Topical Application of Antrodia cinnamomea Ointment in Diabetic Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
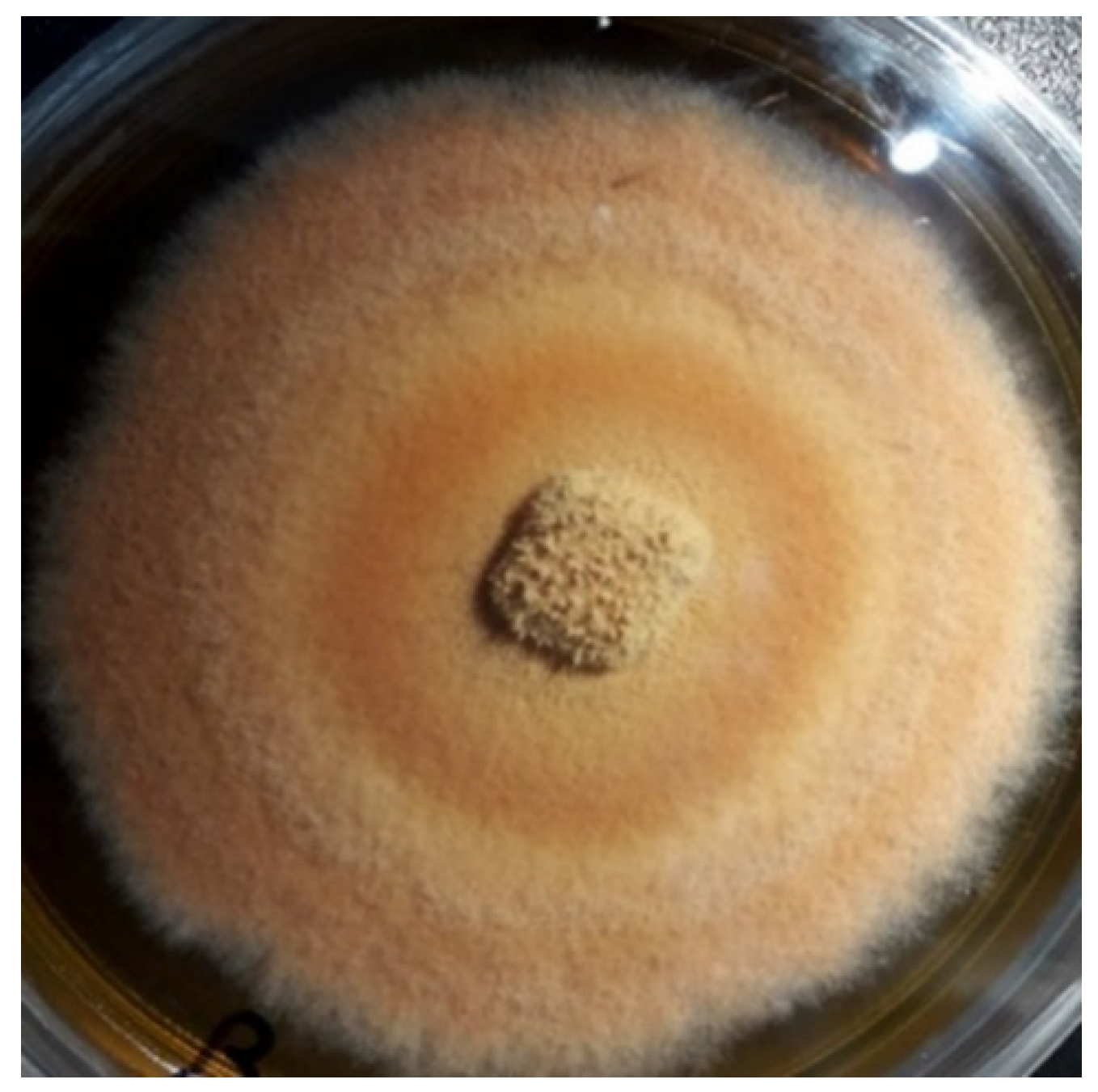
2.1. Culture of Antrodia cinnamomea Mycelia
2.2. Animals
2.3. Induction of Diabetic Animal
2.4. Ointment Preparation
2.5. Measurement of Wound Area
2.6. Immunocytochemical Stain
2.7. Western Blot Analysis
2.8. Statistical Analysis
3. Results
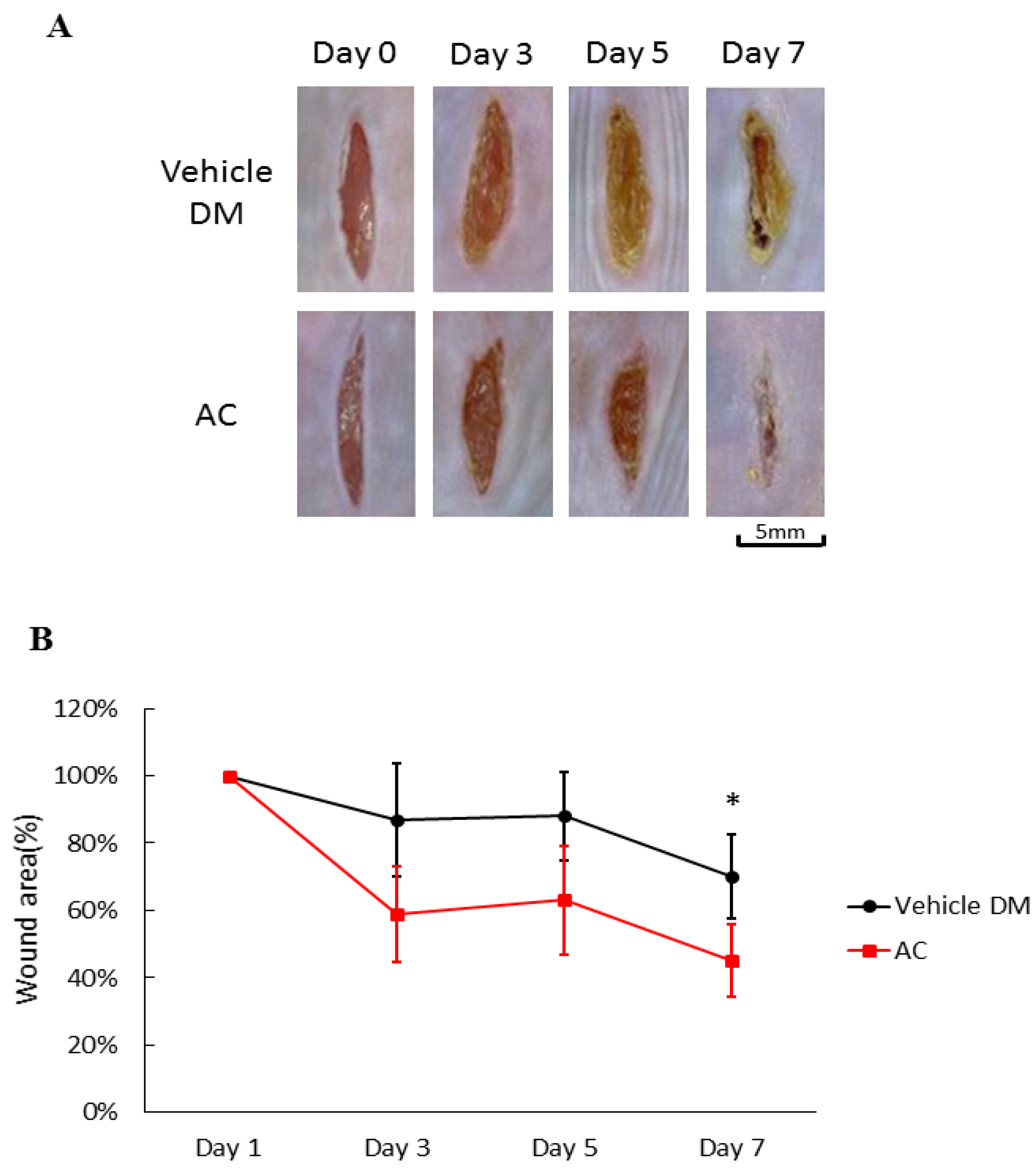
3.1. Effects of Antrodia cinnamomea Broth Filtrate on Linear Diabetic Wound Closure
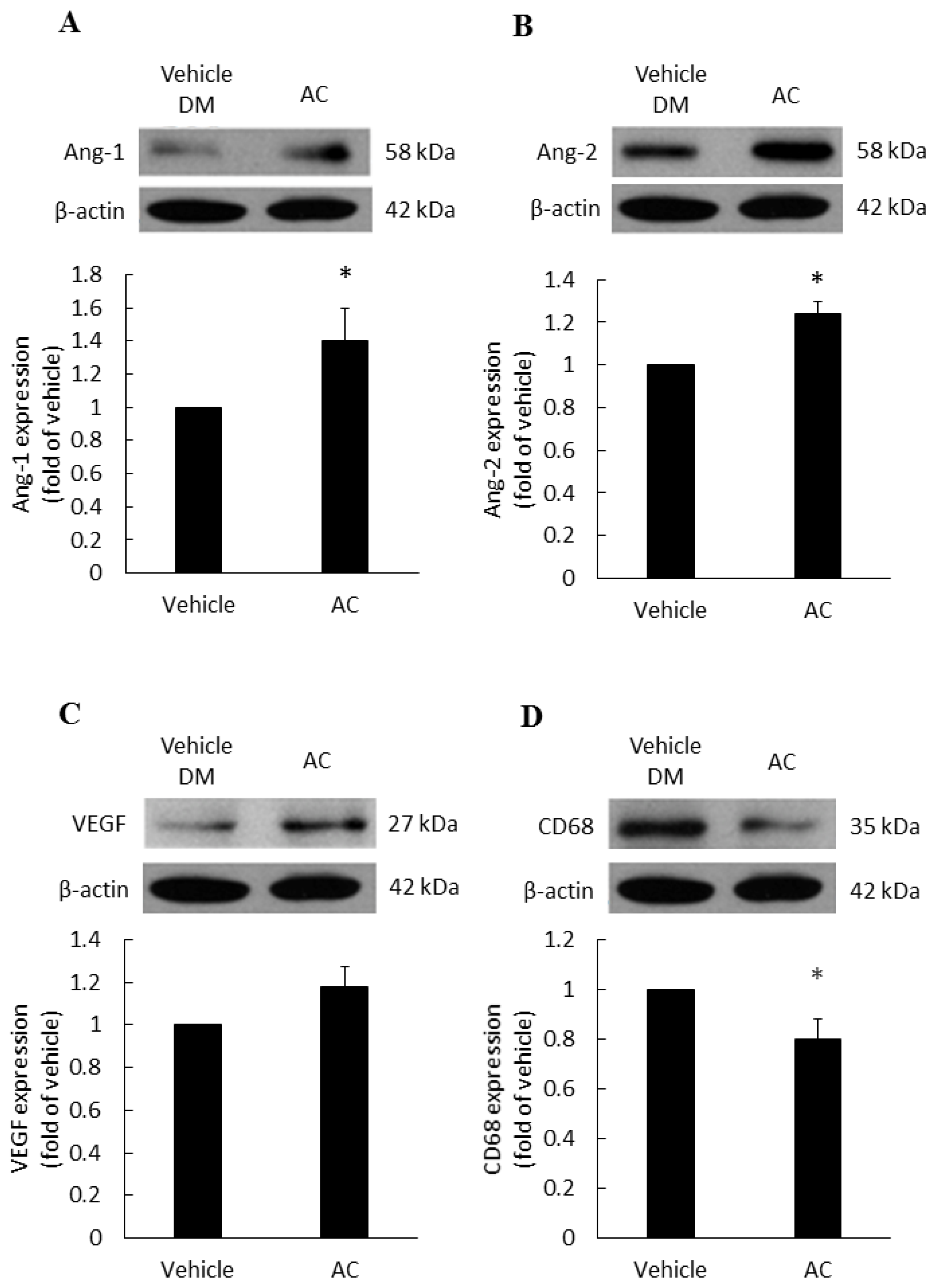
3.2. The Relationship between Antrodia cinnamomea Broth Filtrate and Angiogenesis
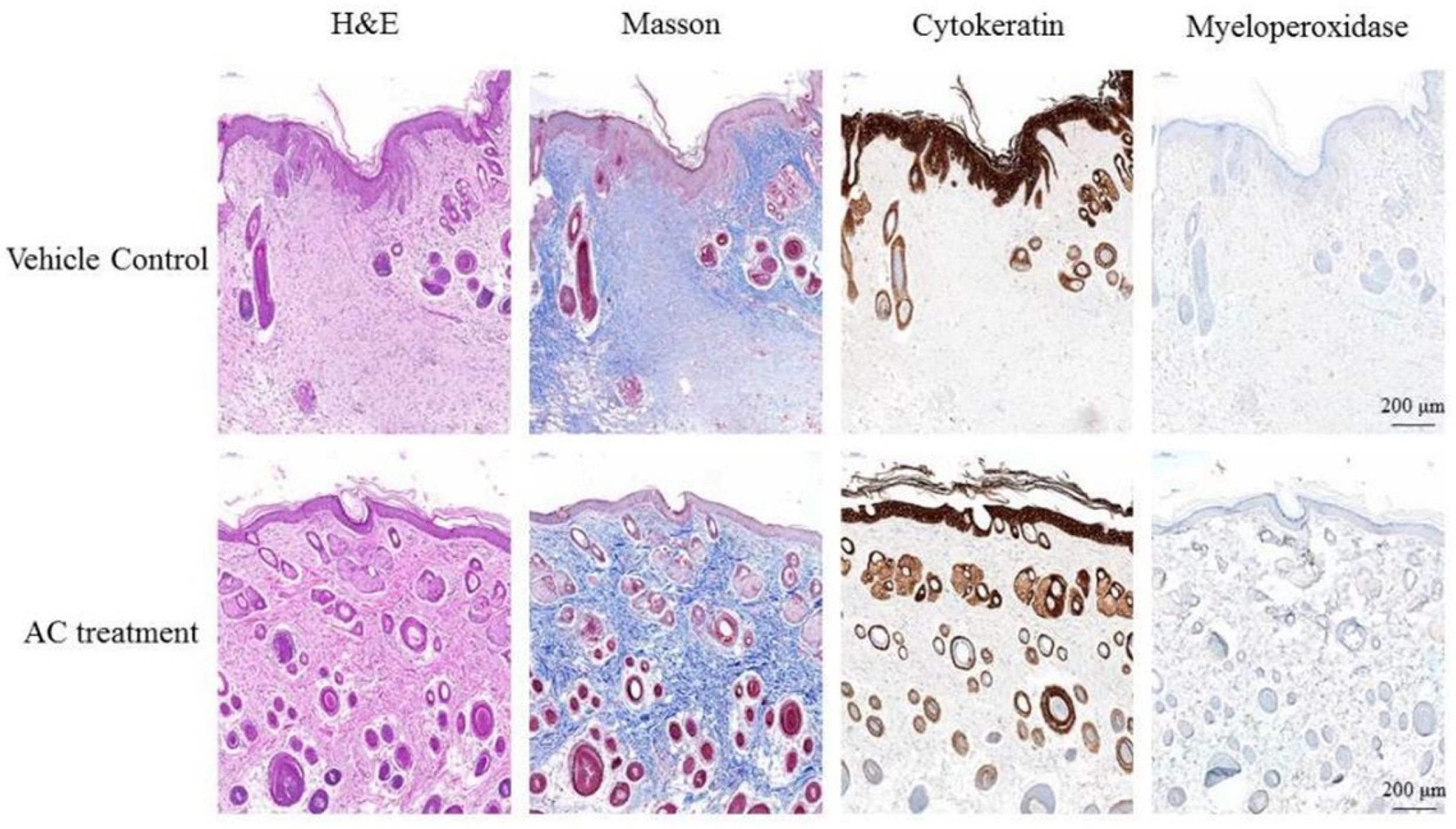
3.3. Anti-Inflammatory Effect of Antrodia cinnamomea Broth Filtrate
3.4. Combined Oral and Topical Antrodia cinnamomea Broth Filtrate Treatment
3.5. The Effects of Antrodia cinnamomea Broth Filtrate on the EPOR and PPARδ Expressions in Wound Area
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silhi, N. Diabetes and wound healing. J. Wound Care 1998, 7, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, K.; Asai, J.; Ii, M.; Thorne, T.; Losordo, D.W.; D’Amore, P.A. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am. J. Pathol. 2007, 170, 1178–1191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arya, A.K.; Tripathi, R.; Kumar, S.; Tripathi, K. Recent advances on the association of apoptosis in chronic non healing diabetic wound. World J. Diabetes 2014, 5, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Agren, M.S.; Steenfos, H.H.; Dabelsteen, S.; Hansen, J.B.; Dabelsteen, E. Proliferation and mitogenic response to PDGF-BB of fibroblasts isolated from chronic venous leg ulcers is ulcer-age dependent. J. Investig. Dermatol. 1999, 112, 463–469. [Google Scholar]
- Arya, A.K.; Tripathi, K.; Das, P. Promising role of ANGPTL4 gene in diabetic wound healing. Int. J. Low Extrem. Wounds. 2014, 13, 58–63. [Google Scholar] [CrossRef]
- Gallagher, K.A.; Liu, Z.J.; Xiao, M.; Chen, H.; Goldstein, L.J.; Buerk, D.G.; Nedeau, A.; Thom, S.R.; Velazquez, O.C. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J. Clin. Investig. 2007, 117, 1249–1259. [Google Scholar] [CrossRef] [Green Version]
- Berlanga-Acosta, J.; Schultz, G.S.; Lopez-Mola, E.; Guillen-Nieto, G.; Garcia-Siverio, M.; Herrera-Martinez, L. Glucose toxic effects on granulation tissue productive cells: The diabetics’ impaired healing. Biomed. Res. Int. 2013, 2013, 256043. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.T.; Chou, W.N. Antrodia cinnamomea reconsidered and A. salmonea sp. nov. on Cunninghamia konishii in Taiwan. Bot. Bull. Acad. Sin. 2004, 45, 347–352. [Google Scholar]
- Huang, Y.L.; Chu, Y.L.; Ho, C.T.; Chung, J.G.; Lai, C.I.; Su, Y.C.; Kuo, Y.H.; Sheen, L.Y.; Antcin, K. An Active Triterpenoid from the Fruiting Bodies of Basswood-Cultivated Antrodia cinnamomea, Inhibits Metastasis via Suppression of Integrin-Mediated Adhesion, Migration, and Invasion in Human Hepatoma Cells. J. Agric. Food Chem. 2015, 63, 4561–4569. [Google Scholar] [CrossRef]
- Hsiao, G.; Shen, M.Y.; Lin, K.H.; Lan, M.H.; Wu, L.Y.; Chou, D.S.; Lin, C.H.; Su, C.H.; Sheu, J.R. Antioxidative and hepatoprotective effects of Antrodia camphorata extract. J. Agric. Food Chem. 2003, 51, 3302–3308. [Google Scholar] [CrossRef]
- Wen, C.L.; Chang, C.C.; Huang, S.S.; Kuo, C.L.; Hsu, S.L.; Deng, J.S.; Huang, G.J. Anti-inflammatory effects of methanol extract of Antrodia cinnamomea mycelia both in vitro and in vivo. J. Ethnopharmacol. 2011, 137, 575–584. [Google Scholar] [CrossRef]
- Johnson, A.; Cheng, S.C.; Tsou, D.; Kong, Z.L. Attenuation of reproductive dysfunction in diabetic male rats with timber cultured Antrodia cinnamomea ethanol extract. Biomed. Pharmacother. 2019, 112, 1–13. [Google Scholar] [CrossRef]
- Galeano, M.; Altavilla, D.; Cucinotta, D.; Russo, G.T.; Calo, M.; Bitto, A.; Marini, H.; Marini, R.; Adamo, E.B.; Seminara, P.; et al. Recombinant human erythropoietin stimulates angiogenesis and wound healing in the genetically diabetic mouse. Diabetes 2004, 53, 2509–2517. [Google Scholar] [CrossRef] [Green Version]
- Youssoufian, H.; Longmore, G.; Neumann, D.; Yoshimura, A.; Lodish, H.F. Structure, function, and activation of the erythropoietin receptor. Blood 1993, 81, 2223–2236. [Google Scholar] [CrossRef]
- Arcasoy, M.O. The non-haematopoietic biological effects of erythropoietin. Br. J. Haematol. 2008, 141, 14–31. [Google Scholar] [CrossRef]
- Siebert, N.; Xu, W.; Grambow, E.; Zechner, D.; Vollmar, B. Erythropoietin improves skin wound healing and activates the TGF-beta signaling pathway. Lab. Investig. 2011, 91, 1753–1765. [Google Scholar] [CrossRef] [Green Version]
- Sorg, H.; Krueger, C.; Schulz, T.; Menger, M.D.; Schmitz, F.; Vollmar, B. Effects of erythropoietin in skin wound healing are dose related. FASEB J. 2009, 23, 3049–3058. [Google Scholar] [CrossRef]
- Hamed, S.; Ullmann, Y.; Masoud, M.; Hellou, E.; Khamaysi, Z.; Teot, L. Topical erythropoietin promotes wound repair in diabetic rats. J. Investig. Dermatol. 2010, 130, 287–294. [Google Scholar] [CrossRef] [Green Version]
- Wagner, N.; Wagner, K.D. The Role of PPARs in Disease. Cells 2020, 9, 2367. [Google Scholar] [CrossRef]
- Liang, Y.J.; Jian, J.H.; Chen, C.Y.; Hsu, C.Y.; Shih, C.Y.; Leu, J.G. L-165,041, troglitazone and their combination treatment to attenuate high glucose-induced receptor for advanced glycation end products (RAGE) expression. Eur. J. Pharmacol. 2013, 715, 33–38. [Google Scholar] [CrossRef]
- Liang, Y.J.; Chen, C.Y.; Juang, S.J.; Lai, L.P.; Shyu, K.G.; Wang, B.W.; Liu, S.Y.; Leu, J.G. Peroxisome proliferator-activated receptor delta agonists attenuated the C-reactive protein-induced pro-inflammation in cardiomyocytes and H9c2 cardiomyoblasts. Eur. J. Pharmacol. 2020, 643, 84–92. [Google Scholar] [CrossRef]
- Amin, Z.A.; Ali, H.M.; Alshawsh, M.A.; Darvish, P.H.; Abdulla, M.A. Application of Antrodia camphorata Promotes Rat’s Wound Healing In Vivo and Facilitates Fibroblast Cell Proliferation In Vitro. Evid. Based Complement. Altern. Med. 2015, 2015, 317693. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.C.; Kuo, Y.C.; Huang, R.L.; Lin, L.C.; Don, M.J.; Chang, T.T.; Chou, C.J. New ergostane and lanostane from Antrodia camphorata. J. Chin. Med. 2003, 14, 247–258. [Google Scholar] [CrossRef]
- Leu, J.G.; Su, W.H.; Chen, Y.C.; Liang, Y.J. Hydralazine attenuates renal inflammation in diabetic rats with ischemia/reperfusion acute kidney injury. Eur. J. Pharmacol. 2021, 910, 174468. [Google Scholar] [CrossRef]
- Gal, P.; Kilik, R.; Mokry, M.; Vidinsky, B.; Vasilenko, T.; Mozes, S.; Bobrov, N.; Tomori, Z.; Bober, J.; Lenhardt, L. Simple method of open skin wound healing model in corticosteroid-treated and diabetic rats: Standardization of semi-quantitative and quantitative histological assessments. Vet. Med. 2008, 53, 652–659. [Google Scholar] [CrossRef] [Green Version]
- Leu, J.G.; Wang, C.M.; Chen, C.Y.; Yang, Y.F.; Shih, C.Y.; Lin, J.T.; Chen, H.M.; Liang, Y.J. The Cell Protective Effect of Adenine on Hypoxia-Reoxygenation Injury through PPAR Delta Activation. Life 2021, 11, 1408. [Google Scholar] [CrossRef]
- Heather, B.C.; David, M.M. Extrinsic and intrinsic control of macrophage inflammatory responses. J. Leukoc. Biol. 2013, 94, 913–919. [Google Scholar]
- Ding, N.Z.; Teng, C.B.; Ma, H.; Ni, H.; Ma, X.H.; Xu, L.B.; Yang, Z.M. Peroxisome proliferator-activated receptor delta expression and regulation in mouse uterus during embryo implantation and decidualization. Mol. Reprod. Dev. 2003, 66, 218–224. [Google Scholar] [CrossRef]
- Scholtysek, C.; Katzenbeisser, J.; Fu, H.; Uderhardt, S.; Ipseiz, N.; Stoll, C.; Zaiss, M.M.; Stock, M.; Donhauser, L.; Bohm, C.; et al. PPARbeta/delta governs Wnt signaling and bone turnover. Nat. Med. 2013, 19, 608–613. [Google Scholar] [CrossRef]
- Burdick, A.D.; Kim, D.J.; Peraza, M.A.; Gonzalez, F.J.; Peters, J.M. The role of peroxisome proliferator-activated receptor-beta/delta in epithelial cell growth and differentiation. Cell Signal. 2006, 18, 9–20. [Google Scholar] [CrossRef]
- Tan, N.S.; Michalik, L.; Desvergne, B.; Wahli, W. Genetic- or transforming growth factor-beta 1-induced changes in epidermal peroxisome proliferator-activated receptor beta/delta expression dictate wound repair kinetics. J. Biol Chem. 2005, 280, 18163–18170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavard, J.; Patel, V.; Gutkind, J.S. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev. Cell. 2008, 14, 25–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobov, I.B.; Brooks, P.C.; Lang, R.A. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 11205–11210. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.L.; Shin, I.S.; Kim, J.M.; Choi, J.H.; Byun, J.; Jeon, E.S.; Suh, W.; Kim, D.K. Interaction between Tie receptors modulates angiogenic activity of angiopoietin2 in endothelial progenitor cells. Cardiovasc. Res. 2006, 72, 394–402. [Google Scholar] [CrossRef]
- Magadum, A.; Engel, F.B. PPARβ/δ: Linking Metabolism to Regeneration. Int. J. Mol. Sci. 2018, 10, 2013. [Google Scholar] [CrossRef] [Green Version]
- Tobita, Y.; Arima, T.; Nakano, Y.; Uchiyama, M.; Shimizu, A.; Takahashi, H. Peroxisome Proliferator-Activated Receptor Beta/Delta Agonist Suppresses Inflammation and Promotes Neovascularization. Int. J. Mol. Sci. 2020, 21, 5296. [Google Scholar] [CrossRef]
- Bell-Syer, S.E.; Hart, R.; Crawford, F.; Torgerson, D.J.; Young, P.; Tyrrell, W.; Williams, H.; Russell, I. A systematic review of oral treatments for fungal infections of the skin of the feet. J. Dermatol. Treat. 2001, 12, 69–74. [Google Scholar]


| Grading | Epithelization | PMN | Fibroblast | Collagen |
|---|---|---|---|---|
| 0 | Thickness of cut edges | Absent | Absent | Absent |
| 1 | Migration of cells (<50%) | Mild ST | Mild-ST | Minimal-GT |
| 2 | Migration of cells (>50%) | Mild DL/GT | Mild-GT | Mild-GT |
| 3 | Bridging the excision | Moderate DL/GT | Moderate-GT | Moderate-GT |
| 4 | Keratinization | Marked DL/GT | Marked-GT | Marked-GT |
| Control | AC | p Value | |
|---|---|---|---|
| Epithelization | 2.8 ± 0.13 | 3.7 ± 0.15 | <0.001 |
| PMN infiltration | 2.0 ± 0.21 | 0.4 ± 0.16 | <0.001 |
| Fibroblast | 2.0 ± 0.15 | 0.5 ± 0.17 | <0.001 |
| Collagen | 1.9 ± 0.10 | 0.6 ± 0.16 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, R.-C.; Leu, J.-G.; Chen, Y.-H.; Chen, C.-Y.; Yang, Y.-F.; Yen, C.-C.; Chou, S.-H.; Liang, Y.-J. Topical Application of Antrodia cinnamomea Ointment in Diabetic Wound Healing. Life 2022, 12, 507. https://doi.org/10.3390/life12040507
Su R-C, Leu J-G, Chen Y-H, Chen C-Y, Yang Y-F, Yen C-C, Chou S-H, Liang Y-J. Topical Application of Antrodia cinnamomea Ointment in Diabetic Wound Healing. Life. 2022; 12(4):507. https://doi.org/10.3390/life12040507
Chicago/Turabian StyleSu, Ruey-Chih, Jyh-Gang Leu, Yuan-Hsin Chen, Chao-Yi Chen, Yi-Feng Yang, Chih-Cheng Yen, Shiu-Huey Chou, and Yao-Jen Liang. 2022. "Topical Application of Antrodia cinnamomea Ointment in Diabetic Wound Healing" Life 12, no. 4: 507. https://doi.org/10.3390/life12040507
APA StyleSu, R.-C., Leu, J.-G., Chen, Y.-H., Chen, C.-Y., Yang, Y.-F., Yen, C.-C., Chou, S.-H., & Liang, Y.-J. (2022). Topical Application of Antrodia cinnamomea Ointment in Diabetic Wound Healing. Life, 12(4), 507. https://doi.org/10.3390/life12040507






