SD-OCT Biomarkers and the Current Status of Artificial Intelligence in Predicting Progression from Intermediate to Advanced AMD
Abstract
:1. Introduction
2. Method of Literature Search
2.1. Study Selection
2.2. Quality Assessment of Included Studies
3. Degeneration of RPE in AMD’s Pathogenesis
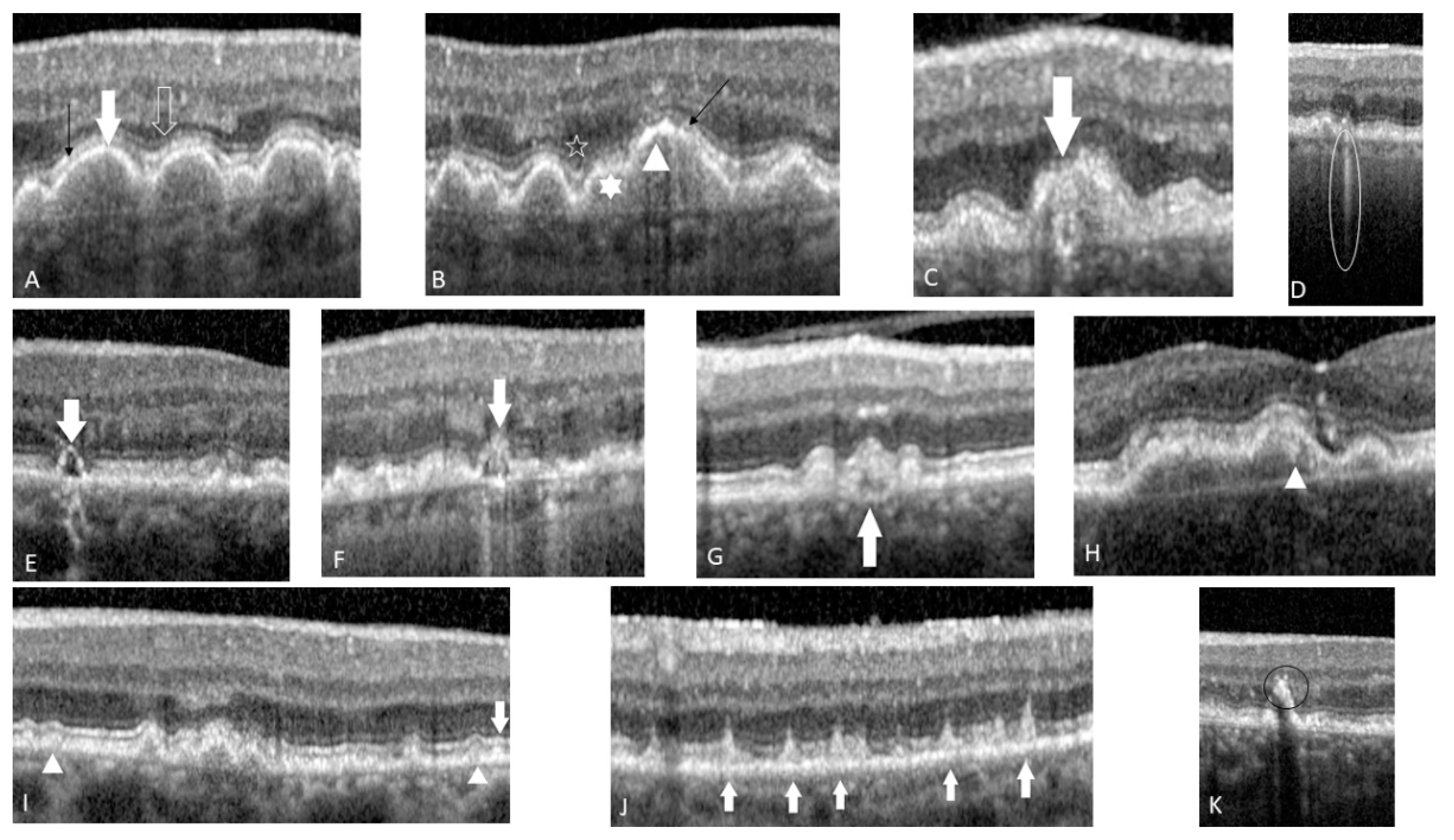
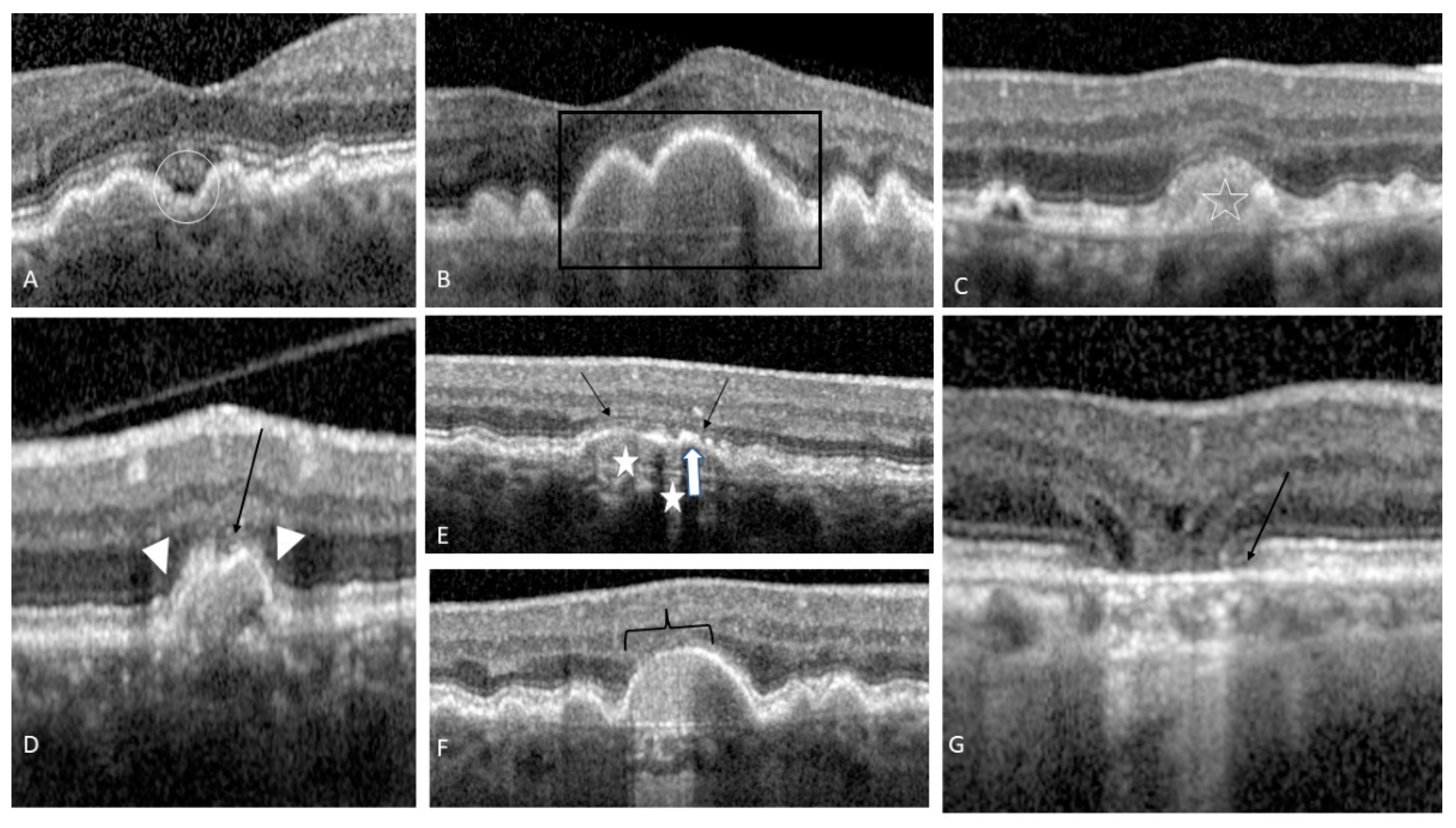
4. Predictive OCT Biomarkers
4.1. Druse Qualitative and Quantitative Features
4.2. OCT-Reflective Drusen Substructures
4.3. Reticular Pseudo-Drusen or Subretinal Drusenoid Deposits
4.4. Hyperreflective Foci
4.5. Sub-RPE Hyper-Reflective Columns
4.6. Ellipsoid Zone Disruption
4.7. Drusen with Sub-Retinal Fluid
4.8. Acquired Vitelliform Lesion
4.9. RPE Thickening
4.10. Drusenoid PED
4.11. Focal Irregular Thinning or the Retina
4.12. Nascent GA
5. Artificial Intelligence’s Contribution in Intermediate AMD Progression
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- Colijn, J.M.; Meester-Smoor, M.; Verzijden, T.; de Breuk, A.; Silva, R.; Merle, B.M.J.; Cougnard-Grégoire, A.; Hoyng, C.B.; Fauser, S.; Coolen, A.; et al. Genetic Risk, Lifestyle, and Age-Related Macular Degeneration in Europe: The EYE-RISK Consortium. Ophthalmology 2021, 128, 1039–1049. [Google Scholar] [CrossRef]
- McLeod, D.S.; Grebe, R.; Bhutto, I.; Merges, C.; Baba, T.; Lutty, G.A. Relationship between RPE and choriocapillaris in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4982–4991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leuschen, J.N.; Schuman, S.G.; Winter, K.P.; McCall, M.N.; Wong, W.T.; Chew, E.Y.; Hwang, T.; Srivastava, S.; Sarin, N.; Clemons, T.; et al. Spectral-Domain optical coherence tomography characteristics of intermediate age-related macular degeneration. Ophthalmology 2013, 120, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Bearelly, S.; Chau, F.Y.; Koreishi, A.; Stinnett, S.S.; Izatt, J.A.; Toth, C.A. Spectral domain optical coherence tomography imaging of geographic atrophy margins. Ophthalmology 2009, 116, 1762–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brar, M.; Kozak, I.; Cheng, L.; Bartsch, D.U.; Yuson, R.; Nigam, N.; Oster, S.F.; Mojana, F.; Freeman, W.R. Correlation between spectral-domain optical coherence tomography and fundus autofluorescence at the margins of geographic atrophy. Am. J. Ophthalmol. 2009, 148, 439–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivert, L.; Kong, J.; Gouras, P. Changes in the choroidal circulation of rabbit following RPE removal. Graefes Arch. Clin. Exp. Ophthalmol. 2003, 241, 656–666. [Google Scholar] [CrossRef]
- Del Priore, L.V.; Kaplan, H.J.; Hornbeck, R.; Swinn, M. Retinal pigment epithelial debridement as a model for the pathogenesis and treatment of macular degeneration. Am. J. Ophthalmol. 1996, 122, 629–643. [Google Scholar] [CrossRef]
- Blaauwgeers, H.G.; Holtkamp, G.M.; Rutten, H.; Witmer, A.N.; Koolwijk, P.; Partanen, T.A.; Alitalo, K.; Kroon, M.E.; Kijlstra, A.; van Hinsbergh, V.W.; et al. Polarized vascular endothelial growth factor secretion by human retinal pigment epithelium and localization of vascular endothelial growth factor receptors on the inner choriocapillaris. Evidence for a trophic paracrine relation. Am. J. Pathol. 1999, 155, 421–428. [Google Scholar] [CrossRef]
- Fritsche, L.G.; Igl, W.; Bailey, J.N.; Grassmann, F.; Sengupta, S.; Bragg-Gresham, J.L.; Burdon, K.P.; Hebbring, S.J.; Wen, C.; Gorski, M.; et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and. common variants. Nat. Genet. 2016, 48, 134–143. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.V.; Leitner, W.P.; Rivest, A.J.; Staples, M.K.; Radeke, M.J.; Anderson, D.H. The Alzheimer’s A β-peptide is deposited at sites of complement activation in pathologic deposits associated with aging and age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 11830–11835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Sisternes, L.; Simon, N.; Tibshirani, R.; Leng, T.; Rubin, D.L. Quantitative SD-OCT imaging biomarkers as indicators of age-related macular degeneration progression. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7093–7103. [Google Scholar] [CrossRef] [PubMed]
- Yehoshua, Z.; Wang, F.; Rosenfeld, P.J.; Penha, F.M.; Feuer, W.J.; Gregori, G. Natural history of drusen morphology in age-related macular degeneration using spectral domain optical coherence tomography. Ophthalmology 2011, 118, 2434–2441. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Erfurth, U.; Klimscha, S.; Waldstein, S.M.; Bogunović, H. A view of the current and future role of optical coherence tomography in the management of age-related macular degeneration. Eye 2017, 31, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Khanifar, A.A.; Koreishi, A.F.; Izatt, J.A.; Toth, C.A. Drusen ultrastructure imaging with spectral domain optical coherence tomography in age-related macular degeneration. Ophthalmology 2008, 115, 1883–1890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veerappan, M.; El-Hage-Sleiman, A.M.; Tai, V.; Chiu, S.J.; Winter, K.P.; Stinnett, S.S.; Hwang, T.S.; Hubbard, G.B., 3rd; Michelson, M.; Gunther, R.; et al. Age-Related Eye Disease Study 2 Ancillary Spectral Domain Optical Coherence Tomography Study Group. Optical Coherence Tomography Reflective Drusen Substructures Predict Progression to Geographic Atrophy in Age-related Macular Degeneration. Ophthalmology 2016, 123, 2554–2570. [Google Scholar] [CrossRef] [Green Version]
- Abdelfattah, N.S.; Zhang, H.; Boyer, D.S.; Rosenfeld, P.J.; Feuer, W.J.; Gregori, G.; Sadda, S.R. Drusen Volume as a Predictor of Disease Progression in Patients with Late Age-Related Macular Degeneration in the Fellow Eye. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1839–1846. [Google Scholar] [CrossRef]
- Schlanitz, F.G.; Baumann, B.; Kundi, M.; Sacu, S.; Baratsits, M.; Scheschy, U.; Shahlaee, A.; Mittermüller, T.J.; Montuoro, A.; Roberts, P.; et al. Drusen volume development over time and its relevance to the course of age-related macular degeneration. Br. J. Ophthalmol. 2017, 101, 198–203. [Google Scholar] [CrossRef]
- Ly, A.; Yapp, M.; Nivison-Smith, L.; Assaad, N.; Hennessy, M.; Kalloniatis, M. Developing prognostic biomarkers in intermediate age-related macular degeneration: Their clinical use in predicting progression. Clin. Exp. Optom. 2018, 101, 172–181. [Google Scholar] [CrossRef] [Green Version]
- Klein, M.L.; Ferris, F.L., 3rd; Armstrong, J.; Hwang, T.S.; Chew, E.Y.; Bressler, S.B.; Chandra, S.R. AREDS Research Group. Retinal precursors and the development of geographic atrophy in age-related macular degeneration. Ophthalmology 2008, 115, 1026–1031. [Google Scholar] [CrossRef]
- Johnson, L.V.; Leitner, W.P.; Staples, M.K.; Anderson, D.H. Complement activation and inflammatory processes in Drusen formation and age-related macular degeneration. Exp. Eye. Res. 2001, 73, 887–896. [Google Scholar] [CrossRef]
- Anderson, D.H.; Ozaki, S.; Nealon, M.; Neitz, J.; Mullins, R.F.; Hageman, G.S.; Johnson, L.V. Local cellular sources of apolipoprotein E in the human retina and retinal pigmented epithelium: Implications for the process of drusen formation. Am. J. Ophthalmol. 2001, 131, 767–781. [Google Scholar] [CrossRef]
- Curcio, C.A.; Messinger, J.D.; Sloan, K.R.; McGwin, G.; Medeiros, N.E.; Spaide, R.F. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: Morphology, prevalence, topography, and biogenesis model. Retina 2013, 33, 265–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarubina, A.V.; Neely, D.C.; Clark, M.E.; Huisingh, C.E.; Samuels, B.C.; Zhang, Y.; McGwin, G., Jr.; Owsley, C.; Curcio, C.A. Prevalence of Subretinal Drusenoid Deposits in Older Persons with and without Age-Related Macular Degeneration, by Multimodal Imaging. Ophthalmology 2016, 123, 1090–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zweifel, S.A.; Spaide, R.F.; Curcio, C.A.; Malek, G.; Imamura, Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology 2010, 117, 303–312.e1. [Google Scholar] [CrossRef] [PubMed]
- Querques, G.; Canouï-Poitrine, F.; Coscas, F.; Massamba, N.; Querques, L.; Mimoun, G.; Bandello, F.; Souied, E.H. Analysis of progression of reticular pseudodrusen by spectral domain-optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1264–1270. [Google Scholar] [CrossRef]
- Finger, R.P.; Wu, Z.; Luu, C.D.; Kearney, F.; Ayton, L.N.; Lucci, L.M.; Hubbard, W.C.; Hageman, J.L.; Hageman, G.S.; Guymer, R.H. Reticular pseudodrusen: A risk factor for geographic atrophy in fellow eyes of individuals with unilateral choroidal neovascularization. Ophthalmology 2014, 121, 1252–1256. [Google Scholar] [CrossRef] [Green Version]
- Christenbury, J.G.; Folgar, F.A.; O’Connell, R.V.; Chiu, S.J.; Farsiu, S.; Toth, C.A.; Age-related Eye Disease Study 2 Ancillary Spectral Domain Optical Coherence Tomography Study Group Age-Related Eye Disease Study 2 Ancillary Spectral Domain Optical Coherence Tomography Study Group. Progression of intermediate age-related macular degeneration with proliferation and inner retinal migration of hyperreflective foci. Ophthalmology 2013, 120, 1038–1045. [Google Scholar] [CrossRef] [Green Version]
- Nassisi, M.; Fan, W.; Shi, Y.; Lei, J.; Borrelli, E.; Ip, M.; Sadda, S.R. Quantity of Intraretinal Hyperreflective Foci in Patients with Intermediate Age-Related Macular Degeneration Correlates With 1-Year Progression. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3431–3439. [Google Scholar] [CrossRef] [Green Version]
- Schuman, S.G.; Koreishi, A.F.; Farsiu, S.; Jung, S.H.; Izatt, J.A.; Toth, C.A. Photoreceptor layer thinning over drusen in eyes with age-related macular degeneration imaged in vivo with spectral-domain optical coherence tomography. Ophthalmology 2009, 116, 488–496. [Google Scholar] [CrossRef] [Green Version]
- Buch, H.; Vinding, T.; la Cour, M.; Jensen, G.B.; Prause, J.U.; Nielsen, N.V. Risk factors for age-related maculopathy in a 14-year follow-up study: The Copenhagen City Eye Study. Acta Ophthalmol. Scand. 2005, 83, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Hageman, G.S.; Luthert, P.J.; Victor Chong, N.H.; Johnson, L.V.; Anderson, D.H.; Mullins, R.F. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch’s membrane interface in aging and age-related macular degeneration. Prog. Retin. Eye. Res. 2001, 20, 705–732. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, L.; Fontainhas, A.M.; Fariss, R.N.; Wong, W.T. Microglia in the mouse retina alter the structure and function of retinal pigmented epithelial cells: A potential cellular interaction relevant to AMD. PLoS ONE 2009, 4, e7945. [Google Scholar] [CrossRef] [PubMed]
- Pieroni, C.G.; Witkin, A.J.; Ko, T.H.; Fujimoto, J.G.; Chan, A.; Schuman, J.S.; Ishikawa, H.; Reichel, E.; Duker, J. S Ultrahigh resolution optical coherence tomography in non-exudative age-related macular degeneration. Br. J. Ophthalmol. 2006, 90, 191–197. [Google Scholar] [CrossRef]
- Mitsuhiro, M.R.; Eguchi, S.; Yamashita, H. Regulation mechanisms of retinal pigment epithelial cell migration by the TGF-βa superfamily. Acta. Ophthalmol. Scand. 2003, 81, 630–638. [Google Scholar] [CrossRef] [Green Version]
- Folgar, F.A.; Yuan, E.L.; Sevilla, M.B.; Chiu, S.J.; Farsiu, S.; Chew, E.Y.; Toth, C.A. Age Related Eye Disease Study 2 Ancillary Spectral-Domain Optical Coherence Tomography Study GroupDrusen Volume and Retinal Pigment Epithelium Abnormal Thinning Volume Predict 2-Year Progression of Age-Related Macular Degeneration. Ophthalmology 2016, 123, 39–50.e1. [Google Scholar] [CrossRef]
- Padnick-Silver, L.; Weinberg, A.B.; Lafranco, F.P.; Macsai, M.S. Pilot study for the detection of early exudative age-related macular degeneration with optical coherence tomography. Retina 2012, 32, 1045–1056. [Google Scholar] [CrossRef]
- Toprak, I.; Yaylalı, V.; Yildirim, C. Early deterioration in ellipsoid zone in eyes with non-neovascular age-related macular degeneration. Int. Ophthalmol. 2017, 37, 801–806. [Google Scholar] [CrossRef]
- Ferrara, D.; Silver, R.E.; Louzada, R.N.; Novais, E.A.; Collins, G.K.; Seddon, J.M. Optical Coherence Tomography Features Preceding the Onset of Advanced Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3519–3529. [Google Scholar] [CrossRef]
- Sikorski, B.L.; Bukowska, D.; Kaluzny, J.J.; Szkulmowski, M.; Kowalczyk, A.; Wojtkowski, M. Drusen with accompanying fluid underneath the sensory retina. Ophthalmology 2011, 118, 82–92. [Google Scholar] [CrossRef]
- Brinkmann, M.P.; Bacci, T.; Messinger, J.; Kar, D.; Sloan, K.R.; Chen, L.; Hamann, T.; Wiest, M.; Freund, K.B.; Zweifel, S.A.; et al. Histology and clinical lifecycle of acquired vitelliform lesion, a pathway to atrophy in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2021, 62, 301. [Google Scholar]
- Saade, C.; Greenberg, P.B. Acquired Vitelliform Lesions in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2061. [Google Scholar]
- Roquet, W.; Roudot-Thoraval, F.; Coscas, G.; Soubrane, G. Clinical features of drusenoid pigment epithelial detachment in age related macular degeneration. Br. J. Ophthalmol. 2004, 88, 638–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadigh, S.; Cideciyan, A.V.; Sumaroka, A.; Huang, W.C.; Luo, X.; Swider, M.; Steinberg, J.D.; Stambolian, D.; Jacobson, S.G. Abnormal Thickening as well as Thinning of the Photoreceptor Layer in Intermediate Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1603–1612. [Google Scholar] [CrossRef]
- Curcio, C.A.; Medeiros, N.E.; Millican, C.L. Photoreceptor loss in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 1996, 37, 1236–1249. [Google Scholar]
- Wu, Z.; Luu, C.D.; Ayton, L.N.; Goh, J.K.; Lucci, L.M.; Hubbard, W.C.; Hageman, J.L.; Hageman, G.S.; Guymer, R.H. Optical coherence tomography-defined changes preceding the development of drusen-associated atrophy in age-related macular degeneration. Ophthalmology 2014, 121, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Luu, C.D.; Ayton, L.N.; Goh, J.K.; Lucci, L.M.; Hubbard, W.C.; Hageman, J.L.; Hageman, G.S.; Guymer, R.H. Fundus autofluorescence characteristics of nascent geographic atrophy in age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1546–1552. [Google Scholar] [CrossRef] [Green Version]
- Sadda, S.R.; Guymer, R.; Holz, F.G.; Schmitz-Valckenberg, S.; Curcio, C.A.; Bird, A.C.; Blodi, B.A.; Bottoni, F.; Chakravarthy, U.; Chew, E.Y.; et al. Consensus Definition for Atrophy Associated with Age-Related Macular Degeneration on OCT: Classification of Atrophy Report 3. Ophthalmology 2018, 125, 537–548. [Google Scholar] [CrossRef] [Green Version]
- Bilc, S.; Groza, A.; Muntean, G.; Nicoara, S.D. Interleaving Automatic Segmentation and Expert Opinion for Retinal Conditions. Diagnostics 2022, 12, 22. [Google Scholar] [CrossRef]
- Gregori, G.; Wang, F.; Rosenfeld, P.J.; Yehoshua, Z.; Gregori, N.Z.; Lujan, B.J.; Puliafito, C.A.; Feuer, W.J. e Spectral domain optical coherence tomography imaging of drusen in nonexudative age-related macular degeneration. Ophthalmology 2011, 118, 1373–1379. [Google Scholar] [CrossRef] [Green Version]
- Chiu, S.J.; Izatt, J.A.; O’Connell, R.V.; Winter, K.P.; Toth, C.A.; Farsiu, S. Validated automatic segmentation of AMD pathology including drusen and geographic atrophy in SD-OCT images. Investig. Ophthalmol. Vis. Sci. 2012, 53, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Bogunovic, H.; Montuoro, A.; Baratsits, M.; Karantonis, M.G.; Waldstein, S.M.; Schlanitz, F.; Schmidt-Erfurth, U. Machine Learning of the Progression of Intermediate Age-Related Macular Degeneration Based on OCT Imaging. Investig. Ophthalmol. Vis. Sci. 2017, 58, BIO141–BIO150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt-Erfurth, U.; Waldstein, S.M.; Klimscha, S.; Sadeghipour, A.; Hu, X.; Gerendas, B.S.; Osborne, A.; Bogunovic, H. Prediction of Individual Disease Conversion in Early AMD Using Artificial Intelligence. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3199–3208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, S.; de Sisternes, L.; Chen, Q.; Rubin, D.L.; Leng, T. Fully Automated Prediction of Geographic Atrophy Growth Using Quantitative Spectral-Domain Optical Coherence Tomography Biomarkers. Ophthalmology 2016, 123, 1737–1750. [Google Scholar] [CrossRef]
- Sevilla, M.B.; McGwin, G., Jr.; Lad, E.M.; Clark, M.; Yuan, E.L.; Farsiu, S.; Curcio, C.A.; Owsley, C.; Toth, C.A. Relating Retinal Morphology and Function in Aging and Early to Intermediate Age-related Macular Degeneration Subjects. Am. J. Ophthalmol. 2016, 165, 65–77. [Google Scholar] [CrossRef] [Green Version]
- Tepelus, T.C.; Hariri, A.H.; Al-Sheikh, M.; Sadda, S.R. Correlation Between Mesopic Retinal Sensitivity and Optical Coherence Tomographic Metrics of the Outer Retina in Patients with Non-Atrophic Dry Age-Related Macular Degeneration. Ophthalmic. Surg. Lasers Imaging Retina 2017, 48, 312–318. [Google Scholar] [CrossRef]
- Waldstein, S.M.; Vogl, W.D.; Bogunovic, H.; Sadeghipour, A.; Riedl, S.; Schmidt-Erfurth, U. Characterization of Drusen and Hyperreflective Foci as Biomarkers for Disease Progression in Age-Related Macular Degeneration Using Artificial Intelligence in Optical Coherence Tomography. JAMA Ophthalmol. 2020, 138, 740–747. [Google Scholar] [CrossRef]
- Banerjee, I.; de Sisternes, L.; Hallak, J.A.; Leng, T.; Osborne, A.; Rosenfeld, P.J.; Gregori, G.; Durbin, M.; Rubin, D. Prediction of age-related macular degeneration disease using a sequential deep learning approach on longitudinal SD-OCT imaging biomarkers. Sci. Rep. 2020, 10, 15434. [Google Scholar] [CrossRef]
- Hallak, J.A.; de Sisternes, L.; Osborne, A.; Yaspan, B.; Rubin, D.L.; Leng, T. Imaging, Genetic, and Demographic Factors Associated with Conversion to Neovascular Age-Related Macular Degeneration: Secondary Analysis of a Randomized Clinical Trial. JAMA Ophthalmol. 2019, 137, 738–744. [Google Scholar] [CrossRef]
- Yim, J.; Chopra, R.; Spitz, T.; Winkens, J.; Obika, A.; Kelly, C.; Askham, H.; Lukic, M.; Huemer, J.; Fasler, K.; et al. Predicting conversion to wet age-related macular degeneration using deep learning. Nat. Med. 2020, 26, 892–899. [Google Scholar] [CrossRef]
- Russakoff, D.B.; Lamin, A.; Oakley, J.D.; Dubis, A.M.; Sivaprasad, S.S. Deep Learning for Prediction of AMD Progression: A Pilot Study. Investig. Ophthalmol. Vis. Sci. 2019, 60, 712–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, T.K.; Choi, J.Y.; Seo, J.G.; Ramasubramanian, B.; Selvaperumal, S.; Kim, D.W. The possibility of the combination of OCT and fundus images for improving the diagnostic accuracy of deep learning for age-related macular degeneration: A preliminary experiment. Med. Biol. Eng. Comput. 2019, 57, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Bogunović, H.; Asgari, R.; Schmidt-Erfurth, U.; Guymer, R.H. Predicting Progression of Age-Related Macular Degeneration Using OCT and Fundus Photography. Ophthalmol. Retin. 2021, 5, 118–125. [Google Scholar] [CrossRef] [PubMed]
| Stage | Definition |
|---|---|
| no AMD | No drusen or small drusen ≤ 63 µm, no pigment abnormalities |
| Early AMD | Medium drusen > 63 µm and ≤125 µm, no pigment abnormalities |
| Intermediate AMD | Large drusen > 125 µm or any pigment abnormalities |
| Advanced AMD | nvAMD or GA |
| First Author | Publication Year | Country | Database | Total Images | Outcome | Performance |
|---|---|---|---|---|---|---|
| Bogunovic [52] | 2017 | Austria | private dataset | 61 eyes/944 drusen | drusen regression | AUC 0.75 (2 years) |
| Schmidt-Erfurth [53] | 2018 | Austria | HARBOR | 495 eyes | iAMD conversion | AUC 0.68 (CNV) AUC 0.8 (GA) |
| Russakoff [61] | 2019 | United States | private dataset | 71 eyes | AMD progression | AMDnet: AUC 0.89 B-scan and AUC 0.91 volumes VGG16: AUC 0.82 B-scan and AUC 0.87 volume |
| Yoo [62] | 2019 | South Korea | Project Macula | 98 eyes | AMD diagnosis | AUC 0.906 (OCT) AUC 0.914 (fundus) AUC 0.969 (mix) |
| Waldstein [57] | 2020 | Austria | HARBOR | 518 eyes/ 8529 images | progression to CNV/MA | CNV: AUC 0.66 drusen MA: AUC 0.73 HRF |
| Banerjee [58] | 2020 | United States | HARBOR | 671 eyes/13.954 | risk of exudation in iAMD | AUC 0.96 (3 months) AUC 0.97 (21 months) |
| Yim [60] | 2020 | UK | private dataset | 2795 patients | fellow eye nvAMD prediction | TP in 78% and 41% FP in 56% and 17% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damian, I.; Nicoară, S.D. SD-OCT Biomarkers and the Current Status of Artificial Intelligence in Predicting Progression from Intermediate to Advanced AMD. Life 2022, 12, 454. https://doi.org/10.3390/life12030454
Damian I, Nicoară SD. SD-OCT Biomarkers and the Current Status of Artificial Intelligence in Predicting Progression from Intermediate to Advanced AMD. Life. 2022; 12(3):454. https://doi.org/10.3390/life12030454
Chicago/Turabian StyleDamian, Ioana, and Simona Delia Nicoară. 2022. "SD-OCT Biomarkers and the Current Status of Artificial Intelligence in Predicting Progression from Intermediate to Advanced AMD" Life 12, no. 3: 454. https://doi.org/10.3390/life12030454
APA StyleDamian, I., & Nicoară, S. D. (2022). SD-OCT Biomarkers and the Current Status of Artificial Intelligence in Predicting Progression from Intermediate to Advanced AMD. Life, 12(3), 454. https://doi.org/10.3390/life12030454







