SARS-CoV-2 Leads to Significantly More Severe Olfactory Loss than Other Seasonal Cold Viruses
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Study Population
3.2. Subjective Smell and/or Taste Loss
3.3. Psychophysical Testing of Smell and/or Taste Function
3.4. Identification of Pathogens Other than SARS-CoV-2
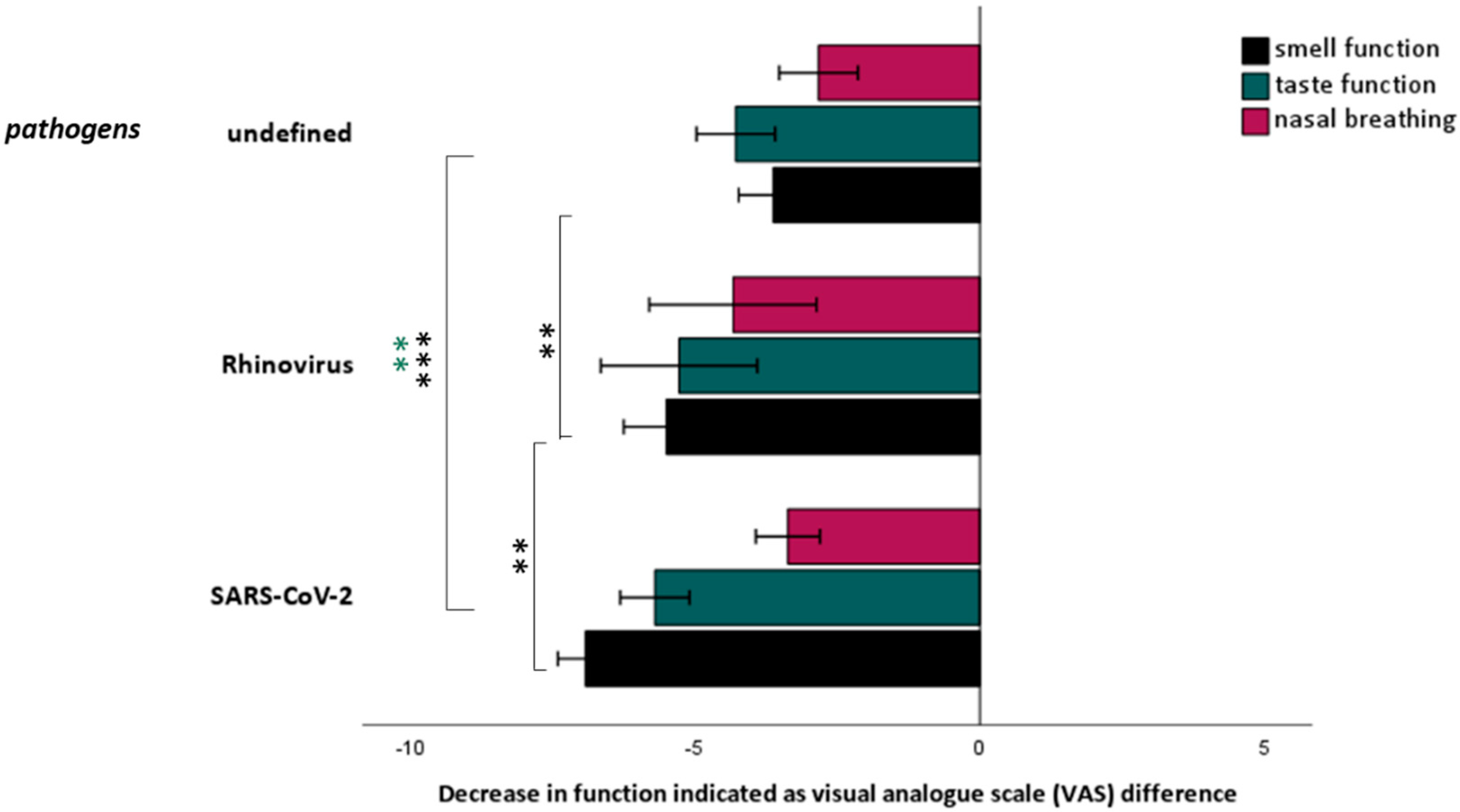
3.5. Comparison between Smell and Taste Loss Related to SARS-CoV-2 and Other Causes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heikkinen, T.; Jarvinen, A. The common cold. Lancet 2003, 361, 51–59. [Google Scholar] [CrossRef]
- Sugiura, M.; Aiba, T.; Mori, J.; Nakai, Y. An epidemiological study of postviral olfactory disorder. Acta Oto-Laryngol. 1998, 538, 191–196. [Google Scholar]
- Wang, J.H.; Kwon, H.J.; Jang, Y.J. Detection of parainfluenza virus 3 in turbinate epithelial cells of postviral olfactory dysfunction patients. Laryngoscope 2007, 117, 1445–1449. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidis, I.; Haehner, A.; Frasnelli, J.; Reden, J.; Quante, G.; Damm, M.; Hummel, T. Post-infectious olfactory dysfunction exhibits a seasonal pattern. Rhinology 2006, 44, 135–139. [Google Scholar] [PubMed]
- Suzuki, M.; Saito, K.; Min, W.P.; Vladau, C.; Toida, K.; Itoh, H.; Murakami, S. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope 2007, 117, 272–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lechien, J.R.; Chiesa-Estomba, C.M.; Siati, D.R.D.; Horoi, M.; Bon, S.D.; Rodriguez, A.; Dequanter, D.; Blecic, S.; Afia, F.E.; Distinguin, L.; et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. Eur. Arch. Oto-Rhino-Laryngol. 2020, 277, 2251–2261. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, A.; Pezzati, L.; Conti, F.; Bernacchia, D.; Siano, M.; Oreni, L.; Rusconi, S.; Gervasoni, C.; Ridolfo, A.L.; Rizzardini, G.; et al. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: A cross-sectional study. Clin. Infect. Dis. 2020, 71, 889–890. [Google Scholar] [CrossRef] [Green Version]
- Vaira, L.A.; Deiana, G.; Fois, A.G.; Pirina, P.; Madeddu, G.; De Vito, A.; Babudieri, S.; Petrocelli, M.; Serra, A.; Bussu, F.; et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: Single-center experience on 72 cases. Head Neck 2020, 42, 1252–1258. [Google Scholar] [CrossRef]
- Gane, S.B.; Kelly, C.; Hopkins, C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology 2020, 58, 299–301. [Google Scholar] [CrossRef]
- Khan, M.; Yoo, S.J.; Clijsters, M.; Backaert, W.; Vanstapel, A.; Speleman, K.; Lietaer, C.; Choi, S.; Hether, T.D.; Marcelis, L.; et al. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell 2021, 184, 5932–5949. [Google Scholar] [CrossRef]
- Beltrán-Corbellini, Á.; Chico-García, J.L.; Martínez-Poles, J.; Rodríguez-Jorge, F.; Natera-Villalba, E.; Gómez-Corral, J.; Gómez-López, A.; Monreal, E.; Parra-Díaz, P.; Cortés-Cuevas, J.L.; et al. Acute-onset smell and taste disorders in the context of COVID-19: A pilot multicenter PCR-based case-control study. Eur. J. Neurol. 2020, 27, 1738–1741. [Google Scholar] [CrossRef] [PubMed]
- Huart, C.; Philpott, C.; Konstantinidis, I.; Altundag, A.; Whitcroft, K.L.; Trecca, E.M.C.; Cassano, M.; Rombaux, P.; Hummel, T. Comparison of COVID-19 and common cold chemosensory dysfunction. Rhinology 2020, 58, 623–625. [Google Scholar]
- Yan, C.H.; Faraji, F.; Prajapati, D.P.; Boone, C.E.; Conde, A.S.D. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int. Forum Allergy Rhinol. 2020, 10, 806–813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, C.; Renner, B. A new procedure for the short screening of olfactory function using five items from the “Sniffin’ Sticks” identification test kit. Am. J. Rhinol. 2006, 20, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Landis, B.N.; Welge-Luessen, A.; Brämerson, A.; Bende, M.; Mueller, C.A.; Nordin, S.; Hummel, T. “Taste Strips”—A rapid, lateralized, gustatory bedside identification test based on impregnated filter papers. J. Neurol. 2009, 256, 242–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BioFire Diagnostics LLC. FilmArray Respiratory Panel 2 (RP2) Instruction Booklet RFIT-ASY-0129; BioFire Diagnostics LLC: Salt Lake City, UT, USA, 2017. [Google Scholar]
- RKI Testkriterien. Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Teststrategie/Testkriterien_Herbst_Winter.html (accessed on 25 September 2020).
- Hintschich, C.A.; Wenzel, J.J.; Hummel, T.; Hankir, M.K.; Kühnel, T.; Vielsmeier, V.; Bohr, C. Psychophysical tests reveal impaired olfaction but preserved gustation in COVID-19 patients. Int. Forum Allergy Rhinol. 2020, 10, 1105–1107. [Google Scholar] [CrossRef] [PubMed]
- Sorokowska, A.; Oleszkiewicz, A.; Minovi, A.; Konnerth, C.G.; Hummel, T. Fast Screening of Olfactory Function Using the Q-Sticks Test. ORL J. Oto-Rhino-Laryngol. Relat. Spec. 2019, 81, 245–251. [Google Scholar] [CrossRef]
- Petrocelli, M.; Ruggiero, F.; Baietti, A.M.; Pandolfi, P.; Salzano, G.; Salzano, F.A. Remote psychophysical evaluation of olfactory and gustatory functions in early-stage coronavirus disease 2019 patients: The Bologna experience of 300 cases. J. Laryngol. Otol. 2020, 134, 571–576. [Google Scholar] [CrossRef]
- Konstantinidis, I.; Delides, A.; Tsakiropoulou, E.; Maragoudakis, P.; Sapounas, S.; Tsiodras, S. Short-term follow-up of self-isolated COVID-19 patients with smell and taste dysfunction in Greece: Two phenotypes of recovery. ORL J. Oto-Rhino-Laryngol. Relat. Spec. 2020, 82, 95–303. [Google Scholar] [CrossRef]
- Leuzinger, K.; Roloff, T.; Gosert, R.; Sogaard, K.; Naegele, K.; Rentsch, K.; Bingisser, R.; Nickel, C.H.; Pargger, H.; Bassetti, S.; et al. Epidemiology of Severe Acute Respiratory Syndrome Coronavirus 2 Emergence Amidst Community-Acquired Respiratory Viruses. J. Infect. Dis. 2020, 222, 1270–1279. [Google Scholar] [CrossRef]
- Akerlund, A.; Bende, M.; Murphy, C. Olfactory threshold and nasal mucosal changes in experimentally induced common cold. Acta Otolaryngol. 1995, 115, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Gurrola, J.G.; Chang, J.L.; Roland, L.T.; Loftus, P.A.; Cheung, S.W. Short-term chemosensory distortions and phantoms in COVID-19. Laryngoscope Investig. Otolaryngol. 2021, 6, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Parma, V.; Ohla, K.; Veldhuizen, M.G.; Niv, M.Y.; Kelly, C.E.; Bakke, A.J.; Cooper, K.W.; Bouysset, C.; Pirastu, N.; Dibattista, M.; et al. More Than Smell-COVID-19 Is Associated With Severe Impairment of Smell, Taste, and Chemesthesis. Chem. Senses 2020, 45, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Raad, N.; Ghorbani, J.; Naeini, A.S.; Tajik, N.; Karimi-Galougahi, M. Parosmia in patients with COVID-19 and olfactory dysfunction. Int. Forum Allergy Rhinol. 2021, 11, 1497–1500. [Google Scholar] [CrossRef]
- Haehner, A.; Draf, J.; Dräger, S.; With, K.d.; Hummel, T. Predictive Value of Sudden Olfactory Loss in the Diagnosis of COVID-19. ORL J. Oto-Rhino-Laryngol. Relat. Spec. 2020, 82, 175–180. [Google Scholar] [CrossRef]
| SARS-CoV-2 | Total | ||||
|---|---|---|---|---|---|
| Negative | Positive | ||||
| Subjective smell and/or taste loss | No | n | 1316 | 490 | 1806 |
| % | 72.9 | 27.1 | 100 | ||
| Yes | n | 177 | 137 | 314 | |
| % | 56.4 | 43.6 | 100 | ||
| Total | n | 1493 | 627 | 2120 | |
| SARS-CoV-2 | Total | ||||
|---|---|---|---|---|---|
| Negative | Positive | ||||
| Psychophysically determined smell loss | No | n | 61 | 13 | 74 |
| % | 82.4 | 17.6 | 100 | ||
| Yes | n | 64 | 100 | 164 | |
| % | 39.0 | 61.0 | 100 | ||
| Total | n | 125 | 113 | 238 | |
| SARS-CoV-2 | Total | ||||
|---|---|---|---|---|---|
| Negative | Positive | ||||
| Psychophysically determined taste loss | No | n | 91 | 86 | 177 |
| % | 51.4 | 48.6 | 100 | ||
| Yes | n | 34 | 27 | 61 | |
| % | 55.7 | 44.3 | 100 | ||
| Total | n | 125 | 113 | 238 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haehner, A.; Marquardt, B.; Kardashi, R.; de With, K.; Rößler, S.; Landis, B.N.; Welge-Luessen, A.; Hummel, T. SARS-CoV-2 Leads to Significantly More Severe Olfactory Loss than Other Seasonal Cold Viruses. Life 2022, 12, 461. https://doi.org/10.3390/life12030461
Haehner A, Marquardt B, Kardashi R, de With K, Rößler S, Landis BN, Welge-Luessen A, Hummel T. SARS-CoV-2 Leads to Significantly More Severe Olfactory Loss than Other Seasonal Cold Viruses. Life. 2022; 12(3):461. https://doi.org/10.3390/life12030461
Chicago/Turabian StyleHaehner, Antje, Belinda Marquardt, Romina Kardashi, Katja de With, Susann Rößler, Basile Nicolas Landis, Antje Welge-Luessen, and Thomas Hummel. 2022. "SARS-CoV-2 Leads to Significantly More Severe Olfactory Loss than Other Seasonal Cold Viruses" Life 12, no. 3: 461. https://doi.org/10.3390/life12030461
APA StyleHaehner, A., Marquardt, B., Kardashi, R., de With, K., Rößler, S., Landis, B. N., Welge-Luessen, A., & Hummel, T. (2022). SARS-CoV-2 Leads to Significantly More Severe Olfactory Loss than Other Seasonal Cold Viruses. Life, 12(3), 461. https://doi.org/10.3390/life12030461






