Evaporation Patterns of Dextran–Poly(Ethylene Glycol) Droplets with Changes in Wettability and Compatibility
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sessile Droplet Formation and Its Observation
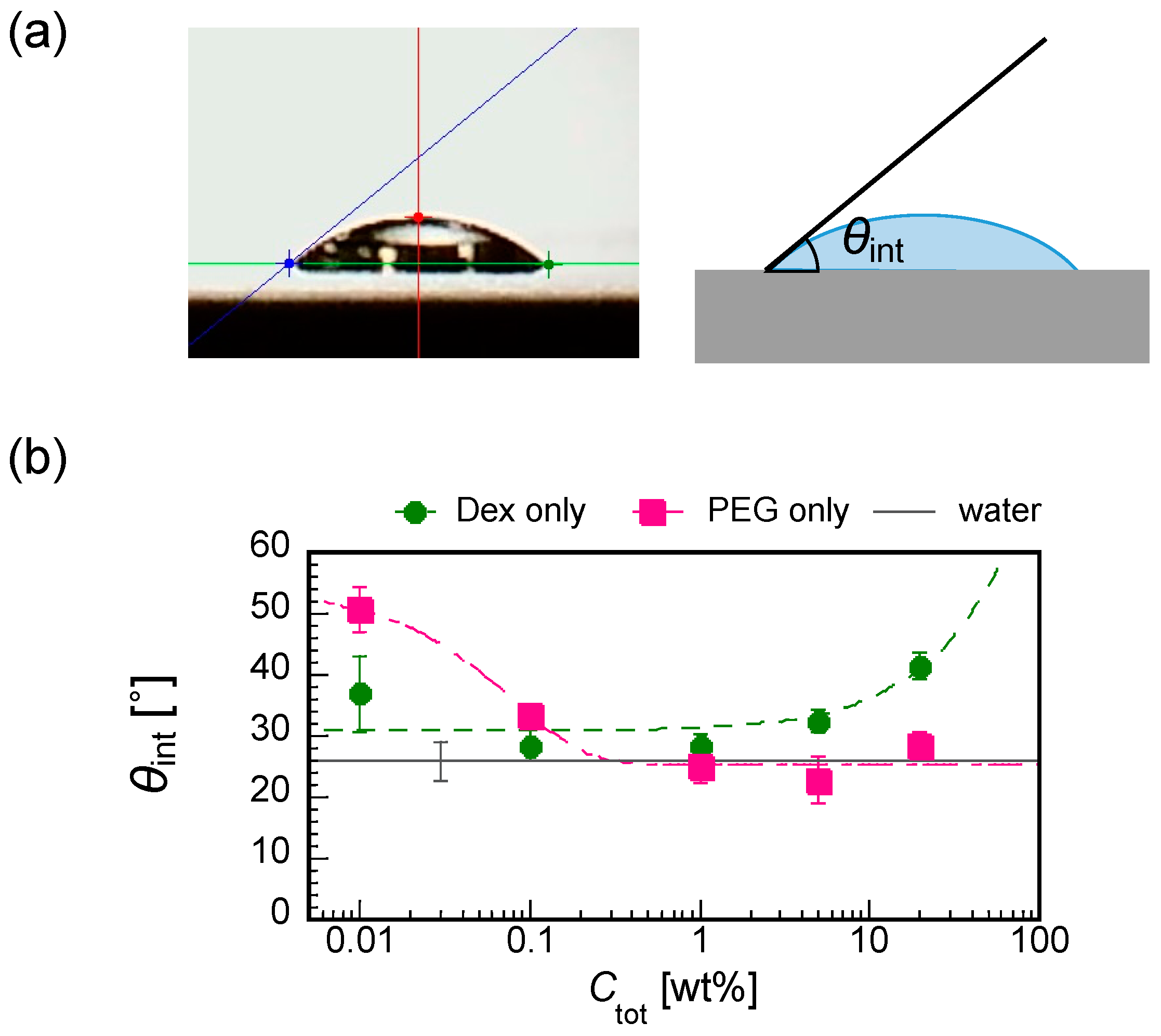
2.3. Contact Angle Measurement
3. Results and Discussion
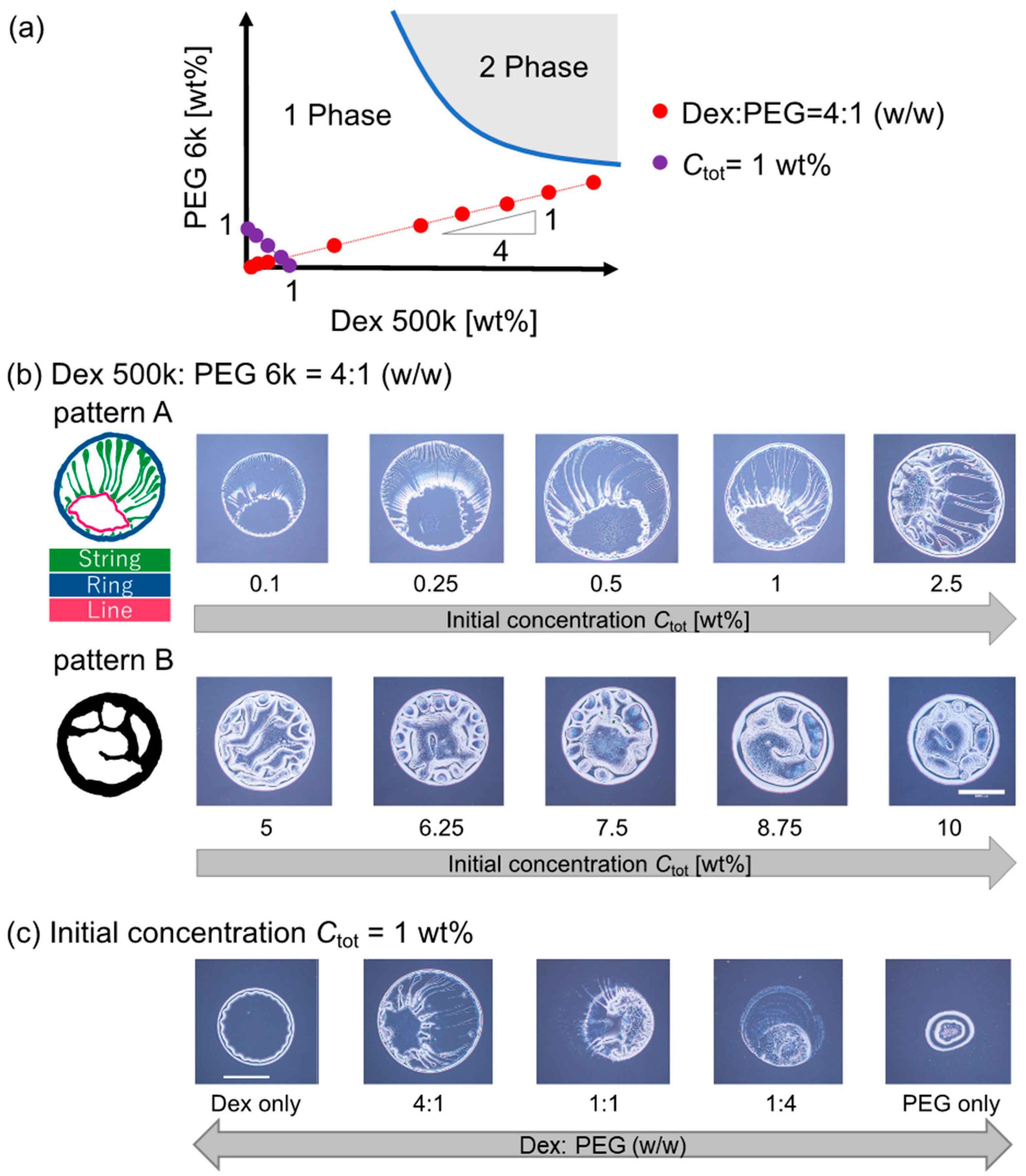
3.1. Evaporation Patterns for Dextran–PEG Droplets
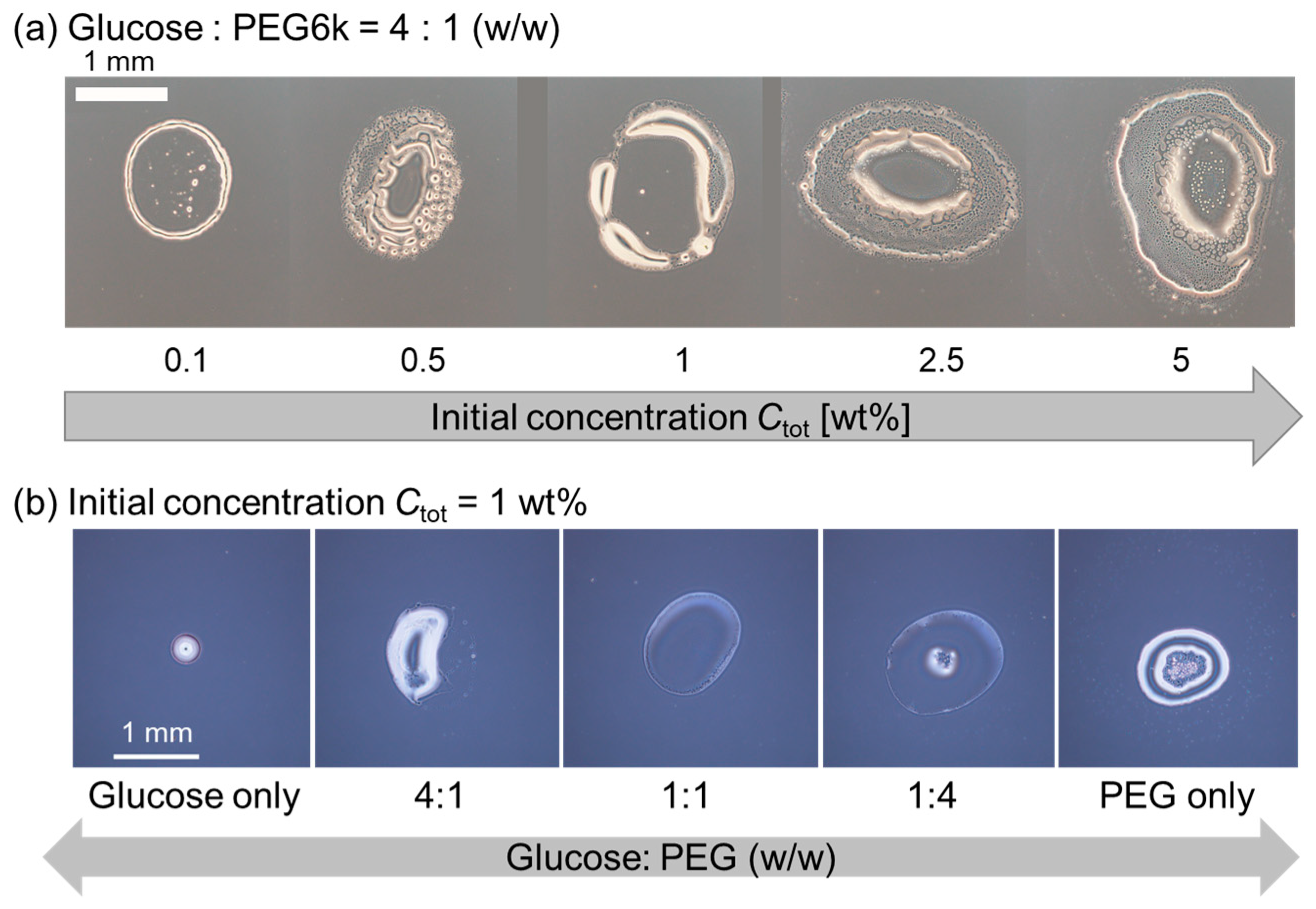
3.2. Evaporation Patterns for Glucose-PEG Droplets
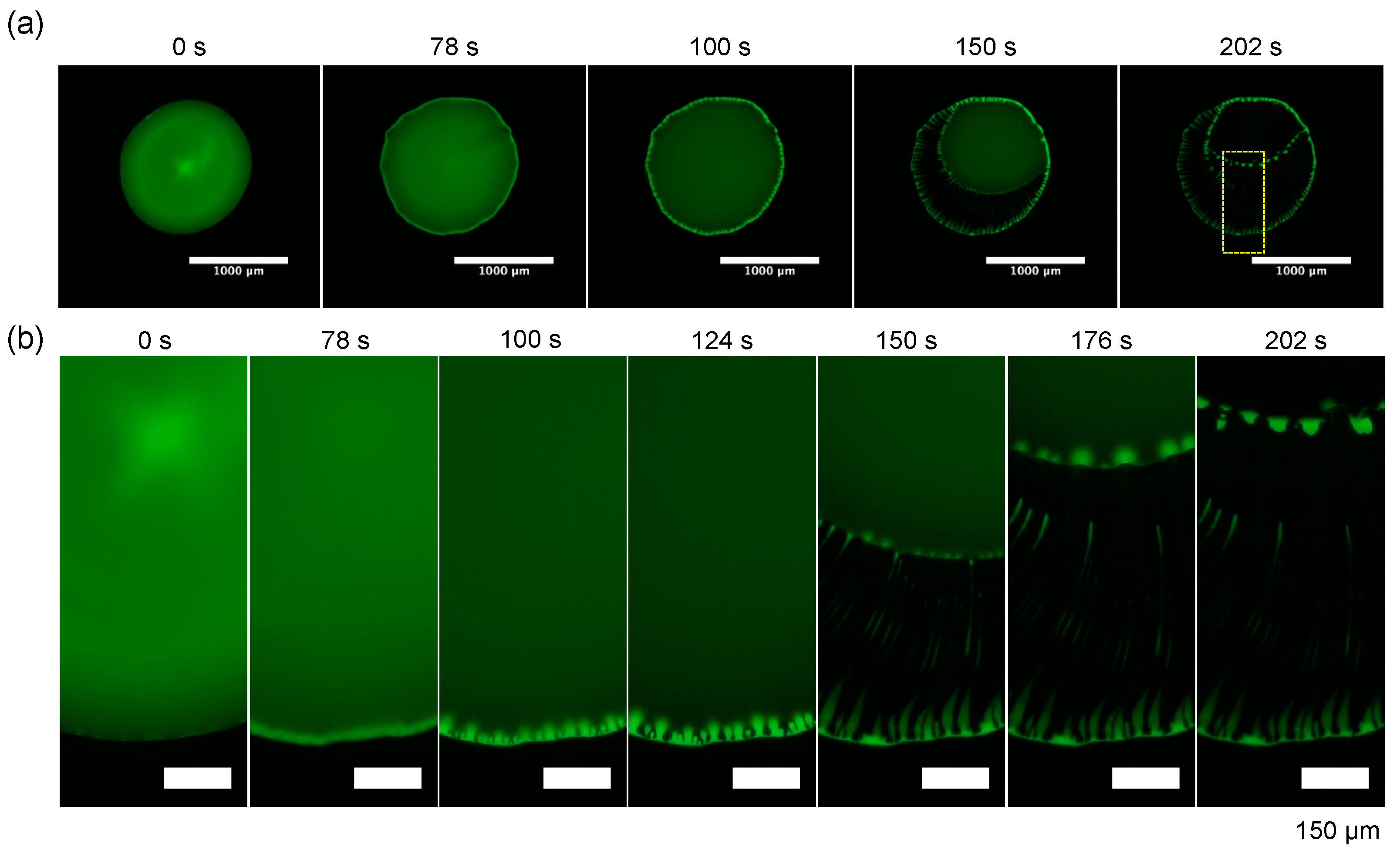
3.3. Fluctuation of Polymer Concentration in Evaporating Dextran–PEG Droplets
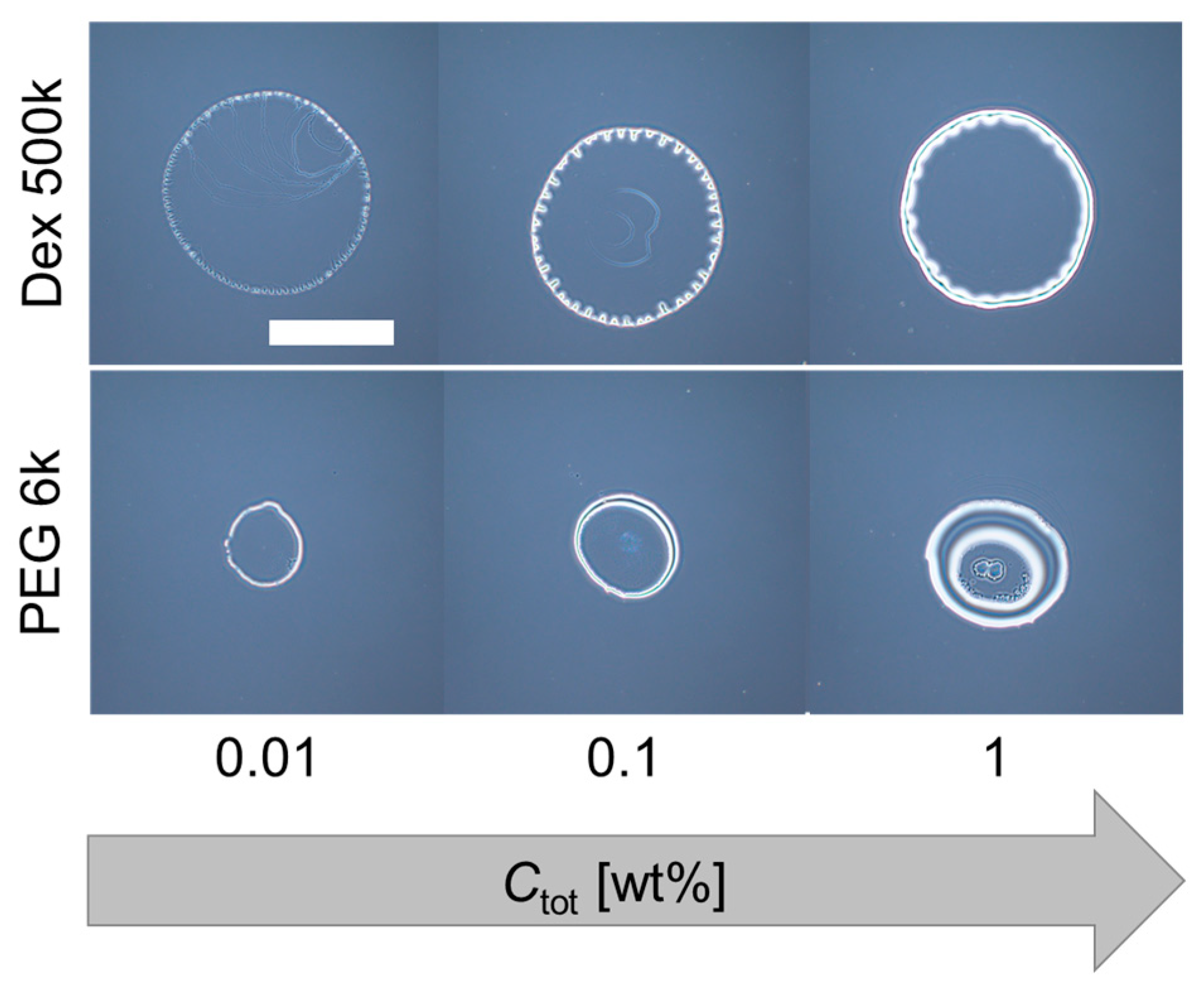
3.4. Initial Contact Angles of Dextran and PEG Droplet
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary Flow as the Cause of Ring Stains from Dried Liquid Drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Larson, R.G. Twenty Years of Drying Droplets. Nature 2017, 550, 466–467. [Google Scholar] [CrossRef] [PubMed]
- Parsa, M.; Harmand, S.; Sefiane, K. Mechanisms of Pattern Formation from Dried Sessile Drops. Adv. Colloid Interface Sci. 2018, 254, 22–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulard, C.; Damman, P. Control of Spreading and Drying of a Polymer Solution from Marangoni Flows. Europhys. Lett. 2007, 80, 64001. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, L.; Zang, D.; Shen, W. Blood Drop Patterns: Formation and Applications. Adv. Colloid Interface Sci. 2016, 231, 1–14. [Google Scholar] [CrossRef]
- Pal, A.; Gope, A.; Obayemi, J.D.; Iannacchione, G.S. Concentration-Driven Phase Transition and Self-Assembly in Drying Droplets of Diluting Whole Blood. Sci. Rep. 2020, 10, 18908. [Google Scholar] [CrossRef] [PubMed]
- Sefiane, K.; Duursma, G.; Arif, A. Patterns from Dried Drops as a Characterisation and Healthcare Diagnosis Technique, Potential and Challenges: A Review. Adv. Colloid Interface Sci. 2021, 298, 102546. [Google Scholar] [CrossRef]
- Carreón, Y.J.P.; Díaz-Hernández, O.; Escalera Santos, G.J.; Cipriano-Urbano, I.; Solorio-Ordaz, F.J.; González-Gutiérrez, J.; Zenit, R. Texture Analysis of Dried Droplets for the Quality Control of Medicines. Sensors 2021, 21, 4048. [Google Scholar] [CrossRef]
- Alberti, S.; Gladfelter, A.; Mittag, T. Considerations and Challenges in Studying Liquid-Liquid Phase Separation and Biomolecular Condensates. Cell 2019, 176, 419–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wegmann, S.; Eftekharzadeh, B.; Tepper, K.; Zoltowska, K.M.; Bennett, R.E.; Dujardin, S.; Laskowski, P.R.; MacKenzie, D.; Kamath, T.; Commins, C.; et al. Tau Protein Liquid–Liquid Phase Separation Can Initiate Tau Aggregation. EMBO J. 2018, 37, e98049. [Google Scholar] [CrossRef] [PubMed]
- Rebane, A.A.; Ziltener, P.; LaMonica, L.C.; Bauer, A.H.; Zheng, H.; López-Montero, I.; Pincet, F.; Rothman, J.E.; Ernst, A.M. Liquid–Liquid Phase Separation of the Golgi Matrix Protein GM130. FEBS Lett. 2020, 594, 1132–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, T.Z.; Chandru, K.; Hongo, Y.; Afrin, R.; Usui, T.; Myojo, K.; Cleaves, H.J. Membraneless Polyester Microdroplets as Primordial Compartments at the Origins of Life. Proc. Natl. Acad. Sci. USA 2019, 116, 15830–15835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, W.; Kinghorn, A.B.; Zhang, Y.; Li, Q.; Poonam, A.D.; Tanner, J.A.; Shum, H.C. Non-Associative Phase Separation in an Evaporating Droplet as a Model for Prebiotic Compartmentalization. Nat. Commun. 2021, 12, 3194. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, C.; Yanagisawa, M. Cell-Size Confinement Effect on Protein Diffusion in Crowded Poly(Ethylene)Glycol Solution. Phys. Chem. Chem. Phys. 2018, 20, 8842–8847. [Google Scholar] [CrossRef] [PubMed]
- Harusawa, K.; Watanabe, C.; Kobori, Y.; Tomita, K.; Kitamura, A.; Kinjo, M.; Yanagisawa, M. Membrane Surface Modulates Slow Diffusion in Small Crowded Droplets. Langmuir 2021, 37, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Nittmann, J.; Daccord, G.; Stanley, H.E. Fractal Growth Viscous Fingers: Quantitative Characterization of a Fluid Instability Phenomenon. Nature 1985, 314, 141–144. [Google Scholar] [CrossRef]
- Biswas, N.; Ichikawa, M.; Datta, A.; Sato, Y.T.; Yanagisawa, M.; Yoshikawa, K. Phase Separation in Crowded Micro-Spheroids: DNA–PEG System. Chem. Phys. Lett. 2012, 539–540, 157–162. [Google Scholar] [CrossRef]
- Dewangan, J.K.; Basu, N.; Chowdhury, M. Cationic Surfactant-Directed Structural Control of NaCl Crystals from Evaporating Sessile Droplets. Soft Matter 2022, 18, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Inanlu, M.J.; Shojaan, B.; Farhadi, J.; Bazargan, V. Effect of Particle Concentration on Surfactant-Induced Alteration of the Contact Line Deposition in Evaporating Sessile Droplets. Langmuir 2021, 37, 2658–2666. [Google Scholar] [CrossRef] [PubMed]




| PEG 6 k | Dex 500 k | |
|---|---|---|
| Rg [nm] | 3.1 | 17 |
| c* [wt%] | 8 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, C.; Yanagisawa, M. Evaporation Patterns of Dextran–Poly(Ethylene Glycol) Droplets with Changes in Wettability and Compatibility. Life 2022, 12, 373. https://doi.org/10.3390/life12030373
Watanabe C, Yanagisawa M. Evaporation Patterns of Dextran–Poly(Ethylene Glycol) Droplets with Changes in Wettability and Compatibility. Life. 2022; 12(3):373. https://doi.org/10.3390/life12030373
Chicago/Turabian StyleWatanabe, Chiho, and Miho Yanagisawa. 2022. "Evaporation Patterns of Dextran–Poly(Ethylene Glycol) Droplets with Changes in Wettability and Compatibility" Life 12, no. 3: 373. https://doi.org/10.3390/life12030373
APA StyleWatanabe, C., & Yanagisawa, M. (2022). Evaporation Patterns of Dextran–Poly(Ethylene Glycol) Droplets with Changes in Wettability and Compatibility. Life, 12(3), 373. https://doi.org/10.3390/life12030373






