The Integration of Cell Therapy and Biomaterials as Treatment Strategies for Remyelination
Abstract
:1. Introduction
1.1. Microglia
1.2. Astrocytes
1.3. Relationship between Microglia and Astrocytes
1.4. Circulating Factors
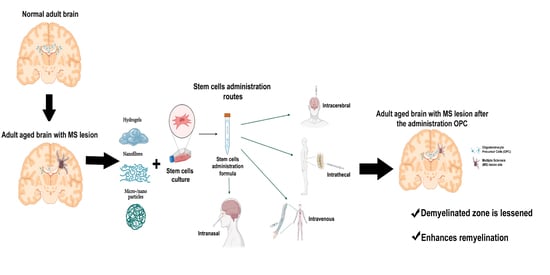
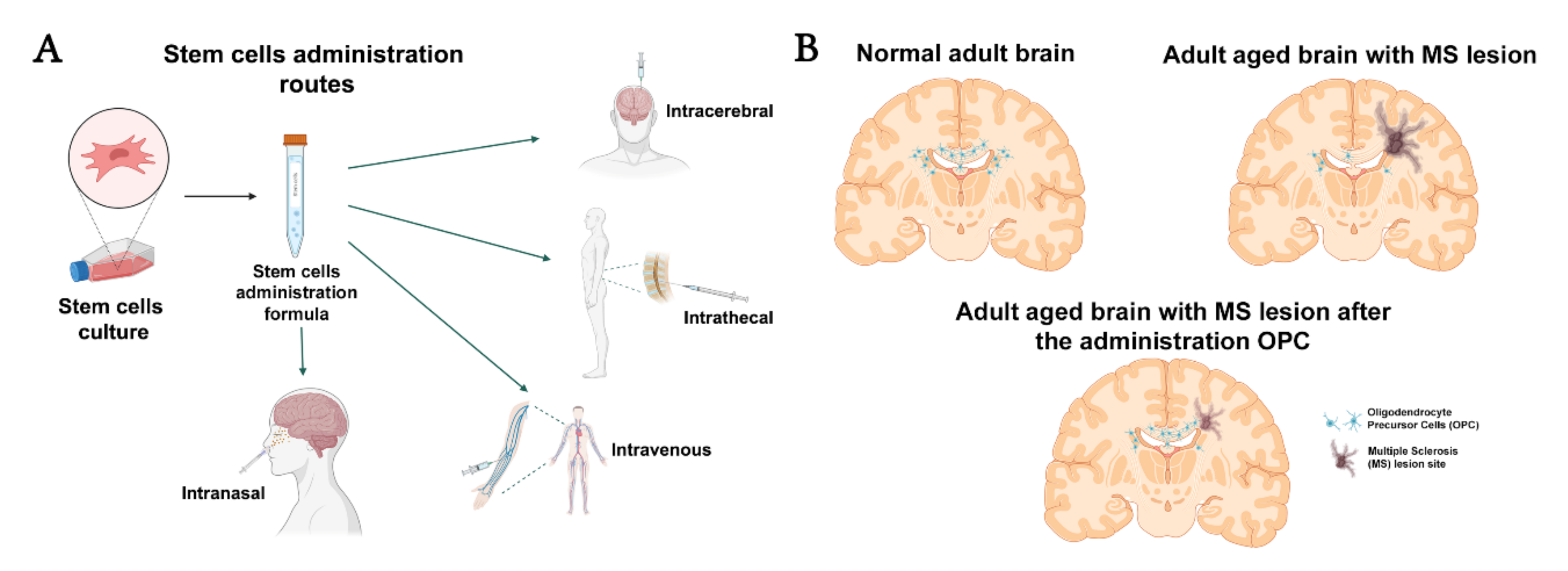
2. Cell Therapy and Types of Cells
2.1. Hematopoietic Stem Cells
2.2. Non-Hematopoietic Stem Cells
2.2.1. Mesenchymal Stem Cells
2.2.2. Adipose-Derived Stem Cells
2.2.3. Neural Stem Cells
2.2.4. Oligodendrocyte Precursor Cells
2.2.5. Oligodendrocytes
2.2.6. Induced Pluripotent Stem Cells
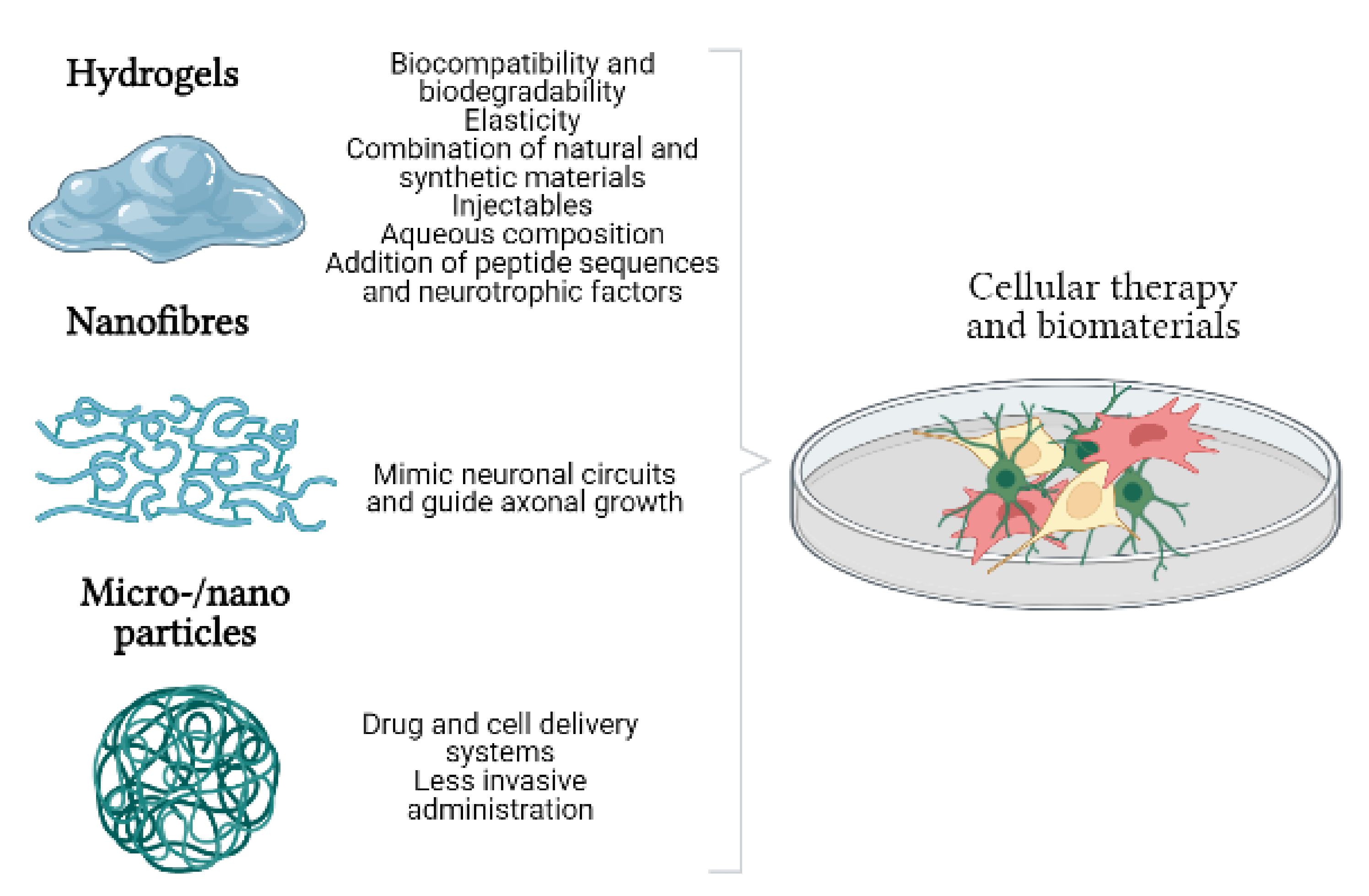
3. Biomaterials and Cell Therapy. Generation of Biohybrids
3.1. Hydrogels
3.2. Nanofibres
3.3. Micro- and Nanoparticles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goldenberg, M.M. Multiple Sclerosis Review. Pharm. Ther. 2012, 37, 175–184. [Google Scholar]
- Pluchino, S.; Smith, J.A.; Peruzzotti-Jametti, L. Promises and Limitations of Neural Stem Cell Therapies for Progressive Multiple Sclerosis. Trends Mol. Med. 2020, 26, 898–912. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, A.; Ghasemi-Kasman, M.; Javan, M. Possible regenerative effects of fingolimod (FTY720) in multiple sclerosis disease: An overview on remyelination process. J. Neurosci. Res. 2020, 98, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.A.; Chun, J. Mechanisms of fingolimod’s efficacy and adverse effects in multiple sclerosis. Ann. Neurol. 2011, 69, 759–777. [Google Scholar] [CrossRef]
- Cadavid, D.; Mellion, M.; Hupperts, R.; Edwards, K.R.; Calabresi, P.A.; Drulović, J.; Giovannoni, G.; Hartung, H.-P.; Arnold, D.L.; Fisher, E.; et al. Safety and efficacy of opicinumab in patients with relapsing multiple sclerosis (SYNERGY): A randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2019, 18, 845–856. [Google Scholar] [CrossRef]
- Gomez-Pinedo, U.; Cuevas, J.A.; Benito-Martín, M.S.; Moreno-Jiménez, L.; Esteban-Garcia, N.; Torre-Fuentes, L.; Matías-Guiu, J.A.; Pytel, V.; Montero, P.; Matías-Guiu, J. Vitamin D increases remyelination by promoting oligodendrocyte lineage differentiation. Brain Behav. 2020, 10, e01498. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Ma, Z.; Qin, H.; Yao, Z. Thyroid Hormone Potentially Benefits Multiple Sclerosis via Facilitating Remyelination. Mol. Neurobiol. 2016, 53, 4406–4416. [Google Scholar] [CrossRef]
- Neumann, B.; Segel, M.; Chalut, K.J.; Franklin, R.J. Remyelination and ageing: Reversing the ravages of time. Mult. Scler. 2019, 25, 1835–1841. [Google Scholar] [CrossRef] [Green Version]
- Patrikios, P.; Stadelmann, C.; Kutzelnigg, A.; Rauschka, H.; Schmidbauer, M.; Laursen, H.; Sorensen, P.S.; Bruck, W.; Lucchinetti, C.; Lassmann, H. Remyelination is extensive in a subset of multiple sclerosis patients. Brain 2006, 129, 3165–3172. [Google Scholar] [CrossRef] [Green Version]
- Heß, K.; Starost, L.; Kieran, N.W.; Thomas, C.; Vincenten, M.C.J.; Antel, J.; Martino, G.; Huitinga, I.; Healy, L.; Kuhlmann, T. Lesion stage-dependent causes for impaired remyelination in MS. Acta Neuropathol. 2020, 140, 359–375. [Google Scholar] [CrossRef]
- Saitoh, S.S.; Tanabe, S.; Muramatsu, R. Circulating factors that influence the central nervous system remyelination. Curr. Opin. Pharmacol. 2022, 62, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, A.F.; Miron, V.E. The pro-remyelination properties of microglia in the central nervous system. Nat. Rev. Neurol. 2019, 15, 447–458. [Google Scholar] [CrossRef] [PubMed]
- Rawji, K.S.; Gonzalez Martinez, G.A.; Sharma, A.; Franklin, R.J.M. The Role of Astrocytes in Remyelination. Trends Neurosci. 2020, 43, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; He, C. Polarization of macrophages and microglia in inflammatory demyelination. Neurosci. Bull. 2013, 29, 189–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franklin, R.J.M.; Frisén, J.; Lyons, D.A. Revisiting remyelination: Towards a consensus on the regeneration of CNS myelin. Semin. Cell Dev. Biol. 2021, 116, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Molina-Gonzalez, I.; Miron, V.E. Astrocytes in myelination and remyelination. Neurosci. Lett. 2019, 713, 134532. [Google Scholar] [CrossRef] [PubMed]
- Mancardi, G.; Saccardi, R. Autologous haematopoietic stem-cell transplantation in multiple sclerosis. Lancet Neurol. 2008, 7, 626–636. [Google Scholar] [CrossRef]
- Gregory, C.A.; Prockop, D.J.; Spees, J.L. Non-hematopoietic bone marrow stem cells: Molecular control of expansion and differentiation. Exp. Cell Res. 2005, 306, 330–335. [Google Scholar] [CrossRef]
- Kassem, M.; Kristiansen, M.; Abdallah, B.M. Mesenchymal Stem Cells: Cell Biology and Potential Use in Therapy. Basic Clin. Pharmacol. Toxicol. 2004, 95, 209–214. [Google Scholar] [CrossRef]
- Rogers, I.; Casper, R.F. Umbilical cord blood stem cells. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 893–908. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Wang, H.; Liu, X.; Zhang, T.; Wang, Y.; Hu, D. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res. Ther. 2015, 6, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.A.; Nicaise, A.M.; Ionescu, R.-B.; Hamel, R.; Peruzzotti-Jametti, L.; Pluchino, S. Stem Cell Therapies for Progressive Multiple Sclerosis. Front. Cell Dev. Biol. 2021, 9, 696434. [Google Scholar] [CrossRef] [PubMed]
- Witherick, J.; Wilkins, A.; Scolding, N.; Kemp, K. Mechanisms of oxidative damage in multiple sclerosis and a cell therapy approach to treatment. Autoimmune Dis. 2010, 2011, 164608. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Huang, Y.; Liu, J.; Lan, Z.; Tang, X.; Hu, Z. The Efficacy of Mesenchymal Stem Cell Therapies in Rodent Models of Multiple Sclerosis: An Updated Systematic Review and Meta-Analysis. Front. Immunol. 2021, 12, 3398. [Google Scholar] [CrossRef]
- Laso-García, F.; Ramos-Cejudo, J.; Carrillo-Salinas, F.J.; Otero-Ortega, L.; Feliú, A.; Frutos, M.G.; Mecha, M.; Díez-Tejedor, E.; Guaza, C.; Gutiérrez-Fernández, M. Therapeutic potential of extracellular vesicles derived from human mesenchymal stem cells in a model of progressive multiple sclerosis. PLoS ONE 2018, 13, e0202590. [Google Scholar] [CrossRef] [Green Version]
- Hasan, A.; Deeb, G.; Rahal, R.; Atwi, K.; Mondello, S.; Marei, H.E.; Gali, A.; Sleiman, E. Mesenchymal Stem Cells in the Treatment of Traumatic Brain Injury. Front. Neurol. 2017, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Constantin, G.; Marconi, S.; Rossi, B.; Angiari, S.; Calderan, L.; Anghileri, E.; Gini, B.; Dorothea Bach, S.; Martinello, M.; Bifari, F.; et al. Adipose-Derived Mesenchymal Stem Cells Ameliorate Chronic Experimental Autoimmune Encephalomyelitis. Stem Cells 2009, 27, 2624–2635. [Google Scholar] [CrossRef]
- Semon, J.A.; Maness, C.; Zhang, X.; Sharkey, S.A.; Beuttler, M.M.; Shah, F.S.; Pandey, A.C.; Gimble, J.M.; Zhang, S.; Scruggs, B.A.; et al. Comparison of human adult stem cells from adipose tissue and bone marrow in the treatment of experimental autoimmune encephalomyelitis. Stem Cell Res. Ther. 2014, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Bowles, A.C.; Strong, A.L.; Wise, R.M.; Thomas, R.C.; Gerstein, B.Y.; Dutreil, M.F.; Hunter, R.S.; Gimble, J.M.; Bunnell, B.A. Adipose Stromal Vascular Fraction-Mediated Improvements at Late-Stage Disease in a Murine Model of Multiple Sclerosis. Stem Cells 2017, 35, 532–544. [Google Scholar] [CrossRef] [Green Version]
- Stepien, A.; Dabrowska, N.L.; Maciagowska, M.; Macoch, R.P.; Zolocinska, A.; Mazur, S.; Siennicka, K.; Frankowska, E.; Kidzinski, R.; Chalimoniuk, M.; et al. Clinical Application of Autologous Adipose Stem Cells in Patients with Multiple Sclerosis: Preliminary Results. Mediat. Inflamm. 2016, 2016, e5302120. [Google Scholar] [CrossRef]
- Grochowski, C.; Radzikowska, E.; Maciejewski, R. Neural stem cell therapy—Brief review. Clin. Neurol. Neurosurg. 2018, 173, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Willis, C.M.; Nicaise, A.M.; Peruzzotti-Jametti, L.; Pluchino, S. The neural stem cell secretome and its role in brain repair. Brain Res. 2020, 1729, 146615. [Google Scholar] [CrossRef] [PubMed]
- Pluchino, S.; Quattrini, A.; Brambilla, E.; Gritti, A.; Salani, G.; Dina, G.; Galli, R.; Del Carro, U.; Amadio, S.; Bergami, A.; et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature 2003, 422, 688–694. [Google Scholar] [CrossRef]
- McIntyre, L.L.; Greilach, S.A.; Othy, S.; Sears-Kraxberger, I.; Wi, B.; Ayala-Angulo, J.; Vu, E.; Pham, Q.; Silva, J.; Dang, K.; et al. Regulatory T cells promote remyelination in the murine experimental autoimmune encephalomyelitis model of multiple sclerosis following human neural stem cell transplant. Neurobiol. Dis. 2020, 140, 104868. [Google Scholar] [CrossRef]
- Bae, D.-K.; Park, D.; Lee, S.H.; Yang, G.; Kyung, J.; Kim, D.; Shin, K.; Choi, E.-K.; Kim, G.; Hong, J.T.; et al. Comparative Effects of Human Neural Stem Cells and Oligodendrocyte Progenitor Cells on the Neurobehavioral Disorders of Experimental Autoimmune Encephalomyelitis Mice. Stem Cells Int. 2016, 2016, 4079863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.-M.; Zhang, L.-F.; Ding, P.-S.; Liu, Y.-J.; Wu, X.; Zhou, J.-N. Microglial activation mediates host neuronal survival induced by neural stem cells. J. Cell Mol. Med. 2014, 18, 1300–1312. [Google Scholar] [CrossRef]
- Scolding, N.J.; Pasquini, M.; Reingold, S.C.; Cohen, J.A. Cell-based therapeutic strategies for multiple sclerosis. Brain 2017, 140, 2776–2796. [Google Scholar] [CrossRef] [Green Version]
- Czepiel, M.; Balasubramaniyan, V.; Schaafsma, W.; Stancic, M.; Mikkers, H.; Huisman, C.; Boddeke, E.; Copray, S. Differentiation of induced pluripotent stem cells into functional oligodendrocytes. Glia 2011, 59, 882–892. [Google Scholar] [CrossRef]
- Mikaeili Agah, E.; Parivar, K.; Joghataei, M.T. Therapeutic Effect of Transplanted Human Wharton’s Jelly Stem Cell-Derived Oligodendrocyte Progenitor Cells (hWJ-MSC-derived OPCs) in an Animal Model of Multiple Sclerosis. Mol. Neurobiol. 2014, 49, 625–632. [Google Scholar] [CrossRef]
- Zhang, H.-T.; Fan, J.; Cai, Y.-Q.; Zhao, S.-J.; Xue, S.; Lin, J.-H.; Jiang, X.-D.; Xu, R.-X. Human Wharton’s jelly cells can be induced to differentiate into growth factor-secreting oligodendrocyte progenitor-like cells. Differentiation 2010, 79, 15–20. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Denham, J.; Thies, R.S. Oligodendrocyte Progenitor Cells Derived from Human Embryonic Stem Cells Express Neurotrophic Factors. Stem Cells Dev. 2006, 15, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Hatch, M.N.; Schaumburg, C.S.; Lane, T.E.; Keirstead, H.S. Endogenous remyelination is induced by transplant rejection in a viral model of multiple sclerosis. J. Neuroimmunol. 2009, 212, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Pinedo, U.; Matías-Guiu, J.A.; Benito-Martín, M.S.; Moreno-Jiménez, L.; Sanclemente-Alamán, I.; Selma-Calvo, B.; Pérez-Suarez, S.; Sancho-Bielsa, F.; Canales-Aguirre, A.; Mateos-Díaz, J.C.; et al. Intranasal Administration of Undifferentiated Oligodendrocyte Lineage Cells as a Potential Approach to Deliver Oligodendrocyte Precursor Cells into Brain. Int. J. Mol. Sci. 2021, 22, 10738. [Google Scholar] [CrossRef] [PubMed]
- Matías-Guiu, J.; Matías-Guiu, J.A.; Montero-Escribano, P.; Barcia, J.A.; Canales-Aguirre, A.A.; Mateos-Diaz, J.C.; Gómez-Pinedo, U. Particles Containing Cells as a Strategy to Promote Remyelination in Patients With Multiple Sclerosis. Front. Neurol. 2020, 11, 638. [Google Scholar] [CrossRef]
- Kuhn, S.; Gritti, L.; Crooks, D.; Dombrowski, Y. Oligodendrocytes in Development, Myelin Generation and Beyond. Cells 2019, 8, 1424. [Google Scholar] [CrossRef] [Green Version]
- Dulamea, A.O. The contribution of oligodendrocytes and oligodendrocyte progenitor cells to central nervous system repair in multiple sclerosis: Perspectives for remyelination therapeutic strategies. Neural. Regen. Res. 2017, 12, 1939–1944. [Google Scholar] [CrossRef]
- García-León, J.A.; García-Díaz, B.; Eggermont, K.; Cáceres-Palomo, L.; Neyrinck, K.; Madeiro da Costa, R.; Dávila, J.C.; Baron-Van Evercooren, A.; Gutiérrez, A.; Verfaillie, C.M. Generation of oligodendrocytes and establishment of an all-human myelinating platform from human pluripotent stem cells. Nat. Protoc. 2020, 15, 3716–3744. [Google Scholar] [CrossRef]
- Mozafari, S.; Starost, L.; Manot-Saillet, B.; Garcia-Diaz, B.; Xu, Y.K.T.; Roussel, D.; Levy, M.J.F.; Ottoboni, L.; Kim, K.-P.; Schöler, H.R.; et al. Multiple sclerosis iPS-derived oligodendroglia conserve their properties to functionally interact with axons and glia in vivo. Sci. Adv. 2020, 6, eabc6983. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, J.; Li, X.; Xu, H.; Wang, W.; Wang, L.; Zhao, X.; Li, W.; Jiao, J.; Hu, B.; et al. Treatment of multiple sclerosis by transplantation of neural stem cells derived from induced pluripotent stem cells. Sci. China Life Sci. 2016, 59, 950–957. [Google Scholar] [CrossRef] [Green Version]
- Barbar, L.; Jain, T.; Zimmer, M.; Kruglikov, I.; Sadick, J.; Wang, M.; Kalpana, K.; Rose, I.V.L.; Burstein, S.R.; Rusielewicz, T.; et al. CD49f is a novel marker of functional and reactive human iPSC-derived astrocytes. Neuron 2020, 107, 436–453. [Google Scholar] [CrossRef]
- Mutukula, N.; Man, Z.; Takahashi, Y.; Iniesta Martinez, F.; Morales, M.; Carreon-Guarnizo, E.; Hernandez Clares, R.; Garcia-Bernal, D.; Martinez Martinez, L.; Lajara, J.; et al. Generation of RRMS and PPMS specific iPSCs as a platform for modeling Multiple Sclerosis. Stem Cell Res. 2021, 53, 102319. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Liu, Y.-Q.; Guan, Y.-T.; Zhang, G.-X. Induced Stem Cells as a Novel Multiple Sclerosis Therapy. Curr. Stem Cell Res. Ther. 2016, 11, 313–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assunção-Silva, R.C.; Gomes, E.D.; Sousa, N.; Silva, N.A.; Salgado, A.J. Hydrogels and Cell Based Therapies in Spinal Cord Injury Regeneration. Stem Cells Int. 2015, 2015, 948040. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Cui, L.; Brunson, C.; Zhao, W.; Bhat, N.R.; Zhang, N.; Wen, X. Engineering an in situ crosslinkable hydrogel for enhanced remyelination. FASEB J. 2013, 27, 1127–1136. [Google Scholar] [CrossRef] [Green Version]
- Hoveizi, E.; Tavakol, S.; Ebrahimi-Barough, S. Neuroprotective Effect of Transplanted Neural Precursors Embedded on PLA/CS Scaffold in an Animal Model of Multiple Sclerosis. Mol. Neurobiol. 2015, 51, 1334–1342. [Google Scholar] [CrossRef]
- Rezaei, S.; Dabirmanesh, B.; Zare, L.; Golestani, A.; Javan, M.; Khajeh, K. Enhancing myelin repair in experimental model of multiple sclerosis using immobilized chondroitinase ABC I on porous silicon nanoparticles. Int. J. Biol. Macromol. 2020, 146, 162–170. [Google Scholar] [CrossRef]
- Naeimi, R.; Safarpour, F.; Hashemian, M.; Tashakorian, H.; Ahmadian, S.R.; Ashrafpour, M.; Ghasemi-Kasman, M. Curcumin-loaded nanoparticles ameliorate glial activation and improve myelin repair in lyolecithin-induced focal demyelination model of rat corpus callosum. Neurosci. Lett. 2018, 674, 1–10. [Google Scholar] [CrossRef]
| Cell Type | Disease | Clinical Trial Code | Patients | Procedure | Date | Results |
|---|---|---|---|---|---|---|
| HSCs | MS | NCT00273364 | n = 110 18–55 years | Immunosuppression followed by autologous hematopoietic cell transplantation | Completed 5 January 2017–30 August 2019 | Severe adverse reactions: febrile neutropenia, hypophosphatemia, hypokalemia, hyperglycemia |
| MSCs | MS | NCT04823000 | n = 24 18–65 years MS (RRMS, SPMS, PPMS) | Multiple intrathecal or intravenous transplants of autologous MSCs at 6–12 month intervals | Completed 1 January 2013–1 April 2020 | No published results |
| MS | NCT01377870 | n = 22 18–55 years RRMS | Intravenous transplantation of MSCs | Completed June 2011–April 2014 | No published results | |
| NSCs | MS | NCT03269071 | n = 4 18–55 years Progressive MS | Intrathecal transplantation of 4 different volumes of NSCs | Completed 17 May 2021–31 July 2021 | No published results |
| MS | NCT03282760 | n = 24 18–60 years SPMS | Intraventricular transplantation of different volumes of NSCs for 3 months followed by immunosuppression for 6 months | Completed 9 September 2017–29 May 2021 | No published results | |
| OPCs | Cervical spinal cord injury | NCT02302157 | n = 25 18–69 years | AST-OPC transplantation. A total of 5 cohorts (3 dosages: one injection of 2 million or 10 million cells; or 2 injections of 10 million cells) | Completed March 2015–December 2018 | Adverse reactions: urinary tract infection, sepsis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Muguruza, E.; Villar-Gómez, N.; Matias-Guiu, J.A.; Selma-Calvo, B.; Moreno-Jiménez, L.; Sancho-Bielsa, F.; Lopez-Carbonero, J.; Benito-Martín, M.S.; García-Flores, S.; Bonel-García, N.; et al. The Integration of Cell Therapy and Biomaterials as Treatment Strategies for Remyelination. Life 2022, 12, 474. https://doi.org/10.3390/life12040474
López-Muguruza E, Villar-Gómez N, Matias-Guiu JA, Selma-Calvo B, Moreno-Jiménez L, Sancho-Bielsa F, Lopez-Carbonero J, Benito-Martín MS, García-Flores S, Bonel-García N, et al. The Integration of Cell Therapy and Biomaterials as Treatment Strategies for Remyelination. Life. 2022; 12(4):474. https://doi.org/10.3390/life12040474
Chicago/Turabian StyleLópez-Muguruza, Eneritz, Natalia Villar-Gómez, Jordi A. Matias-Guiu, Belen Selma-Calvo, Lidia Moreno-Jiménez, Francisco Sancho-Bielsa, Juan Lopez-Carbonero, María Soledad Benito-Martín, Silvia García-Flores, Natalia Bonel-García, and et al. 2022. "The Integration of Cell Therapy and Biomaterials as Treatment Strategies for Remyelination" Life 12, no. 4: 474. https://doi.org/10.3390/life12040474
APA StyleLópez-Muguruza, E., Villar-Gómez, N., Matias-Guiu, J. A., Selma-Calvo, B., Moreno-Jiménez, L., Sancho-Bielsa, F., Lopez-Carbonero, J., Benito-Martín, M. S., García-Flores, S., Bonel-García, N., Kamal, O. M.-F., Ojeda-Hernández, D., Matías-Guiu, J., & Gómez-Pinedo, U. (2022). The Integration of Cell Therapy and Biomaterials as Treatment Strategies for Remyelination. Life, 12(4), 474. https://doi.org/10.3390/life12040474