Endothelial Dysfunction and Cardiovascular Risk in Obstructive Sleep Apnea: A Review Article
Abstract
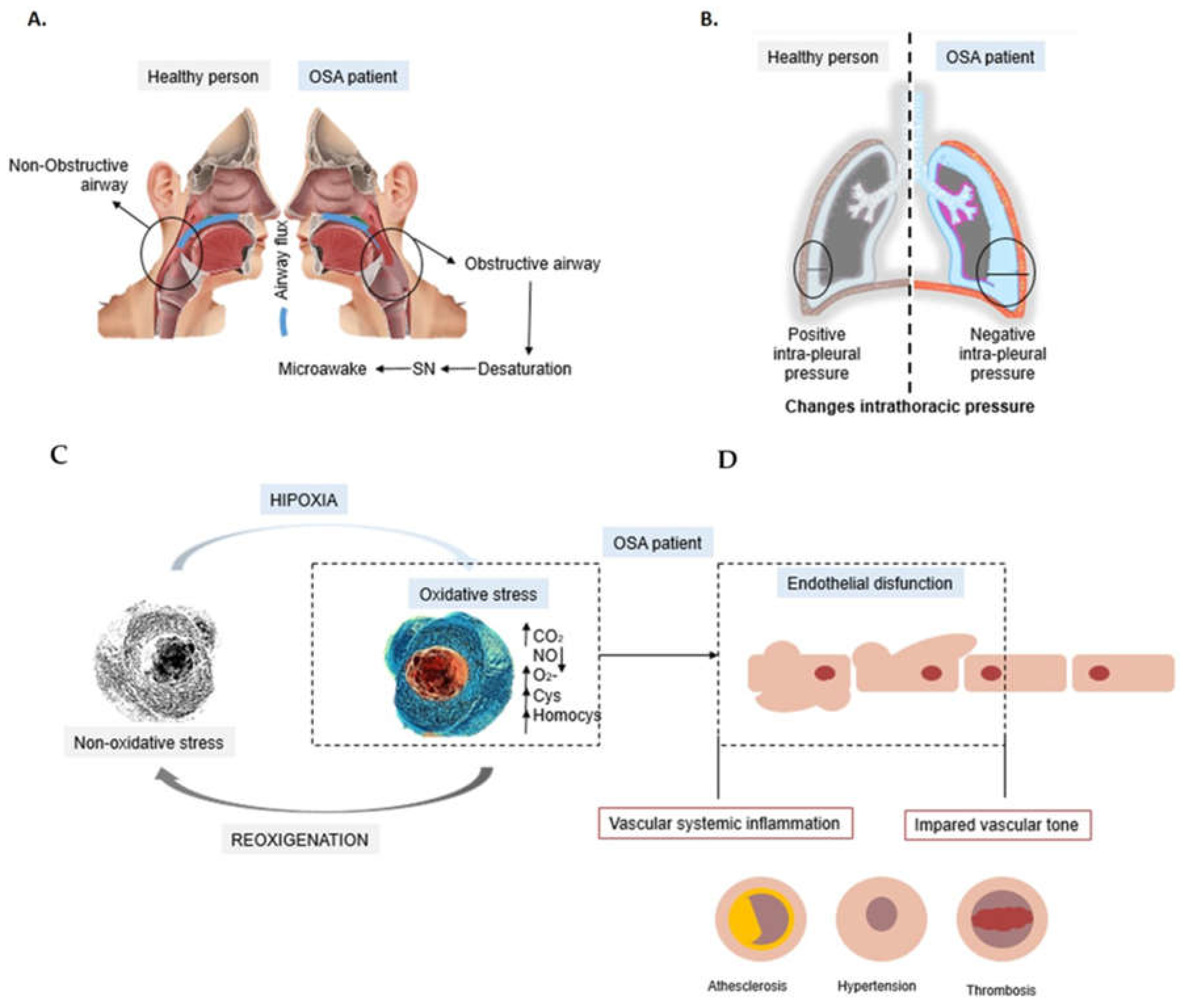
1. Obstructive Sleep Apnea (OSA)
2. Diagnosis and Severity
3. Endothelial Dysfunction in OSA Patients
4. Animal Models and In Vitro Cellular Models of OSA
5. Role of the Endothelial Progenitor Cells (EPCs)
6. Potential Biomarkers of OSA and CV Alterations
7. Effects of Endothelial Dysfunction in OSA and CV Events
8. Effect of OSA in Hypertension
9. Effect of OSA in Heart Failure and Arrhythmias
10. Effect of OSA in Coronary Syndromes
11. Effect of OSA in Metabolic Disorders
12. Therapies for OSA Patients
13. Influence of CPAP in OSA Improvement
14. Future Directions
15. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Durán, J.; Esnaola, S.; Rubio, R.; Iztueta, Á. Obstructive Sleep Apnea–Hypopnea and Related Clinical Features in a Population-based Sample of Subjects Aged 30 to 70 Yr. Am. J. Respir. Crit. Care Med. 2001, 163, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Mediano, O.; González Mangado, N.; Montserrat, J.M.; Alonso-Álvarez, M.L.; Almendros, I.; Alonso-Fernández, A.; Barbé, F.; Borsini, E.; Caballero-Eraso, C.; Cano-Pumarega, I.; et al. International Consensus Document on Obstructive Sleep Apnea. Arch Bronconeumol. 2021, 58, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Evans, L.; Finn, L.; Palta, M. Estimation of the Clinically Diagnosed Proportion of Sleep Apnea Syndrome in Middle-aged Men and Women. Sleep 1997, 20, 705–706. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.B.; Nieto, F.J.; Guidry, U.; Lind, B.K.; Redline, S.; Shahar, E.; Pickering, T.G.; Stuart, F.S.F. Quan for the Sleep Heart Health Study Research Group Relation of Sleep-disordered Breathing to Cardiovascular Disease Risk Factors: The Sleep Heart Health Study. Am. J. Epidemiol. 2001, 154, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Lavie, P.; Herer, P.; Lavie, L. Mortality risk factors in sleep apnoea: A matched case–control study. J. Sleep Res. 2007, 16, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Rundo, J.V. Obstructive sleep apnea basics. Clevel. Clin. J. Med. 2019, 86, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Douglas, N.J.; Luke, M.; Mathur, R. Is the sleep apnoea/hypopnoea syndrome inherited? Thorax 1993, 48, 719–721. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Colt, H.G.; Haas, H.; Rich, G.B. Hypoxemia vs. Sleep Fragmentation as Cause of Excessive Daytime Sleepiness in Obstructive Sleep Apnea. Chest 1991, 100, 1542–1548. [Google Scholar] [CrossRef]
- Passali, D.; Corallo, G.; Yaremchuk, S.; Longini, M.; Proietti, F.; Passali, G.; Bellussi, L. Stress ossidativo nei pazienti con diagnosi di sindrome delle apnee ostruttive notturne. Acta Otorhinolaryngol. Ital. 2015, 35, 420–425. [Google Scholar] [CrossRef]
- Lattimore, J.-D.L.; Celermajer, D.S.; Wilcox, I. Obstructive sleep apnea and cardiovascular disease. J. Am. Coll. Cardiol. 2003, 41, 1429–1437. [Google Scholar] [CrossRef]
- Micheu, M.M.; Rosca, A.-M.; Deleanu, O.-C. Stem/progenitor cells and obstructive sleep apnea syndrome—New insights for clinical applications. World J. Stem Cells 2016, 8, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Brevetti, G.; Silvestro, A.; Schiano, V.; Chiariello, M. Endothelial Dysfunction and Cardiovascular Risk Prediction in Peripheral Arterial Disease: Additive Value of Flow-Mediated Dilation to Ankle-Brachial Pressure Index. Circulation 2003, 108, 2093–2098. [Google Scholar] [CrossRef] [PubMed]
- Lan, C.K.; Rose, M.W. Perioperative Management of Obstructive Sleep Apnea. In Sleep Medicine Clinics; Kim, K.B., Movahed, R., Malhotra, R.K., Stanley, J.J., Eds.; Springer International Publishing: Cham, Switzerland, 2006; Volume 1, pp. 541–548. Available online: http://link.springer.com/10.1007/978-3-030-54146-0 (accessed on 11 February 2022).
- Chung, F.; Abdullah, H.R.; Liao, P. STOP-Bang Questionnaire: A Practical Approach to Screen for Obstructive Sleep Apnea. Chest 2016, 149, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Jelic, S.; Padeletti, M.; Kawut, S.M.; Higgins, C.; Canfield, S.M.; Onat, D.; Colombo, P.C.; Basner, R.C.; Factor, P.; LeJemtel, T.H. Inflammation, Oxidative Stress, and Repair Capacity of the Vascular Endothelium in Obstructive Sleep Apnea. Circulation 2008, 117, 2270–2278. [Google Scholar] [CrossRef] [PubMed]
- Patt, B.T.; Jarjoura, D.; Haddad, D.N.; Sen, C.K.; Roy, S.; Flavahan, N.A.; Khayat, R.N. Endothelial Dysfunction in the Microcirculation of Patients with Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2010, 182, 1540–1545. [Google Scholar] [CrossRef]
- Khayat, R.N.; Varadharaj, S.; Porter, K.; Sow, A.; Jarjoura, D.; Gavrilin, M.A.; Zweier, J.L. Angiotensin Receptor Expression and Vascular Endothelial Dysfunction in Obstructive Sleep Apnea. Am. J. Hypertens. 2017, 31, 355–361. [Google Scholar] [CrossRef]
- Félétou, M. The Endothelium: Part 1: Multiple Functions of the Endothelial Cells—Focus on Endothelium-Derived Vasoactive Mediators; Morgan & Claypool Life Sciences, 2020; pp. 10–12. Available online: https://www.ncbi.nlm.nih.gov/books/NBK57148/ (accessed on 31 December 2021).
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef]
- Medina-leyte, D.J.; Zepeda-garc, O.; Dom, M. Endothelial Dysfunction, Inflammation and Coronary Artery Disease: Potential Biomarkers and Promising Therapeutical Approaches and new pharmacological and non-pharmacological promising th The endothelium is formed by a single layer of EC located about 1. Int. J. Mol. Sci. 2021, 22, 3850. [Google Scholar] [CrossRef]
- Leite, A.R.; Borges-Canha, M.; Cardoso, R.; Neves, J.S.; Castro-Ferreira, R.; Leite-Moreira, A. Novel Biomarkers for Evaluation of Endothelial Dysfunction; Angiology; SAGE Publications Inc., 2020; Volume 71, pp. 397–410. Available online: https://journals.sagepub.com/doi/10.1177/0003319720903586?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed (accessed on 18 March 2022).
- Nafisa, A.; Gray, S.G.; Cao, Y.; Wang, T.; Xu, S.; Wattoo, F.H.; Barras, M.; Cohen, N.; Kamato, D.; Little, P.J. Endothelial function and dysfunction: Impact of metformin. Pharmacol. Ther. 2018, 192, 150–162. [Google Scholar] [CrossRef]
- Silva, I.V.G.; De Figueiredo, R.C.; Rios, D.R.A. Effect of Different Classes of Antihypertensive Drugs on Endothelial Function and Inflammation. Int. J. Mol. Sci. 2019, 20, 3458. [Google Scholar] [CrossRef]
- Liang, K.-W.; Sheu, W.H.-H.; Lee, W.-L.; Fu, C.-P.; Wang, J.-S. Differential expression of circulating vascular cell adhesion molecule-1 in subjects with coronary artery disease and cardiac syndrome X without known diabetes mellitus. Biomarkers 2017, 22, 798–804. [Google Scholar] [CrossRef]
- Pober, J.S.; Sessa, W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007, 7, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Durán-Cantolla, J.; Aizpuru, F.; Martínez-Null, C.; Barbé-Illa, F. Obstructive sleep apnea/hypopnea and systemic hypertension. Sleep Med. Rev. 2009, 13, 323–331. [Google Scholar] [CrossRef]
- Varadharaj, S.; Porter, K.; Pleister, A.; Wannemacher, J.; Sow, A.; Jarjoura, D.; Zweier, J.L.; Khayat, R.N. Endothelial nitric oxide synthase uncoupling: A novel pathway in OSA induced vascular endothelial dysfunction. Respir. Physiol. Neurobiol. 2015, 207, 40–47. [Google Scholar] [CrossRef]
- Ip, M.S.M.; Tse, H.-F.; Lam, B.; Tsang, K.W.T.; Lam, W.-K. Endothelial Function in Obstructive Sleep Apnea and Response to Treatment. Am. J. Respir. Crit. Care Med. 2004, 169, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Philippi, N.R.; Bird, C.E.; Marcus, N.J.; Olson, E.B.; Chesler, N.C.; Morgan, B.J. Time course of intermittent hypoxia-induced impairments in resistance artery structure and function. Respir. Physiol. Neurobiol. 2010, 170, 157–163. [Google Scholar] [CrossRef]
- Gozal, D.; Kheirandish-Gozal, L.; Serpero, L.D.; Capdevila, O.S.; Dayyat, E. Obstructive sleep apnea and endothelial function in school-aged nonobese children: Effect of adenotonsillectomy. Circulation 2007, 116, 2307–2314. [Google Scholar] [CrossRef]
- Epstein, F.H.; Mccord, J.M. Oxygen-Derived Free Radicals in Postischemic Tissue Injury. N. Engl. J. Med. 1985, 312, 159–163. [Google Scholar] [CrossRef]
- Bacci, M.R.; Emboz, J.N.M.; Alves, B.D.C.A.; Da Veiga, G.L.; Murad, N.; Meneghini, A.; Chagas, A.C.P.; Fonseca, F.L.A. Obstructive sleep apnea syndrome and sleep quality in hypertensive patients. Rev. Assoc. Med. Bras. 2017, 63, 1055–1060. [Google Scholar] [CrossRef]
- Rana, D.; Torrilus, C.; Ahmad, W.; Okam, N.A.; Fatima, T.; Jahan, N. Obstructive Sleep Apnea and Cardiovascular Morbidities: A Review Article. Cureus 2020, 12, e10424. [Google Scholar] [CrossRef] [PubMed]
- Porto, F.; Sakamoto, Y.S.; Salles, C. Association between Obstructive Sleep Apnea and Myocardial Infarction: A Systematic Review. Arq. Bras. Cardiol. 2017, 108, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Palta, M.; Skatrud, J. Prospective Study of the Association between Sleep-Disordered Breathing and Hypertension. N. Engl. J. Med. 2000, 342, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Parker, J.; Newton, G.E.; Floras, J.S.; Mak, S.; Chiu, K.-L.; Ruttanaumpawan, P.; Tomlinson, G.; Bradley, T.D. Influence of Obstructive Sleep Apnea on Mortality in Patients with Heart Failure. J. Am. Coll. Cardiol. 2007, 49, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Sin, D.D.; Fitzgerald, F.; Parker, J.; Newton, G.; Floras, J.S.; Bradley, T.D. Risk Factors for Central and Obstructive Sleep Apnea in 450 Men and Women with Congestive Heart Failure. Am. J. Respir. Crit. Care Med. 1999, 160, 1101–1106. [Google Scholar] [CrossRef]
- Mehra, R.; Benjamin, E.J.; Shahar, E.; Gottlieb, D.J.; Nawabit, R.; Kirchner, H.L.; Sahadevan, J.; Redline, S. Association of nocturnal arrhythmias with sleep-disordered breathing: The sleep heart health study. Am. J. Respir. Crit. Care Med. 2006, 173, 910–916. [Google Scholar] [CrossRef]
- Monahan, K.; Storfer-Isser, A.; Mehra, R.; Shahar, E.; Mittleman, M.; Rottman, J.; Punjabi, N.; Sanders, M.; Quan, S.F.; Resnick, H.; et al. Triggering of Nocturnal Arrhythmias by Sleep-Disordered Breathing Events. J. Am. Coll. Cardiol. 2009, 54, 1797–1804. [Google Scholar] [CrossRef]
- Gami, A.S.; Hodge, D.O.; Herges, R.M.; Olson, E.J.; Nykodym, J.; Kara, T.; Somers, V.K. Obstructive Sleep Apnea, Obesity, and the Risk of Incident Atrial Fibrillation. J. Am. Coll. Cardiol. 2007, 49, 565–571. [Google Scholar] [CrossRef]
- Hunyor, I.; Cook, K.M. Models of intermittent hypoxia and obstructive sleep apnea: Molecular pathways and their contribution to cancer. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2018, 315, R669–R687. [Google Scholar] [CrossRef]
- Farré, R.; Rotger, M.; Montserrat, J.M.; Calero, G.; Navajas, D. Collapsible upper airway segment to study the obstructive sleep apnea/hypopnea syndrome in rats. Respir. Physiol. Neurobiol. 2003, 136, 199–209. [Google Scholar] [CrossRef]
- Schofield, C.J.; Ratcliffe, P.J. Oxygen sensing by HIF hydroxylases. Nat. Rev. Mol. Cell Biol. 2004, 5, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Almendros, I.; Farré, R.; Planas, A.M.; Torres, M.; Bonsignore, M.R.; Navajas, D.; Montserrat, J.M. Tissue Oxygenation in Brain, Muscle, and Fat in a Rat Model of Sleep Apnea: Differential Effect of Obstructive Apneas and Intermittent Hypoxia. Sleep 2011, 34, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Padfield, G.J.; Tura, O.; Haeck, M.L.A.; Short, A.; Freyer, E.; Barclay, G.R.; Newby, D.E.; Mills, N. Circulating endothelial progenitor cells are not affected by acute systemic inflammation. Am. J. Physiol.-Heart Circ. Physiol. 2010, 298, H2054–H2061. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Tura-Ceide, O.; Hunter, A.; Mitchell, A.; Vesey, A.; Medine, C.; Gallogly, S.; Hadoke, P.W.; Keith, C.; Sproul, A.; et al. Endothelial progenitor cells do not originate from the bone marrow. Circulation 2019, 140, 1524–1526. [Google Scholar] [CrossRef] [PubMed]
- Tura-Ceide, O.; Skinner, E.M.; Barclay, G.R.; Samuel, K.; Gallagher, R.C.J.; Brittan, M.; Hadoke, P.W.F.; Newby, D.E.; Turner, M.L.; Mills, N.L. Late outgrowth endothelial cells resemble mature endothelial cells and are not derived from bone marrow. Stem Cells 2013, 31, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Peinado, V.I.; Ramírez, J.; Melgosa, T.; Roca, J.; Rodriguez-Roisin, R.; Barbera, J.A. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur. Respir. J. 2002, 19, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Case, J.; Mead, L.E.; Bessler, W.K.; Prater, D.; White, H.A.; Saadatzadeh, M.R.; Bhavsar, J.R.; Yoder, M.C.; Haneline, L.S.; Ingram, D.A. Human CD34 + AC133 + VEGFR-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp. Hematol. 2007, 35, 1109–1118. [Google Scholar] [CrossRef]
- Timmermans, F.; Van Hauwermeiren, F.; De Smedt, M.; Raedt, R.; Plasschaert, F.; De Buyzere, M.L.; Gillebert, T.C.; Plum, J.; Vandekerckhove, B. Endothelial outgrowth cells are not derived from CD133+ Cells or CD45+ hematopoietic precursors. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1572–1579. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Q.; Wu, Q.; Sun, X. Obstructive sleep apnea and endothelial progenitor cells. Patient Prefer. Adherence 2013, 7, 1077–1090. [Google Scholar] [CrossRef]
- Brooks, H.L.; Caballero, S.; Newell, C.K.; Steinmetz, R.L.; Watson, D.; Segal, M.S.; Harrison, J.K.; Scott, E.W.; Grant, M.B. Vitreous levels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic retinopathy and cystoid macular edema before and after intraocular injection of triamcinolone. Arch Ophthalmol. 2004, 122, 1801–1807. [Google Scholar] [CrossRef]
- Burger, D.; Touyz, R. Cellular biomarkers of endothelial health: Microparticles, endothelial progenitor cells, and circulating endothelial cells. J. Am. Soc. Hypertens. 2012, 6, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Yoder, M.C. Defining human endothelial progenitor cells. J. Thromb. Haemost. 2009, 7, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Masuda, H.; Kalka, C.; Takahashi, T.; Yoshida, M.; Wada, M.; Kobori, M.; Itoh, R.; Iwaguro, H.; Eguchi, M.; Iwami, Y.; et al. Estrogen-Mediated Endothelial Progenitor Cell Biology and Kinetics for Physiological Postnatal Vasculogenesis. Circ. Res. 2007, 101, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Hayashi, M.; Takeshita, K.; Numaguchi, Y.; Kobayashi, K.; Iino, S.; Inden, Y.; Murohara, T. Smoking Cessation Rapidly Increases Circulating Progenitor Cells in Peripheral Blood in Chronic Smokers. Arter. Thromb. Vasc. Biol. 2004, 24, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- De La Peña, M.; Barceló, A.; Barbe, F.; Piérola, J.; Pons, J.; Rimbau, E.; Ayllón, O.; Agustí, A.G. Endothelial function and circulating endothelial progenitor cells in patients with sleep apnea syndrome. Respiration 2008, 76, 28–32. [Google Scholar] [CrossRef]
- Murri, M.; García-Delgado, R.; Alcazar-Ramirez, J.; Fernandez-Ramos, A.; Alcaide, J.; Cardona, F.; Tinahones, F.J. Effect of CPAP on Oxidative Stress and Circulating Progenitor Cell Levels in Sleep Patients with Apnea-Hypopnea Syndrome. Respir. Care 2011, 56, 1830–1836. [Google Scholar] [CrossRef]
- Narkiewicz, K.; van de Borne, P.J.H.; Pesek, C.A.; Dyken, M.; Montano, N.; Somers, V.K. Selective Potentiation of Peripheral Chemoreflex Sensitivity in Obstructive Sleep Apnea. Circulation 1999, 99, 1183–1189. [Google Scholar] [CrossRef]
- Werner, N.; Nickenig, G. Influence of cardiovascular risk factors on endothelial progenitor cells: Limitations for therapy? Arterioscler. Thromb. Vasc. Biol. 2006, 26, 257–266. [Google Scholar] [CrossRef]
- Tousoulis, D.; Andreou, I.; Antoniades, C.; Tentolouris, C.; Stefanadis, C. Role of Inflammation and Oxidative Stress in Endothelial Progenitor Cell Function and Mobilization: Therapeutic Implications for Cardiovascular Diseases. Atherosclerosis 2008, 201, 236–247. [Google Scholar] [CrossRef]
- Andreou, I.; Tousoulis, D.; Tentolouris, C.; Antoniades, C.; Stefanadis, C. Potential role of endothelial progenitor cells in the pathophysiology of heart failure: Clinical implications and perspectives. Atherosclerosis 2006, 189, 247–254. [Google Scholar] [CrossRef]
- Thum, T.; Fraccarollo, D.; Schultheiss, M.; Froese, S.; Galuppo, P.; Widder, J.D.; Tsikas, D.; Ertl, G.; Bauersachs, J. Endothelial Nitric Oxide Synthase Uncoupling Impairs Endothelial Progenitor Cell Mobilization and Function in Diabetes. Diabetes 2007, 56, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Miorin, M.; Facco, M.; Bonamico, S.; Baesso, I.; Grego, F.; Menegolo, M.; de Kreutzenberg, S.V.; Tiengo, A.; Agostini, C.; et al. Circulating Endothelial Progenitor Cells Are Reduced in Peripheral Vascular Complications of Type 2 Diabetes Mellitus. J. Am. Coll. Cardiol. 2005, 45, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Werner, N.; Kosiol, S.; Schiegl, T.; Ahlers, P.; Walenta, K.; Link, A.; Bohm, M.C.; Nickenig, G. Circulating Endothelial Progenitor Cells and Cardiovascular Outcomes. N. Engl. J. Med. 2005, 353, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, S.; Ssica García-Lucio, J.; Peinado, V.I.; Tura-Ceide, O.; Díez, M.; Blanco, I.; Sitges, M.; Petriz, J.; Torralba, Y.; Marín, P.; et al. Circulating Progenitor Cells and Vascular Dysfunction in Chronic Obstructive Pulmonary Disease. PLoS ONE 2014, 9, e106163. [Google Scholar] [CrossRef] [PubMed]
- Yaggi, H.K.; Concato, J.; Kernan, W.N.; Lichtman, J.H.; Brass, L.M.; Mohsenin, V. Obstructive Sleep Apnea as a Risk Factor for Stroke and Death. N. Engl. J. Med. 2005, 353, 2034–2041. [Google Scholar] [CrossRef]
- Van Hinsbergh, V.W.M. The endothelium: Vascular control of haemostasis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 95, 198–201. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Z.; Liu, L.; Wang, D.; Zhang, J. The level of circulating endothelial progenitor cells may be associated with the occurrence and recurrence of chronic subdural hematoma. Clinics 2013, 68, 1084–1088. [Google Scholar] [CrossRef]
- Mills, N.L.; Tura, O.; Padfield, G.J.; Millar, C.; Lang, N.; Stirling, D.; Ludlam, C.; Turner, M.L.; Barclay, G.R.; Newby, D.E. Dissociation of phenotypic and functional endothelial progenitor cells in patients undergoing percutaneous coronary intervention. Heart 2009, 95, 2003–2008. [Google Scholar] [CrossRef]
- Carreras, A.; Rojas, M.; Tsapikouni, T.; Montserrat, J.M.; Navajas, D.; Farré, R. Obstructive apneas induce early activation of mesenchymal stem cells and enhancement of endothelial wound healing. Respir. Res. 2010, 11, 91. [Google Scholar] [CrossRef]
- Jean-Louis, G.; Zizi, F.; Clark, L.T.; Brown, C.D.; McFarlane, S.I. Obstructive Sleep Apnea and Cardiovascular Disease: Role of the Metabolic Syndrome and Its Components. J. Clin. Sleep Med. 2008, 4, 261–272. [Google Scholar] [CrossRef]
- Gonzaga, C.; Bertolami, A.; Bertolami, M.; Amodeo, C.; Calhoun, D. Obstructive sleep apnea, hypertension and cardiovascular diseases. J. Hum. Hypertens. 2015, 29, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Somers, V.K.; Dyken, M.; Mark, A.L.; Abboud, F. Sympathetic-Nerve Activity during Sleep in Normal Subjects. N. Engl. J. Med. 1993, 328, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Baguet, J.-P.; Barone-Rochette, G.; Pépin, J.-L. Hypertension and obstructive sleep apnoea syndrome: Current perspectives. J. Hum. Hypertens. 2009, 23, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, J.J.; Cain, M.E.; Hohnloser, S.H.; Kadish, A.H.; Knight, B.P.; Lauer, M.S.; Maron, B.J.; Page, R.L.; Passman, R.S.; Siscovick, D.; et al. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death: A scientific statement from the America. Circulation 2008, 118, 1497–1518. [Google Scholar] [CrossRef] [PubMed]
- Floras, J.S. Sleep apnea and cardiovascular risk. J. Cardiol. 2014, 63, 3–8. [Google Scholar] [CrossRef]
- Narkiewicz, K.; Van De Borne, P.J.H.; Cooley, R.L.; Dyken, M.E.; Somers, V.K. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation 1998, 98, 772–776. [Google Scholar] [CrossRef]
- Fletcher, E.C.; DeBehnke, R.D.; Lovoi, M.S.; Gorin, A.B. Undiagnosed Sleep Apnea in Patients with Essential Hypertension. Ann. Intern. Med. 1985, 103, 190–195. [Google Scholar] [CrossRef]
- Worsnop, C.J.; Naughton, M.T.; Barter, C.E.; Morgan, T.O.; Anderson, A.I.; Pierce, R.J. The Prevalence of Obstructive Sleep Apnea in Hypertensives. Am. J. Respir. Crit. Care Med. 1998, 157, 111–115. [Google Scholar] [CrossRef]
- Tkacova, R.; Niroumand, M.; Lorenzi-Filho, G.; Bradley, T.D. Overnight shift from obstructive to central apneas in patients with heart failure: Role of PCO2 and circulatory delay. Circulation 2001, 103, 238–243. [Google Scholar] [CrossRef]
- Virolainen, J.; Ventila, M.; Turto, H.; Kupari, M. Effect of negative intrathoracic pressure on left ventricular pressure dynamics and relaxation. J. Appl. Physiol. 1995, 79, 455–460. [Google Scholar] [CrossRef]
- Bermúdez, V.; Rojas, J.; Salazar, J.; Bello, L.; Áñez, R.; Toledo, A.; Chacín, M.; Aguirre, M.; Villalobos, M.; Chávez, M.; et al. Variations of lipoprotein(a) levels in the metabolic syndrome: A report from the Maracaibo City metabolic syndrome prevalence study. J. Diabetes Res. 2013, 2013, 12. [Google Scholar] [CrossRef]
- Giles, T.; Lasserson, T.; Smith, B.; White, J.; Wright, J.; Cates, C. Continuous positive airways pressure for obstructive sleep apnoea in adults. In Cochrane Database of Systematic Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006; Available online: https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001106.pub2/full (accessed on 31 December 2021).
- Sutherland, K.; Vanderveken, O.M.; Tsuda, H.; Marklund, M.; Gagnadoux, F.; Kushida, C.A.; Cistulli, P.A. Oral Appliance Treatment for Obstructive Sleep Apnea: An Update. J. Clin. Sleep Med. 2014, 10, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Costantino, A.; Rinaldi, V.; Moffa, A.; Luccarelli, V.; Bressi, F.; Cassano, M.; Casale, M.; Baptista, P. Hypoglossal nerve stimulation long-term clinical outcomes: A systematic review and meta-analysis. Sleep Breath. 2019, 24, 399–411. [Google Scholar] [CrossRef]
- Elshaug, A.; Moss, J.; Southcott, A.; . Hiller, J.E. Redefining success in airway surgery for obstructive sleep apnea: A meta analysis and synthesis of the evidence. Sleep 2007, 30, 461–467. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Yin, G.; Zhan, S.; Xu, J.; Cao, X.; Li, J.; Ye, J. Long-term Efficacy of Uvulopalatopharyngoplasty among Adult Patients with Obstructive Sleep Apnea: A Systematic Review and Meta-analysis. Otolaryngol. Neck Surg. 2019, 161, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Martínez Ruiz de Apodaca, P.; Carrasco Llatas, M.; Valenzuela Gras, M.; Dalmau Galofre, J. Improving surgical results in velopharyngeal surgery: Our experience in the last decade. Acta Otorrinolaringol. Esp. 2020, 71, 197–203. [Google Scholar] [CrossRef] [PubMed]
- MacKay, S.; Carney, A.S.; Catcheside, P.G.; Chai-Coetzer, C.L.; Chia, M.; Cistulli, P.A.; Hodge, J.C.; Jones, A.; Kaambwa, B.; Lewis, R.; et al. Effect of multilevel upper airway surgery vs. medical management on the apnea-hypopnea index and patient-reported daytime sleepiness among patients with moderate or severe obstructive sleep apnea: The SAMS randomized clinical trial. JAMA—J. Am. Med. Assoc. 2020, 324, 1168–1179. [Google Scholar] [CrossRef]
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef]
- Campos-Rodriguez, F.; Martinez-Garcia, M.A.; de la Cruz-Moron, I.; Almeida-Gonzalez, C.; Catalan-Serra, P.; Montserrat, J.M. Cardiovascular mortality in women with obstructive sleep apnea with or without continuous positive airway pressure treatment:A cohort study. Ann. Intern. Med. 2012, 156, 15–122. [Google Scholar]
- Martínez-García, M.A.; Campos-Rodríguez, F.; Catalán-Serra, P.; Soler-Cataluña, J.J.; Almeida-Gonzalez, C.; De La Cruz Morón, I.; Durán-Cantolla, J.; Montserrat, J.M. Cardiovascular mortality in obstructive sleep apnea in the elderly: Role of long-term continuous positive airway pressure treatment: A prospective observational study. Am. J. Respir. Crit. Care Med. 2012, 186, 909–916. [Google Scholar] [CrossRef]
- Lloberes, P.; Durán-Cantolla, J.; Martínez-García, M.Á.; Marín, J.M.; Ferrer, A.; Corral, J.; Masa, J.F.; Parra, O.; Alonso-Álvarez, M.L.; Terán-Santos, J. Diagnóstico y tratamiento del síndrome de apneas-hipopneas del sueño. Arch. Bronconeumol. 2011, 47, 143–156. [Google Scholar] [CrossRef] [PubMed]
| Questions | Answer | |
|---|---|---|
| Does the patient snore loudly? | S | Y/N |
| Does the patient often feel tired during the day? | T | Y/N |
| Has anyone observed the patient stop breathing during their sleep? | O | Y/N |
| Does the patient suffer from high blood pressure? | P | Y/N |
| Does the patient have a BMI > 35? | B | Y/N |
| Is the patient older than 50 (age)? | A | Y/N |
| Has the patient a neck circumference of >40 cm? | N | Y/N |
| Is the patient male (gender)? | G | Y/N |
| Scoring Y ≥ 3 → High risk of OSA Y < 3 → Low risk of OSA |
| Study | Ref. | Method of ED Detected | Participants |
|---|---|---|---|
| Patt, B. T., et al. (2010). | [16] | FMD, Peroxynitrite levels. | N = 14 (NOSA = 7, Nc = 7) |
| Jelic, S., et al. (2008). | [15] | FMD, NOS levels, Phosphorylated eNOS levels, Cyclooxygenase-2 inducible NOS, levels, Nitrotyrosine. Levels, Circulating EPCs. | N = 32 (NOSA = 32, Nc = 15) |
| Khayat, R. N., et al. (2018). | [17] | FMD, AT1 and AT2 receptors, O2− production in ME, NO production in ME. | N = 21 (NOSA = 11, Nc = 10) |
| Varadharaj, S., et al. (2015). | [28] | O2− expression and production in ME, NO expression and production in ME. | N = 31 (NOSA = 19, Nc = 12) |
| Gozal, D., et al. (2007). | [31] | Hyperemic test, sCD40L plasma levels, ADMA plasma levels, Nitrotyrosine plasma levels. | N = 32 (NOSA = 26, Nc = 8) |
| Ip, M. S. M., et al. (2004) | [29] | FMD | N = 40 (NOSA = 28, Nc = 12) |
| Study | Ref. | OSA and CV Findings | Study Design |
|---|---|---|---|
| Peppard, P. E., et al. (2000). | [36] | Association of OSA and the presence of hypertension over a four year period. | Prospective study (N = 709, NSDB = 709) |
| Wang, H., Parker, J. D. et al., (2007). | [37] | Association of untreated OSA with an increased risk of HF. | Prospective study (N = 164, NM-NSA = 113, Nuntreated OSA = 37, Ntreated = 14) |
| Sin, D. D., et al. (1999). | [38] | The presence of OSA is common in CHF population. | Retrospective study (N = 450, NCHF = 450) |
| Mehra, R., et al. (2006). | [39] | Association of severe SDB with complex arrhythmias. | Multicenter longitudinal study (N = 566, NSDB = 228, Nno-SDB = 338) |
| Monahan, K., et al. (2009). | [40] | Association of apneas and hypopneas during sleep with paroxysmal AF, NSVT, nocturnal arrhythmias and cardiac effects. | Multicenter longitudinal study (N = 2816, Nno arrhytmia = 2759, NPAF, NSDVT = 57) |
| Gami, A. S., et al. (2007). | [41] | OSA is recognized as a risk factor for incident AF. | Retrospective study (N = 3542, NOSA = 3542) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peracaula, M.; Torres, D.; Poyatos, P.; Luque, N.; Rojas, E.; Obrador, A.; Orriols, R.; Tura-Ceide, O. Endothelial Dysfunction and Cardiovascular Risk in Obstructive Sleep Apnea: A Review Article. Life 2022, 12, 537. https://doi.org/10.3390/life12040537
Peracaula M, Torres D, Poyatos P, Luque N, Rojas E, Obrador A, Orriols R, Tura-Ceide O. Endothelial Dysfunction and Cardiovascular Risk in Obstructive Sleep Apnea: A Review Article. Life. 2022; 12(4):537. https://doi.org/10.3390/life12040537
Chicago/Turabian StylePeracaula, Miriam, Daniela Torres, Paula Poyatos, Neus Luque, Eric Rojas, Anton Obrador, Ramon Orriols, and Olga Tura-Ceide. 2022. "Endothelial Dysfunction and Cardiovascular Risk in Obstructive Sleep Apnea: A Review Article" Life 12, no. 4: 537. https://doi.org/10.3390/life12040537
APA StylePeracaula, M., Torres, D., Poyatos, P., Luque, N., Rojas, E., Obrador, A., Orriols, R., & Tura-Ceide, O. (2022). Endothelial Dysfunction and Cardiovascular Risk in Obstructive Sleep Apnea: A Review Article. Life, 12(4), 537. https://doi.org/10.3390/life12040537







