Muscle Damage in Dystrophic mdx Mice Is Influenced by the Activity of Ca2+-Activated KCa3.1 Channels
Abstract
1. Introduction
2. Materials and Methods
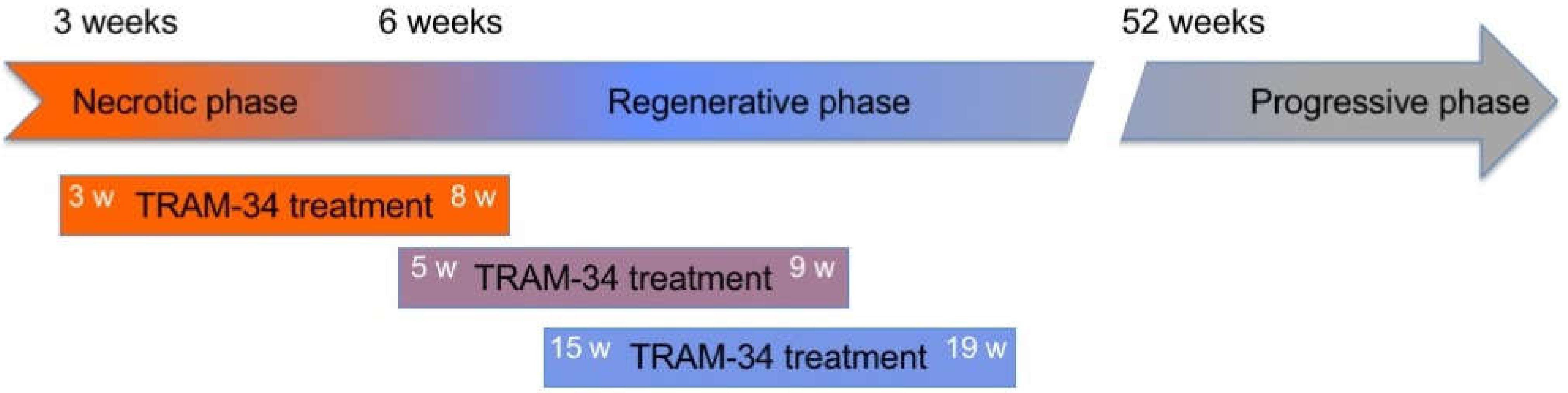
2.1. Animals and Treatments
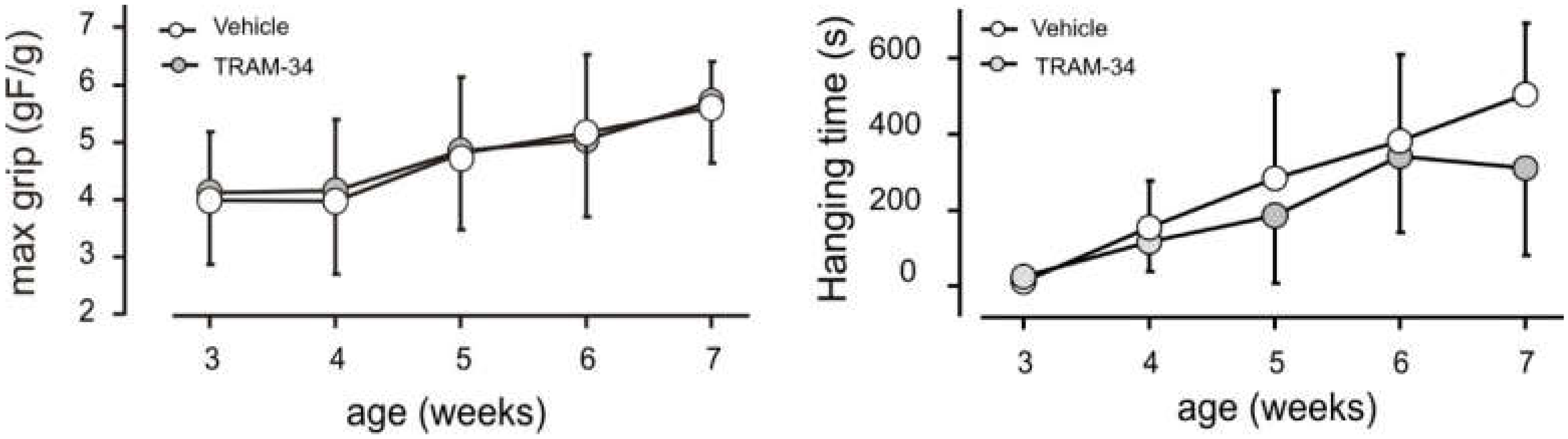
2.2. Grip Strength and Hanging Test
2.3. Immunofluorescence
2.4. Neuromuscular Junction Evaluation
2.5. PCR
2.6. Isolation of Muscle Satellite Cells
2.7. Flow Cytometric Analysis
2.8. Primary Fibroblast Culture and Proliferation Assay
2.9. Proliferation and Fusion of C2C12 Cells
2.10. Statistics
3. Results
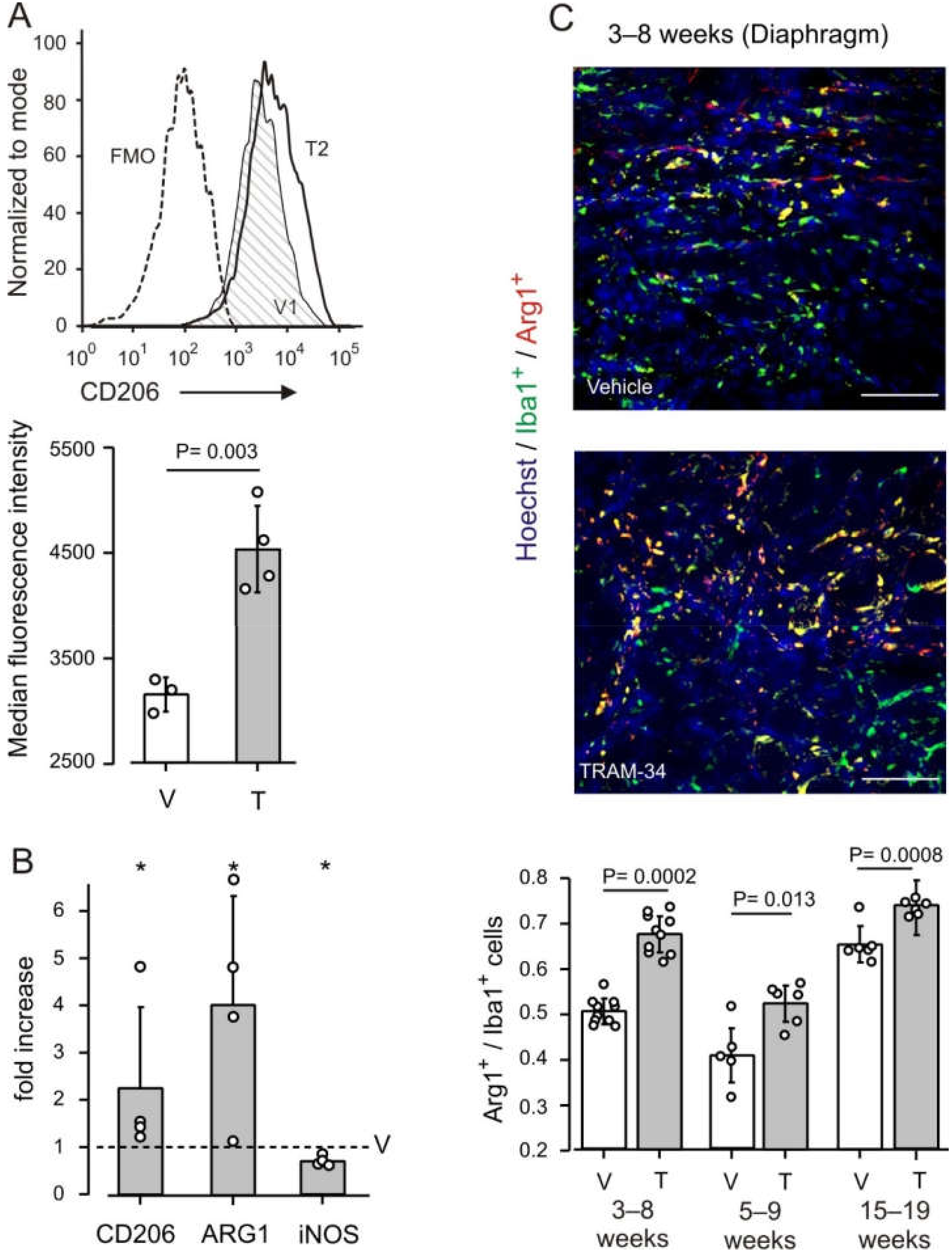
3.1. KCa3.1 Channels Affect Macrophage Phenotype in mdx Muscles
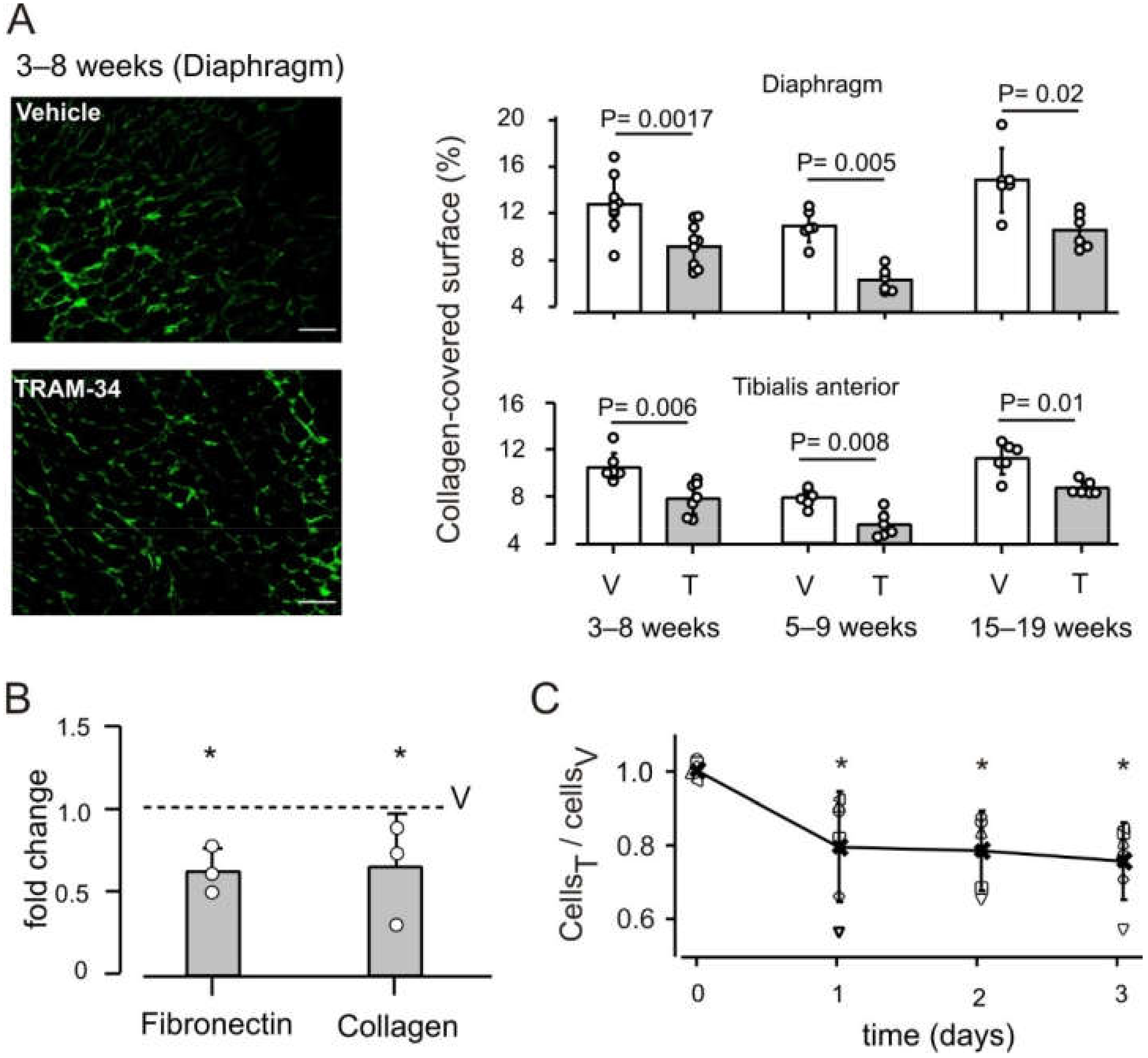
3.2. KCa3.1 Channels Influence Fibrosis in mdx Muscles
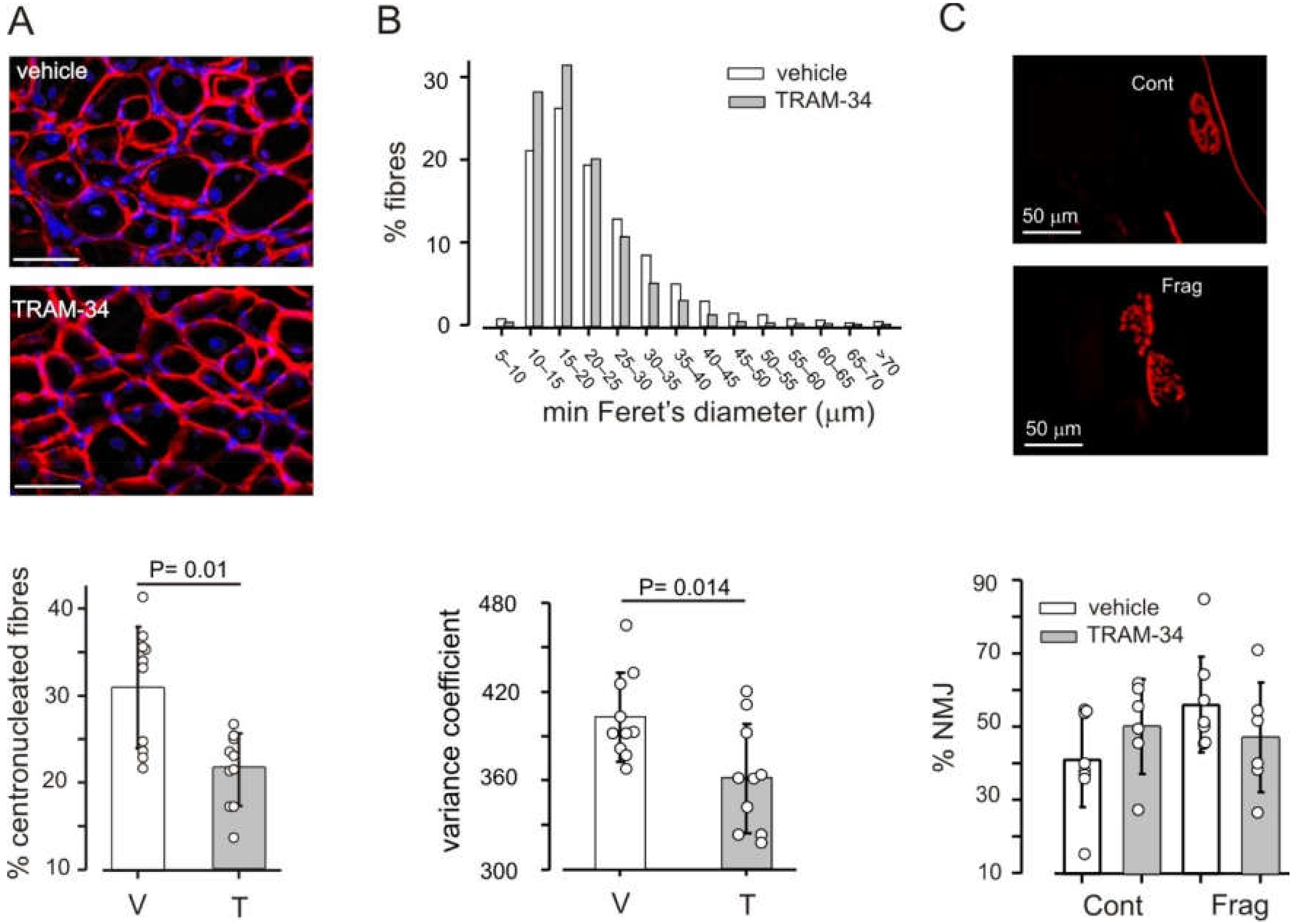
3.3. Block of KCa3.1 Channels Affects Muscle Morphology, but Has No Effect on Performance
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McGreevy, J.W.; Hakim, C.H.; McIntosh, M.A.; Duan, D. Animal Models of Duchenne Muscular Dystrophy: From Basic Mechanisms to Gene Therapy. Dis. Model. Mech. 2015, 8, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Petrof, B.J.; Shrager, J.B.; Stedman, H.H.; Kelly, A.M.; Sweeney, H.L. Dystrophin Protects the Sarcolemma from Stresses Developed during Muscle Contraction. Proc. Natl. Acad. Sci. USA 1993, 90, 3710–3714. [Google Scholar] [CrossRef]
- Stedman, H.H.; Sweeney, H.L.; Shrager, J.B.; Maguire, H.C.; Panettieri, R.A.; Petrof, B.; Narusawa, M.; Leferovich, J.M.; Sladky, J.T.; Kelly, A.M. The Mdx Mouse Diaphragm Reproduces the Degenerative Changes of Duchenne Muscular Dystrophy. Nature 1991, 352, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Theret, M.; Saclier, M.; Messina, G.; Rossi, F.M.V. Macrophages in Skeletal Muscle Dystrophies, An Entangled Partner. J. Neuromuscul. Dis. 2022, 9, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Saclier, M.; Cuvellier, S.; Magnan, M.; Mounier, R.; Chazaud, B. Monocyte/Macrophage Interactions with Myogenic Precursor Cells during Skeletal Muscle Regeneration. FEBS J. 2013, 280, 4118–4130. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, W.; Ransohoff, R.M.; Zhou, L. Infiltrating Macrophages Are Broadly Activated at the Early Stage to Support Acute Skeletal Muscle Injury Repair. J. Neuroimmunol. 2018, 317, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Desgeorges, T.; Caratti, G.; Mounier, R.; Tuckermann, J.; Chazaud, B. Glucocorticoids Shape Macrophage Phenotype for Tissue Repair. Front. Immunol. 2019, 10, 1591. [Google Scholar] [CrossRef]
- Tidball, J.G.; Welc, S.S.; Wehling-Henricks, M. Immunobiology of Inherited Muscular Dystrophies. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2018; pp. 1313–1356. ISBN 978-0-470-65071-4. [Google Scholar]
- Saclier, M.; Yacoub-Youssef, H.; Mackey, A.L.; Arnold, L.; Ardjoune, H.; Magnan, M.; Sailhan, F.; Chelly, J.; Pavlath, G.K.; Mounier, R.; et al. Differentially Activated Macrophages Orchestrate Myogenic Precursor Cell Fate During Human Skeletal Muscle Regeneration. Stem Cells 2013, 31, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, W.; Ransohoff, R.M.; Zhou, L. Identification and Function of Fibrocytes in Skeletal Muscle Injury Repair and Muscular Dystrophy. J. Immunol. 2016, 197, 4750–4761. [Google Scholar] [CrossRef] [PubMed]
- Villalta, S.A.; Nguyen, H.X.; Deng, B.; Gotoh, T.; Tidball, J.G. Shifts in Macrophage Phenotypes and Macrophage Competition for Arginine Metabolism Affect the Severity of Muscle Pathology in Muscular Dystrophy. Hum. Mol. Genet. 2008, 18, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Mojumdar, K.; Liang, F.; Giordano, C.; Lemaire, C.; Danialou, G.; Okazaki, T.; Bourdon, J.; Rafei, M.; Galipeau, J.; Divangahi, M.; et al. Inflammatory Monocytes Promote Progression of Duchenne Muscular Dystrophy and Can Be Therapeutically Targeted via CCR 2. EMBO Mol. Med. 2014, 6, 1476–1492. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.M.; Henricson, E.K.; Abresch, R.T.; Duong, T.; Joyce, N.C.; Hu, F.; Clemens, P.R.; Hoffman, E.P.; Cnaan, A.; Gordish-Dressman, H.; et al. Long-Term Effects of Glucocorticoids on Function, Quality of Life, and Survival in Patients with Duchenne Muscular Dystrophy: A Prospective Cohort Study. Lancet 2018, 391, 451–461. [Google Scholar] [CrossRef]
- Desguerre, I.; Mayer, M.; Leturcq, F.; Barbet, J.-P.; Gherardi, R.K.; Christov, C. Endomysial Fibrosis in Duchenne Muscular Dystrophy: A Marker of Poor Outcome Associated with Macrophage Alternative Activation. J. Neuropathol. Exp. Neurol. 2009, 68, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Wulff, H.; Castle, N.A. Therapeutic Potential of K Ca 3.1 Blockers: Recent Advances and Promising Trends. Expert Rev. Clin. Pharmacol. 2010, 3, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Li, C.; Wu, Y.; Shen, L.; Ma, J.; Qian, J.; Ge, J. Role of KCa3.1 Channels in Macrophage Polarization and Its Relevance in Atherosclerotic Plaque Instability. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 226–236. [Google Scholar] [CrossRef]
- Cocozza, G.; di Castro, M.A.; Carbonari, L.; Grimaldi, A.; Antonangeli, F.; Garofalo, S.; Porzia, A.; Madonna, M.; Mainiero, F.; Santoni, A.; et al. Ca2+-Activated K+ Channels Modulate Microglia Affecting Motor Neuron Survival in HSOD1G93A Mice. Brain. Behav. Immun. 2018, 73, 584–595. [Google Scholar] [CrossRef]
- Cocozza, G.; Garofalo, S.; Morotti, M.; Chece, G.; Grimaldi, A.; Lecce, M.; Scavizzi, F.; Menghini, R.; Casagrande, V.; Federici, M.; et al. The Feeding Behaviour of Amyotrophic Lateral Sclerosis Mouse Models Is Modulated by the Ca2+-activated KCa 3.1 Channels. Br. J. Pharmacol. 2021, 178, 4891–4906. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Raman, G.; Bodendiek, S.; O’Donnell, M.E.; Wulff, H. The KCa3.1 Blocker TRAM-34 Reduces Infarction and Neurological Deficit in a Rat Model of Ischemia/Reperfusion Stroke. J. Cereb. Blood Flow Metab. 2011, 31, 2363–2374. [Google Scholar] [CrossRef]
- Roach, K.M.; Feghali-Bostwick, C.; Wulff, H.; Amrani, Y.; Bradding, P. Human Lung Myofibroblast TGFβ1-Dependent Smad2/3 Signalling Is Ca2+-Dependent and Regulated by KCa3.1 K+ Channels. Fibrogenesis Tissue Repair 2015, 8, 5. [Google Scholar] [CrossRef]
- Adapala, R.K.; Thoppil, R.J.; Luther, D.J.; Paruchuri, S.; Meszaros, J.G.; Chilian, W.M.; Thodeti, C.K. TRPV4 Channels Mediate Cardiac Fibroblast Differentiation by Integrating Mechanical and Soluble Signals. J. Mol. Cell. Cardiol. 2013, 54, 45–52. [Google Scholar] [CrossRef]
- Roach, K.M.; Bradding, P. Ca 2+ Signalling in Fibroblasts and the Therapeutic Potential of KCa 3.1 Channel Blockers in Fibrotic Diseases. Br. J. Pharmacol. 2020, 177, 1003–1024. [Google Scholar] [CrossRef] [PubMed]
- Anumanthan, G.; Gupta, S.; Fink, M.K.; Hesemann, N.P.; Bowles, D.K.; McDaniel, L.M.; Muhammad, M.; Mohan, R.R. KCa3.1 Ion Channel: A Novel Therapeutic Target for Corneal Fibrosis. PLoS ONE 2018, 13, e0192145. [Google Scholar] [CrossRef] [PubMed]
- Grgic, I.; Kiss, E.; Kaistha, B.P.; Busch, C.; Kloss, M.; Sautter, J.; Muller, A.; Kaistha, A.; Schmidt, C.; Raman, G.; et al. Renal Fibrosis Is Attenuated by Targeted Disruption of KCa3.1 Potassium Channels. Proc. Natl. Acad. Sci. USA 2009, 106, 14518–14523. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Lam, J.; Gregory, C.R.; Schrepfer, S.; Wulff, H. The Ca2+-Activated K+ Channel KCa3.1 as a Potential New Target for the Prevention of Allograft Vasculopathy. PLoS ONE 2013, 8, e81006. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Lu, J.; Zhu, Y.; Meng, X.; Wang, R. The KCa3.1 Blocker TRAM-34 Inhibits Proliferation of Fibroblasts in Paraquat-Induced Pulmonary Fibrosis. Toxicol. Lett. 2018, 295, 408–415. [Google Scholar] [CrossRef]
- Wang, L.-P.; Fan, S.-J.; Li, S.-M.; Wang, X.-J.; Gao, J.-L.; Yang, X.-H. Oxidative Stress Promotes Myocardial Fibrosis by Upregulating KCa3.1 Channel Expression in AGT-REN Double Transgenic Hypertensive Mice. Pflüg. Arch.-Eur. J. Physiol. 2017, 469, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- She, G.; Ren, Y.; Wang, Y.; Hou, M.; Wang, H.; Gou, W.; Lai, B.; Lei, T.; Du, X.; Deng, X. KCa 3.1 Channels Promote Cardiac Fibrosis Through Mediating Inflammation and Differentiation of Monocytes Into Myofibroblasts in Angiotensin II–Treated Rats. J. Am. Heart Assoc. 2019, 8, e010418. [Google Scholar] [CrossRef]
- Tajhya, R.B.; Hu, X.; Tanner, M.R.; Huq, R.; Kongchan, N.; Neilson, J.R.; Rodney, G.G.; Horrigan, F.T.; Timchenko, L.T.; Beeton, C. Functional KCa1.1 Channels Are Crucial for Regulating the Proliferation, Migration and Differentiation of Human Primary Skeletal Myoblasts. Cell Death Dis. 2016, 7, e2426. [Google Scholar] [CrossRef][Green Version]
- Wulff, H.; Miller, M.J.; Hansel, W.; Grissmer, S.; Cahalan, M.D.; Chandy, K.G. Design of a Potent and Selective Inhibitor of the Intermediate-Conductance Ca2+-Activated K+ Channel, IKCa1: A Potential Immunosuppressant. Proc. Natl. Acad. Sci. USA 2000, 97, 8151–8156. [Google Scholar] [CrossRef] [PubMed]
- SOP_DMD_M.2.1.004. Available online: https://treat-nmd.org/resources-support/research-overview/preclinical-research/experimental-protocols-for-dmd-animal-models/ (accessed on 22 February 2022).
- Carre-Pierrat, M.; Lafoux, A.; Tanniou, G.; Chambonnier, L.; Divet, A.; Fougerousse, F.; Huchet-Cadiou, C.; Ségalat, L. Pre-Clinical Study of 21 Approved Drugs in the Mdx Mouse. Neuromuscul. Disord. 2011, 21, 313–327. [Google Scholar] [CrossRef]
- Encarnacion-Rivera, L.; Foltz, S.; Hartzell, H.C.; Choo, H. Myosoft: An Automated Muscle Histology Analysis Tool Using Machine Learning Algorithm Utilizing FIJI/ImageJ Software. PLoS ONE 2020, 15, e0229041. [Google Scholar] [CrossRef] [PubMed]
- Briguet, A.; Courdier-Fruh, I.; Foster, M.; Meier, T.; Magyar, J.P. Histological Parameters for the Quantitative Assessment of Muscular Dystrophy in the Mdx-Mouse. Neuromuscul. Disord. 2004, 14, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Morin, S.; de la Porte, S.; Fiszman, M.; Koenig, J. Inhibition of Proliferation in 8-Week-Old Mdx Mouse Muscle Fibroblasts in Vitro. Differentiation 1995, 59, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Lakens, D. Calculating and Reporting Effect Sizes to Facilitate Cumulative Science: A Practical Primer for t-Tests and ANOVAs. Front. Psychol. 2013, 4, 863. [Google Scholar] [CrossRef]
- Coe, R. What Effect Size Is and Why It Is Important. In Proceedings of the British Educational Research Association Annual Conference, Exeter, UK, 12–14 September 2002. [Google Scholar]
- Munder, M.; Eichmann, K.; Modolell, M. Alternative Metabolic States in Murine Macrophages Reflected by the Nitric Oxide Synthase/Arginase Balance: Competitive Regulation by CD4+ T Cells Correlates with Th1/Th2 Phenotype. J. Immunol. Baltim. 1998, 160, 5347–5354. [Google Scholar]
- Munder, M.; Eichmann, K.; Morán, J.M.; Centeno, F.; Soler, G.; Modolell, M. Th1/Th2-Regulated Expression of Arginase Isoforms in Murine Macrophages and Dendritic Cells. J. Immunol. Baltim. 1999, 163, 3771–3777. [Google Scholar]
- Karpati, G.; Carpenter, S.; Prescott, S. Small-Caliber Skeletal Muscle Fibers Do Not Suffer Necrosis in Mdx Mouse Dystrophy. Muscle Nerve 1988, 11, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Coley, W.D.; Bogdanik, L.; Vila, M.C.; Yu, Q.; Van Der Meulen, J.H.; Rayavarapu, S.; Novak, J.S.; Nearing, M.; Quinn, J.L.; Saunders, A.; et al. Effect of Genetic Background on the Dystrophic Phenotype in Mdx Mice. Hum. Mol. Genet. 2016, 25, 130–145. [Google Scholar] [CrossRef] [PubMed]
- Lovering, R.M.; Iyer, S.R.; Edwards, B.; Davies, K.E. Alterations of Neuromuscular Junctions in Duchenne Muscular Dystrophy. Neurosci. Lett. 2020, 737, 135304. [Google Scholar] [CrossRef] [PubMed]
- Hammers, D.W.; Hart, C.C.; Patsalos, A.; Matheny, M.K.; Wright, L.A.; Nagy, L.; Sweeney, H.L. Glucocorticoids Counteract Hypertrophic Effects of Myostatin Inhibition in Dystrophic Muscle. JCI Insight 2020, 5, e133276. [Google Scholar] [CrossRef]
- Turgeman, T.; Hagai, Y.; Huebner, K.; Jassal, D.S.; Anderson, J.E.; Genin, O.; Nagler, A.; Halevy, O.; Pines, M. Prevention of Muscle Fibrosis and Improvement in Muscle Performance in the Mdx Mouse by Halofuginone. Neuromuscul. Disord. 2008, 18, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Cohn, R.D.; van Erp, C.; Habashi, J.P.; Soleimani, A.A.; Klein, E.C.; Lisi, M.T.; Gamradt, M.; Ap Rhys, C.M.; Holm, T.M.; Loeys, B.L.; et al. Angiotensin II Type 1 Receptor Blockade Attenuates TGF-β–Induced Failure of Muscle Regeneration in Multiple Myopathic States. Nat. Med. 2007, 13, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Taniguti, A.P.T.; Pertille, A.; Matsumura, C.Y.; Neto, H.S.; Marques, M.J. Prevention of Muscle Fibrosis and Myonecrosis in Mdx Mice by Suramin, a TGF-Β1 Blocker: Suramin and Antifibrosis Therapy in Mdx Mice. Muscle Nerve 2011, 43, 82–87. [Google Scholar] [CrossRef]
- Juban, G.; Saclier, M.; Yacoub-Youssef, H.; Kernou, A.; Arnold, L.; Boisson, C.; Ben Larbi, S.; Magnan, M.; Cuvellier, S.; Théret, M.; et al. AMPK Activation Regulates LTBP4-Dependent TGF-Β1 Secretion by Pro-Inflammatory Macrophages and Controls Fibrosis in Duchenne Muscular Dystrophy. Cell Rep. 2018, 25, 2163–2176.e6. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Hui, T.; Chen, J.; Yu, Z.; Ren, D.; Zou, S.; Wang, S.; Fei, E.; Jiao, H.; Lai, X. Metformin Increases Sarcolemma Integrity and Ameliorates Neuromuscular Deficits in a Murine Model of Duchenne Muscular Dystrophy. Front. Physiol. 2021, 12, 642908. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.M.; Blomster, L.V.; Christophersen, P.; Wulff, H. Potassium Channel Expression and Function in Microglia: Plasticity and Possible Species Variations. Channels 2017, 11, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, A.; D’Alessandro, G.; Golia, M.T.; Grössinger, E.M.; Di Angelantonio, S.; Ragozzino, D.; Santoro, A.; Esposito, V.; Wulff, H.; Catalano, M.; et al. KCa3.1 Inhibition Switches the Phenotype of Glioma-Infiltrating Microglia/Macrophages. Cell Death Dis. 2016, 7, e2174. [Google Scholar] [CrossRef] [PubMed]
- Fei, Y.-D.; Wang, Q.; Hou, J.-W.; Li, W.; Cai, X.-X.; Yang, Y.-L.; Zhang, L.-H.; Wei, Z.-X.; Chen, T.-Z.; Wang, Y.-P.; et al. Macrophages Facilitate Post Myocardial Infarction Arrhythmias: Roles of Gap Junction and KCa3.1. Theranostics 2019, 9, 6396–6411. [Google Scholar] [CrossRef]
- Kong, J.; Yang, L.; Li, Q.; Cao, J.; Yang, J.; Chen, F.; Wang, Y.; Zhang, C. The Absence of Dystrophin Rather than Muscle Degeneration Causes Acetylcholine Receptor Cluster Defects in Dystrophic Muscle. NeuroReport 2012, 23, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Haddix, S.G.; Lee, Y.I.; Kornegay, J.N.; Thompson, W.J. Cycles of Myofiber Degeneration and Regeneration Lead to Remodeling of the Neuromuscular Junction in Two Mammalian Models of Duchenne Muscular Dystrophy. PLoS ONE 2018, 13, e0205926. [Google Scholar] [CrossRef]
- SOP DMD_M.2.2.001. Available online: https://treat-nmd.org/resources-support/research-overview/preclinical-research/experimental-protocols-for-dmd-animal-models/ (accessed on 22 February 2022).
- Klein, S.M.; Vykoukal, J.; Lechler, P.; Zeitler, K.; Gehmert, S.; Schreml, S.; Alt, E.; Bogdahn, U.; Prantl, L. Noninvasive in Vivo Assessment of Muscle Impairment in the Mdx Mouse Model—A Comparison of Two Common Wire Hanging Methods with Two Different Results. J. Neurosci. Methods 2012, 203, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Keeling, R.M.; Golumbek, P.T.; Streif, E.M.; Connolly, A.M. Weekly Oral Prednisolone Improves Survival and Strength in Malemdx Mice. Muscle Nerve 2007, 35, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Flanigan, K.M.; Ceco, E.; Lamar, K.-M.; Kaminoh, Y.; Dunn, D.M.; Mendell, J.R.; King, W.M.; Pestronk, A.; Florence, J.M.; Mathews, K.D.; et al. LTBP4 Genotype Predicts Age of Ambulatory Loss in Duchenne Muscular Dystrophy: LTBP4 Genotype in DMD. Ann. Neurol. 2013, 73, 481–488. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morotti, M.; Garofalo, S.; Cocozza, G.; Antonangeli, F.; Bianconi, V.; Mozzetta, C.; De Stefano, M.E.; Capitani, R.; Wulff, H.; Limatola, C.; et al. Muscle Damage in Dystrophic mdx Mice Is Influenced by the Activity of Ca2+-Activated KCa3.1 Channels. Life 2022, 12, 538. https://doi.org/10.3390/life12040538
Morotti M, Garofalo S, Cocozza G, Antonangeli F, Bianconi V, Mozzetta C, De Stefano ME, Capitani R, Wulff H, Limatola C, et al. Muscle Damage in Dystrophic mdx Mice Is Influenced by the Activity of Ca2+-Activated KCa3.1 Channels. Life. 2022; 12(4):538. https://doi.org/10.3390/life12040538
Chicago/Turabian StyleMorotti, Marta, Stefano Garofalo, Germana Cocozza, Fabrizio Antonangeli, Valeria Bianconi, Chiara Mozzetta, Maria Egle De Stefano, Riccardo Capitani, Heike Wulff, Cristina Limatola, and et al. 2022. "Muscle Damage in Dystrophic mdx Mice Is Influenced by the Activity of Ca2+-Activated KCa3.1 Channels" Life 12, no. 4: 538. https://doi.org/10.3390/life12040538
APA StyleMorotti, M., Garofalo, S., Cocozza, G., Antonangeli, F., Bianconi, V., Mozzetta, C., De Stefano, M. E., Capitani, R., Wulff, H., Limatola, C., Catalano, M., & Grassi, F. (2022). Muscle Damage in Dystrophic mdx Mice Is Influenced by the Activity of Ca2+-Activated KCa3.1 Channels. Life, 12(4), 538. https://doi.org/10.3390/life12040538








