chan, J.Y.; Kong, A.-N.T. Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Cancer Lett. 2006, 243, 170–192. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, J.; Zhang, X.; Dai, G. Quercetin improves rat brain function after ischemia by regulating DJ-1/Nrf2. Int. J. Clin. Exp. Med. 2019, 12, 8799–8806. [Google Scholar]
- Pereira, R.R.; Abreu, I.C.M.E.d.; Guerra, J.F.d.C.; Lage, N.N.; Lopes, J.M.M.; Silva, M.; Lima, W.G.d.; Silva, M.E.; Pedrosa, M.L. Açai (Euterpe oleracea Mart.) upregulates paraoxonase 1 gene expression and activity with concomitant reduction of hepatic steatosis in high-fat diet-fed rats. Oxidative Med. Cell. Longev. 2016, 2016, 8379105. [Google Scholar] [CrossRef] [Green Version]
- Giordano, G.; Cole, T.B.; Furlong, C.E.; Costa, L.G. Paraoxonase 2 (PON2) in the mouse central nervous system: A neuroprotective role? Toxicol. Appl. Pharmacol. 2011, 256, 369–378. [Google Scholar] [CrossRef] [Green Version]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C.J.O. Mechanisms of neuroprotection by quercetin: Counteracting oxidative stress and more. Oxidative Med. Cell. Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef] [Green Version]
- Murphy, A.; Casey, A.; Byrne, G.; Chambers, G.; Howe, O.J. Silver nanoparticles induce pro-inflammatory gene expression and inflammasome activation in human monocytes. J. Appl. Toxicol. 2016, 36, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Elkhateeb, S.; Mahmoud, A.; Ibrahem, N. Protective Role of Quercetin in Hippocampus Inflammatory and Oxidative Damage Induced By Monosodium Glutamate in Rats. Mansoura J. Forensic Med. Clin. Toxicol. 2020, 28, 15–31. [Google Scholar] [CrossRef]
- Myrzakhanova, M.; Gambardella, C.; Falugi, C.; Gatti, A.M.; Tagliafierro, G.; Ramoino, P.; Bianchini, P.; Diaspro, A. Effects of nanosilver exposure on cholinesterase activities, CD41, and CDF/LIF-like expression in zebrafish (Danio rerio) larvae. BioMed Res. Int. 2013, 2013, 205183. [Google Scholar] [CrossRef] [Green Version]
- Streeter, C.C.; Gerbarg, P.L.; Saper, R.B.; Ciraulo, D.A.; Brown, R.P. Effects of yoga on the autonomic nervous system, gamma-aminobutyric-acid, and allostasis in epilepsy, depression, and post-traumatic stress disorder. Med. Hypotheses 2012, 78, 571–579. [Google Scholar] [CrossRef]
- Jakaria, M.; Azam, S.; Jo, S.H.; Kim, I.S.; Dash, R.; Choi, D.K. Potential Therapeutic Targets of Quercetin and Its Derivatives: Its Role in the Therapy of Cognitive Impairment. J. Clin. Med. 2019, 8, 1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, M.M.; Hussein, M.M.A. Neurotoxic effects of silver nanoparticles and the protective role of rutin. Biomed. Pharmacother. 2017, 90, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, E.K.; El-Sherbiny, M.; Said, E.; Elkattawy, H.A.; Qushawy, M.; Elsherbiny, N. Tranilast ameliorated subchronic silver nanoparticles-induced cerebral toxicity in rats: Effect on TLR4/NLRP3 and Nrf-2. Neurotoxicology 2021, 82, 167–176. [Google Scholar] [CrossRef]
- Ghooshchian, M.; Khodarahmi, P.; Tafvizi, F. Apoptosis-mediated neurotoxicity and altered gene expression induced by silver nanoparticles. Toxicol. Ind. Health 2017, 33, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.G.; Castro-Aceituno, V.; Abbai, R.; Lee, H.A.; Simu, S.Y.; Han, Y.; Hurh, J.; Kim, Y.J.; Yang, D.C. Caspase-3/MAPK pathways as main regulators of the apoptotic effect of the phyto-mediated synthesized silver nanoparticle from dried stem of Eleutherococcus senticosus in human cancer cells. Biomed. Pharmacother. 2018, 99, 128–133. [Google Scholar] [CrossRef]
- Trickler, W.J.; Lantz, S.M.; Murdock, R.C.; Schrand, A.M.; Robinson, B.L.; Newport, G.D.; Schlager, J.J.; Oldenburg, S.J.; Paule, M.G.; Slikker, W., Jr.; et al. Silver nanoparticle induced blood-brain barrier inflammation and increased permeability in primary rat brain microvessel endothelial cells. Toxicol. Sci. 2010, 118, 160–170. [Google Scholar] [CrossRef] [Green Version]
- Yin, N.; Liu, Q.; Liu, J.; He, B.; Cui, L.; Li, Z.; Yun, Z.; Qu, G.; Liu, S.; Zhou, Q.; et al. Silver nanoparticle exposure attenuates the viability of rat cerebellum granule cells through apoptosis coupled to oxidative stress. Small 2013, 9, 1831–1841. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Mahmood, M.; Xu, Y.; Watanabe, F.; Biris, A.S.; Hansen, D.K.; Inselman, A.; Casciano, D.; Patterson, T.A.; Paule, M.G.; et al. Effects of silver nanoparticles on human and rat embryonic neural stem cells. Front. Neurosci. 2015, 9, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hadrup, N.; Loeschner, K.; Mortensen, A.; Sharma, A.K.; Qvortrup, K.; Larsen, E.H.; Lam, H.R. The similar neurotoxic effects of nanoparticulate and ionic silver in vivo and in vitro. Neurotoxicology 2012, 33, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Cullen, D.K.; Simon, C.M.; LaPlaca, M.C. Strain rate-dependent induction of reactive astrogliosis and cell death in three-dimensional neuronal-astrocytic co-cultures. Brain Res. 2007, 1158, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Eng, L.F.; Ghirnikar, R.S.; Lee, Y.L. Glial fibrillary acidic protein: GFAP-thirty-one years (1969-2000). Neurochem. Res. 2000, 25, 1439–1451. [Google Scholar] [CrossRef]
- Ignarro, R.S.; Vieira, A.S.; Sartori, C.R.; Langone, F.; Rogério, F.; Parada, C.A. JAK2 inhibition is neuroprotective and reduces astrogliosis after quinolinic acid striatal lesion in adult mice. J. Chem. Neuroanat. 2013, 48–49, 14–22. [Google Scholar] [CrossRef]
- Bates, K.A.; Fonte, J.; Robertson, T.A.; Martins, R.N.; Harvey, A.R. Chronic gliosis triggers Alzheimer’s disease-like processing of amyloid precursor protein. Neuroscience 2002, 113, 785–796. [Google Scholar] [CrossRef]
- Yin, N.; Zhang, Y.; Yun, Z.; Liu, Q.; Qu, G.; Zhou, Q.; Hu, L.; Jiang, G. Silver nanoparticle exposure induces rat motor dysfunction through decrease in expression of calcium channel protein in cerebellum. Toxicol. Lett. 2015, 237, 112–120. [Google Scholar] [CrossRef]
- Dąbrowska-Bouta, B.; Sulkowski, G.; Strużyński, W.; Strużyńska, L. Prolonged Exposure to Silver Nanoparticles Results in Oxidative Stress in Cerebral Myelin. Neurotox. Res. 2019, 35, 495–504. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Du, G.; Wu, H.; Gao, X.; Yang, Z.; Liu, B.; Cui, S. Protective effects of quercetin on traumatic brain injury induced inflammation and oxidative stress in cortex through activating Nrf2/HO-1 pathway. Restor. Neurol. Neurosci. 2021, 39, 73–84. [Google Scholar] [CrossRef]
- Gasmi, S.; Rouabhi, R.; Kebieche, M.; Boussekine, S.; Salmi, A.; Toualbia, N.; Taib, C.; Bouteraa, Z.; Chenikher, H.; Henine, S.; et al. Effects of Deltamethrin on striatum and hippocampus mitochondrial integrity and the protective role of Quercetin in rats. Environ. Sci. Pollut. Res. Int. 2017, 24, 16440–16457. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Singla, N.; Dhawan, D.K. Neuro-protective potential of quercetin during chlorpyrifos induced neurotoxicity in rats. Drug Chem. Toxicol. 2019, 42, 220–230. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Malik, M.Y.; Rashid, M.; Singh, S.; Tiwari, V.; Gupta, P.; Shukla, S.; Singh, S.; Wahajuddin, M. Mechanistic exploration of quercetin against metronidazole induced neurotoxicity in rats: Possible role of nitric oxide isoforms and inflammatory cytokines. Neurotoxicology 2020, 79, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Škandík, M.; Mrvová, N.; Bezek, Š.; Račková, L. Semisynthetic quercetin-quinone mitigates BV-2 microglia activation through modulation of Nrf2 pathway. Free Radic. Biol. Med. 2020, 152, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, F.J.; Yang, W.L.; Qiao, H.Z.; Zhang, S.J. Quercetin improves cognitive disorder in aging mice by inhibiting NLRP3 inflammasome activation. Food Funct. 2021, 12, 717–725. [Google Scholar] [CrossRef]
- Wu, B.Y.; Yu, A.C. Quercetin inhibits c-fos, heat shock protein, and glial fibrillary acidic protein expression in injured astrocytes. J. Neurosci. Res. 2000, 62, 730–736. [Google Scholar] [CrossRef]



| Gene | Direction | Sequence | Accession Number |
|---|---|---|---|
| Bax | Sense | GGCGAATTGGCGATGAACTG | NM_017059.2 |
| Antisense | ATGGTTCTGATCAGCTCGGG | ||
| Bcl-2 | Sense | GATTGTGGCCTTCTTTGAGT | NM_016993.1 |
| Antisense | ATAGTTCCACAAAGGCATCC | ||
| Cldn5 | Sense | CGTGACGGCGCAGACGACTT | NM_031701.2 |
| Antisense | TGCACTGAGCGCCGGTCAAG | ||
| BDNF | Sense | TTGCCACAGCCCCAGGTGTGA | NM_012513.3 |
| Antisense | ACGCCTGTCACTGCGCCCTA | ||
| Tnf-α | Sense | GTAGCCCACGTCGTAGCAAAC | NM_012675.3 |
| Antisense | ACCACCAGTTGGTTGTCTTTGA | ||
| Nrf2 | Sense | CCCATTGAGGGCTGTGATCT | NM_031789.2 |
| Antisense | GCCTTCAGTGTGCTTCTGGTT | ||
| IL-1β | Sense | TGACAGACCCCAAAAGATTAAGG | NM_031512.2 |
| Antisense | CTCATCTGGACAGCCCAAGTC | ||
| iNOS | Sense | GTTCCCCCAGCGGAGCGATG | NM_012611.3 |
| Antisense | ACTCGAGGCCACCCACCTCC | ||
| paraoxonase 2 | CGCCACCAATGACCACTACT | NM_001013082 | |
| TTGATCCCGTTGGCTGAGTC | |||
| β actin | Sense | TCCACCCGCGAGTACAACCTTC | NM_031144.2 |
| Antisense | GGGCCACACGCAGCTCATTGTA | ||
| GAPDH | Sense | TCAAGAAGGTGGTGAAGCAG | NM_017008.4 |
| Antisense | AGGTGGAAGAATGGGAGTTG |
| Organ | Lesions | Control | Quercetin | AgNPs | AgNPs + Quercetin |
|---|---|---|---|---|---|
| Cerebral cortex | |||||
| Blood vessels congestion | |||||
| Meninges | — | — | xx | x | |
| Cerebral cortex | — | — | x | x | |
| Neuronal pyknosis | — | — | xxx | xx | |
| Intracellular and extracellular vacuolization | — | — | xx | x | |
| Glial cell reaction | — | — | xxx | xx | |
| Cerebral hemorrhage | — | — | x | — | |
| Perivascular cuffing | — | — | xx | x | |
| Cerebellar cortex | |||||
| Blood vessels congestion | |||||
| Meninges | — | — | xx | x | |
| Granular layer | — | — | x | — | |
| Pyknosis of Purkinje cell | — | — | xxx | xx | |
| Perineural vacuolation | — | — | xx | x | |
| Necrosis of Purkinje cell | — | — | xxx | xx | |
| Loss of Purkinje cells | — | — | xx | x | |
| Depletion of the granule cell layer | — | — | x | — | |
| Hemorrhage | — | — | xx | x | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elblehi, S.S.; Abd El-Maksoud, E.M.; Aldhahrani, A.; Alotaibi, S.S.; Ghamry, H.I.; Elgendy, S.A.; Soliman, M.M.; Shukry, M. Quercetin Abrogates Oxidative Neurotoxicity Induced by Silver Nanoparticles in Wistar Rats. Life 2022, 12, 578. https://doi.org/10.3390/life12040578
Elblehi SS, Abd El-Maksoud EM, Aldhahrani A, Alotaibi SS, Ghamry HI, Elgendy SA, Soliman MM, Shukry M. Quercetin Abrogates Oxidative Neurotoxicity Induced by Silver Nanoparticles in Wistar Rats. Life. 2022; 12(4):578. https://doi.org/10.3390/life12040578
Chicago/Turabian StyleElblehi, Samar S., Eman M. Abd El-Maksoud, Adil Aldhahrani, Saqer S. Alotaibi, Heba I. Ghamry, Salwa A. Elgendy, Mohamed Mohamed Soliman, and Mustafa Shukry. 2022. "Quercetin Abrogates Oxidative Neurotoxicity Induced by Silver Nanoparticles in Wistar Rats" Life 12, no. 4: 578. https://doi.org/10.3390/life12040578
APA StyleElblehi, S. S., Abd El-Maksoud, E. M., Aldhahrani, A., Alotaibi, S. S., Ghamry, H. I., Elgendy, S. A., Soliman, M. M., & Shukry, M. (2022). Quercetin Abrogates Oxidative Neurotoxicity Induced by Silver Nanoparticles in Wistar Rats. Life, 12(4), 578. https://doi.org/10.3390/life12040578









