Superstructure Detection in Nucleosome Distribution Shows Common Pattern within a Chromosome and within the Genome
Abstract
1. Introduction
1.1. Data
1.2. Methods
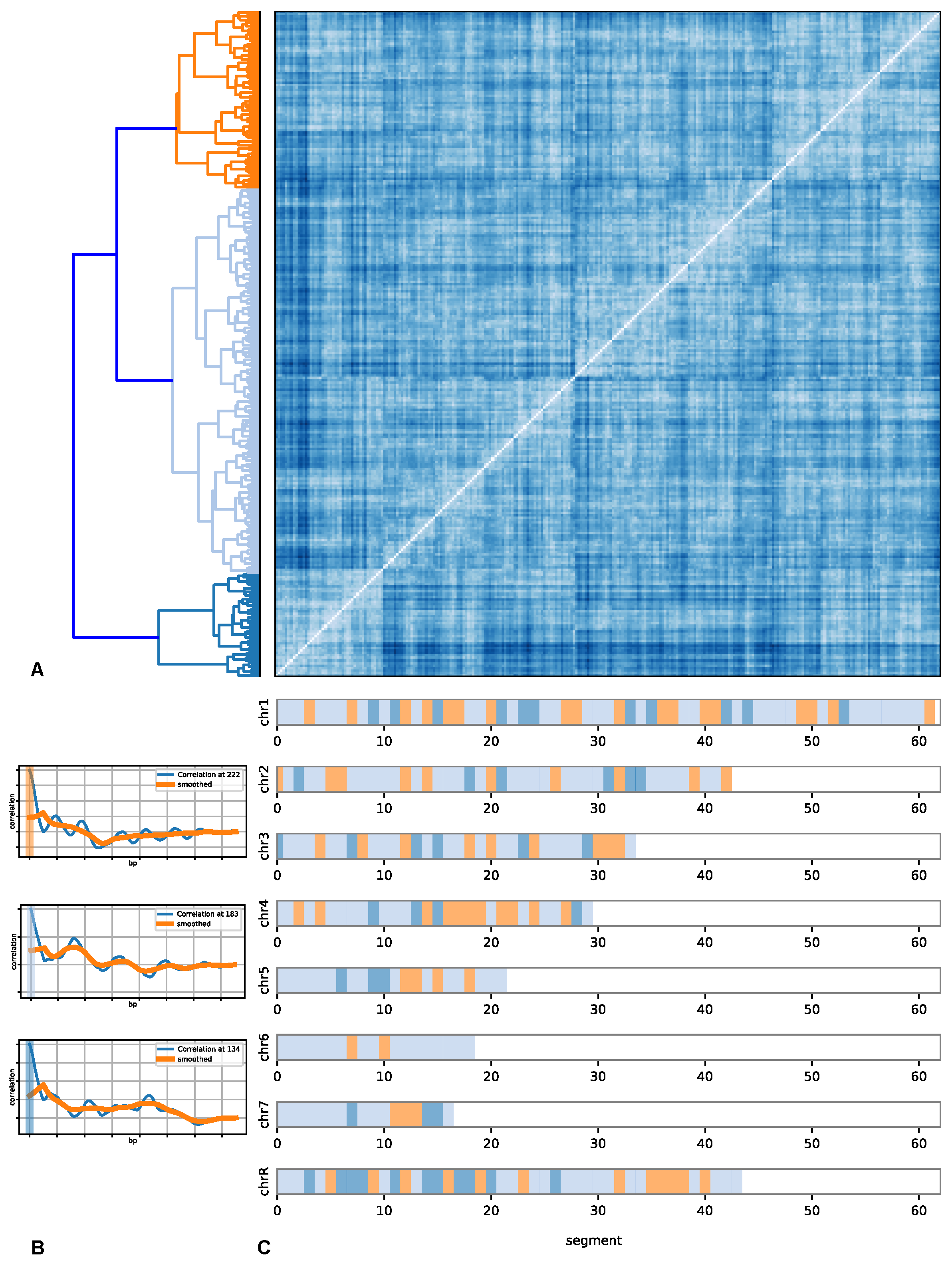
- Each chromosome is divided into segments of 75 kbp of length.
- For every chromosome, the positioning data are coarse-grained.
- The coarse-grained nucleosome positioning data are used to calculate auto-correlation functions over the different sections.
- A distance matrix is calculated over all the auto-correlation function data.
- These segments are clustered. Various distance matrix and clustering algorithms are used to generalize the results.
1.2.1. Genome Section Classification
1.2.2. Coarse Graining
1.2.3. Auto-Correlation Function Calculation
1.2.4. Distance Matrix Calculation
1.2.5. Clustering
1.2.6. Statistical Distributions Fitting
2. Results
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oluwadare, O.; Highsmith, M.; Cheng, J. An Overview of Methods for Reconstructing 3-D Chromosome and Genome Structures from Hi-C Data. Biol. Proced. Online 2019, 21, 7. [Google Scholar] [CrossRef]
- Jerkovic, I.; Cavalli, G. Understanding 3D genome organization by multidisciplinary methods. Nat. Rev. Mol. Cell Biol. 2021, 22, 511–528. [Google Scholar] [CrossRef]
- Routh, A.; Sandin, S.; Rhodes, D. Nucleosome repeat length and linker histone stoichiometry determine chromatin fiber structure. Proc. Natl. Acad. Sci. USA 2008, 105, 8872–8877. [Google Scholar] [CrossRef]
- Bohr, J.; Olsen, K. The size of the nucleosome. arXiv 2012, arXiv:1102.0761. [Google Scholar] [CrossRef]
- Staneva, D.; Georgieva, M.; Miloshev, G. Kluyveromyces lactis genome harbours a functional linker histone encoding gene. FEMS Yeast Res. 2016, 16, fow034. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bohn, M.; Diesinger, P.; Kaufmann, R.; Weiland, Y.; Müller, P.; Gunkel, M.; Ketteler, A.; Lemmer, P.; Hausmann, M.; Heermann, D.; et al. Localization Microscopy Reveals Expression-Dependent Parameters of Chromatin Nanostructure. Biophys. J. 2010, 99, 1358–1367. [Google Scholar] [CrossRef]
- Tchasovnikarova, I.A.; Kingston, R.E. Beyond the Histone Code: A Physical Map of Chromatin States. Mol. Cell 2018, 69, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Struhl, K.; Segal, E. Determinants of nucleosome positioning. Nat. Struct. Mol. Biol. 2013, 20, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, L.; Sato, Y.; Kuznetsova, K.; Bianucci, T.; Kimura, H.; Jülicher, F.; Honigmann, A.; Zaburdaev, V.; Vastenhouw, N.L. Transcription organizes euchromatin via microphase separation. Nat. Commun. 2021, 12, 1360. [Google Scholar] [CrossRef] [PubMed]
- Dekker, J.; Rippe, K.; Dekker, M.; Kleckner, N. Capturing Chromosome Conformation. Science 2002, 295, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- van Berkum, N.L.; Lieberman-Aiden, E.; Williams, L.; Imakaev, M.; Gnirke, A.; Mirny, L.A.; Dekker, J.; Lander, E.S. Hi-C: A method to study the three-dimensional architecture of genomes. J. Vis. Exp. 2010, 6, 1869. [Google Scholar] [CrossRef]
- Beagan, J.A.; Phillips-Cremins, J.E. On the existence and functionality of topologically associating domains. Nat. Genet. 2020, 52, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Ghavi-Helm, Y.; Jankowski, A.; Meiers, S.; Viales, R.R.; Korbel, J.O.; Furlong, E.E.M. Highly rearranged chromosomes reveal uncoupling between genome topology and gene expression. Nat. Genet. 2019, 51, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Nora, E.P.; Lajoie, B.R.; Schulz, E.G.; Giorgetti, L.; Okamoto, I.; Servant, N.; Piolot, T.; van Berkum, N.L.; Meisig, J.; Sedat, J.; et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature 2012, 485, 381–385. [Google Scholar] [CrossRef]
- Quentin, S.; Frédéric, B.; Giacomo, C. Principles of genome folding into topologically associating domains. Sci. Adv. 2022, 5, eaaw1668. [Google Scholar] [CrossRef]
- Wiese, O.; Marenduzzo, D.; Brackley, C.A. Nucleosome positions alone can be used to predict domains in yeast chromosomes. Proc. Natl. Acad. Sci. USA 2019, 116, 17307. [Google Scholar] [CrossRef] [PubMed]
- Kharerin, H.; Bai, L. Thermodynamic modeling of genome-wide nucleosome depleted regions in yeast. PLoS Comput. Biol. 2021, 17, e1008560. [Google Scholar] [CrossRef] [PubMed]
- Drew, H.R.; Travers, A.A. DNA bending and its relation to nucleosome positioning. J. Mol. Biol. 1985, 186, 773–790. [Google Scholar] [CrossRef]
- Chung, H.R.; Vingron, M. Sequence-dependent Nucleosome Positioning. J. Mol. Biol. 2009, 386, 1411–1422. [Google Scholar] [CrossRef]
- Parmar, J.J.; Marko, J.F.; Padinhateeri, R. Nucleosome positioning and kinetics near transcription-start-site barriers are controlled by interplay between active remodeling and DNA sequence. Nucleic Acids Res. 2014, 42, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Angermayr, M.; Oechsner, U.; Bandlow, W. Reb1p-dependent DNA bending effects nucleosome positioning and constitutive transcription at the yeast profilin promoter. J. Biol. Chem. 2003, 278, 17918–17926. [Google Scholar] [CrossRef]
- Rippe, K.; Schrader, A.; Riede, P.; Strohner, R.; Lehmann, E.; Längst, G. DNA sequence- and conformation-directed positioning of nucleosomes by chromatin-remodeling complexes. Proc. Natl. Acad. Sci. USA 2007, 104, 15635–15640. [Google Scholar] [CrossRef] [PubMed]
- Wippo, C.J.; Israel, L.; Watanabe, S.; Hochheimer, A.; Peterson, C.L.; Korber, P. The RSC chromatin remodelling enzyme has a unique role in directing the accurate positioning of nucleosomes. EMBO J. 2011, 30, 1277–1288. [Google Scholar] [CrossRef]
- Shim, Y.S.; Choi, Y.; Kang, K.; Cho, K.; Oh, S.; Lee, J.; Grewal, S.I.; Lee, D. Hrp3 controls nucleosome positioning to suppress non-coding transcription in eu-and heterochromatin. EMBO J. 2012, 31, 4375–4387. [Google Scholar] [CrossRef] [PubMed]
- Helbo, A.S.; Lay, F.D.; Jones, P.A.; Liang, G.; Grønbæk, K. Nucleosome Positioning and NDR Structure at RNA Polymerase III Promoters. Sci. Rep. 2017, 7, 41947. [Google Scholar] [CrossRef]
- Klein, D.C.; Hainer, S.J. Genomic methods in profiling DNA accessibility and factor localization. Chromosome Res. 2020, 28, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Mieczkowski, J.; Cook, A.; Bowman, S.K.; Mueller, B.; Alver, B.H.; Kundu, S.; Deaton, A.M.; Urban, J.A.; Larschan, E.; Park, P.J.; et al. MNase titration reveals differences between nucleosome occupancy and chromatin accessibility. Nat. Commun. 2016, 7, 11485. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Zhu, S.; Green, C.D.; Wei, G.; Han, J.D.J. Improved nucleosome-positioning algorithm iNPS for accurate nucleosome positioning from sequencing data. Nat. Commun. 2014, 5, 4909. [Google Scholar] [CrossRef]
- Georgakilas, G.K.; Perdikopanis, N.; Hatzigeorgiou, A. Solving the transcription start site identification problem with ADAPT-CAGE: A Machine Learning algorithm for the analysis of CAGE data. Sci. Rep. 2020, 10, 877. [Google Scholar] [CrossRef] [PubMed]
- Oiwa, N.N.; Cordeiro, C.E.; Heermann, D.W. The Electronic Behavior of Zinc-Finger Protein Binding Sites in the Context of the DNA Extended Ladder Model. Front. Phys. 2016, 4, 13. [Google Scholar] [CrossRef]
- Singh, A.K.; Mueller-Planitz, F. Nucleosome positioning and spacing: From mechanism to function. J. Mol. Biol. 2021, 433, 166847. [Google Scholar] [CrossRef] [PubMed]
- Schöpflin, R.; Teif, V.B.; Müller, O.; Weinberg, C.; Rippe, K.; Wedemann, G. Modeling nucleosome position distributions from experimental nucleosome positioning maps. Bioinformatics 2013, 29, 2380–2386. [Google Scholar] [CrossRef]
- Flores, O.; Orozco, M. nucleR: A package for non-parametric nucleosome positioning. Bioinformatics 2011, 27, 2149–2150. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xi, Y.; Pan, X.; Li, Z.; Kaestner, K.; Tyler, J.; Dent, S.; He, X.; Li, W. DANPOS: Dynamic analysis of nucleosome position and occupancy by sequencing. Genome Res. 2013, 23, 341–351. [Google Scholar] [CrossRef]
- Price, R.J.; Weindling, E.; Berman, J.; Buscaino, A. Chromatin Profiling of the Repetitive and Nonrepetitive Genomes of the Human Fungal Pathogen Candida albicans. mBio 2019, 10, e01376-19. [Google Scholar] [CrossRef] [PubMed]
- Skrzypek, M.S.; Binkley, J.; Binkley, G.; Miyasato, S.R.; Simison, M.; Sherlock, G. The Candida Genome Database (CGD): Incorporation of Assembly 22, systematic identifiers and visualization of high throughput sequencing data. Nucleic Acids Res. 2017, 45, gkw924. [Google Scholar] [CrossRef]
- Puri, S.; Lai, W.K.M.; Rizzo, J.M.; Buck, M.J.; Edgerton, M. Iron-responsive chromatin remodelling and MAPK signalling enhance adhesion in Candida albicans. Mol. Microbiol. 2014, 93, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, J.; Liang, F.; Liu, Y.; Wang, Q.; Zhang, H.; Jiang, M.; Zhang, Z.; Zhao, W.; Bao, Y.; et al. NucMap: A database of genome-wide nucleosome positioning map across species. Nucleic Acids Res. 2019, 47, D163–D169. [Google Scholar] [CrossRef] [PubMed]
- Kmiecik, S.; Gront, D.; Kolinski, M.; Wieteska, L.; Dawid, A.E.; Kolinski, A. Coarse-Grained Protein Models and Their Applications. Chem. Rev. 2016, 116, 7898–7936. [Google Scholar] [CrossRef]
- Reichl, L.E. A Modern Course in Statistical Physics, 4th ed.; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Pandas Development Team, T. Pandas-Dev/Pandas: Pandas. 2020. Available online: https://doi.org/10.5281/zenodo.3509134 (accessed on 12 February 2022).
- Maimon, O.; Rokach, L. Data Mining and Knowledge Discovery Handbook; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Everitt, B.S.; Landau, S.; Leese, M. Cluster Analysis, 4th ed.; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Finch, J.T.; Klug, A. Solenoidal model for superstructure in chromatin. Proc. Natl. Acad. Sci. USA 1976, 73, 1897–1901. [Google Scholar] [CrossRef] [PubMed]
- Diesinger, P.M.; Kunkel, S.; Langowski, J.; Heermann, D.W. Histone depletion facilitates chromatin loops on the kilobasepair scale. Biophys. J. 2010, 99, 2995–3001. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.P.; Athey, B.D.; Muglia, L.J.; Schappe, R.S.; Gough, A.H.; Langmore, J.P. Chromatin fibers are left-handed double helices with diameter and mass per unit length that depend on linker length. Biophys. J. 1986, 49, 233–248. [Google Scholar] [CrossRef]
- Norio Oiwa, N.; Li, K.; Cordeiro, C.E.; Heermann, D.W. Prediction and Comparative Analysis of CTCF Binding Sites based on a First Principle Approach. arXiv 2021, arXiv:2110.10508. [Google Scholar]
- Gardiner-Garden, M.; Frommer, M. CpG Islands in vertebrate genomes. J. Mol. Biol. 1987, 196, 261–282. [Google Scholar] [CrossRef]
- Pulivarthy, S.R.; Lion, M.; Kuzu, G.; Matthews, A.G.W.; Borowsky, M.L.; Morris, J.; Kingston, R.E.; Dennis, J.H.; Tolstorukov, M.Y.; Oettinger, M.A. Regulated large-scale nucleosome density patterns and precise nucleosome positioning correlate with V(D)J recombination. Proc. Natl. Acad. Sci. USA 2016, 113, 201605543. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, S.K.; Li, K.; Brauburger, S.; Bhattacherjee, A.; Oiwa, N.N.; Heermann, D.W. Superstructure Detection in Nucleosome Distribution Shows Common Pattern within a Chromosome and within the Genome. Life 2022, 12, 541. https://doi.org/10.3390/life12040541
Mishra SK, Li K, Brauburger S, Bhattacherjee A, Oiwa NN, Heermann DW. Superstructure Detection in Nucleosome Distribution Shows Common Pattern within a Chromosome and within the Genome. Life. 2022; 12(4):541. https://doi.org/10.3390/life12040541
Chicago/Turabian StyleMishra, Sujeet Kumar, Kunhe Li, Simon Brauburger, Arnab Bhattacherjee, Nestor Norio Oiwa, and Dieter W. Heermann. 2022. "Superstructure Detection in Nucleosome Distribution Shows Common Pattern within a Chromosome and within the Genome" Life 12, no. 4: 541. https://doi.org/10.3390/life12040541
APA StyleMishra, S. K., Li, K., Brauburger, S., Bhattacherjee, A., Oiwa, N. N., & Heermann, D. W. (2022). Superstructure Detection in Nucleosome Distribution Shows Common Pattern within a Chromosome and within the Genome. Life, 12(4), 541. https://doi.org/10.3390/life12040541





