High Dose Intravenous Vitamin C as Adjunctive Therapy for COVID-19 Patients with Cancer: Two Cases
Abstract
:1. Introduction
2. Case Report
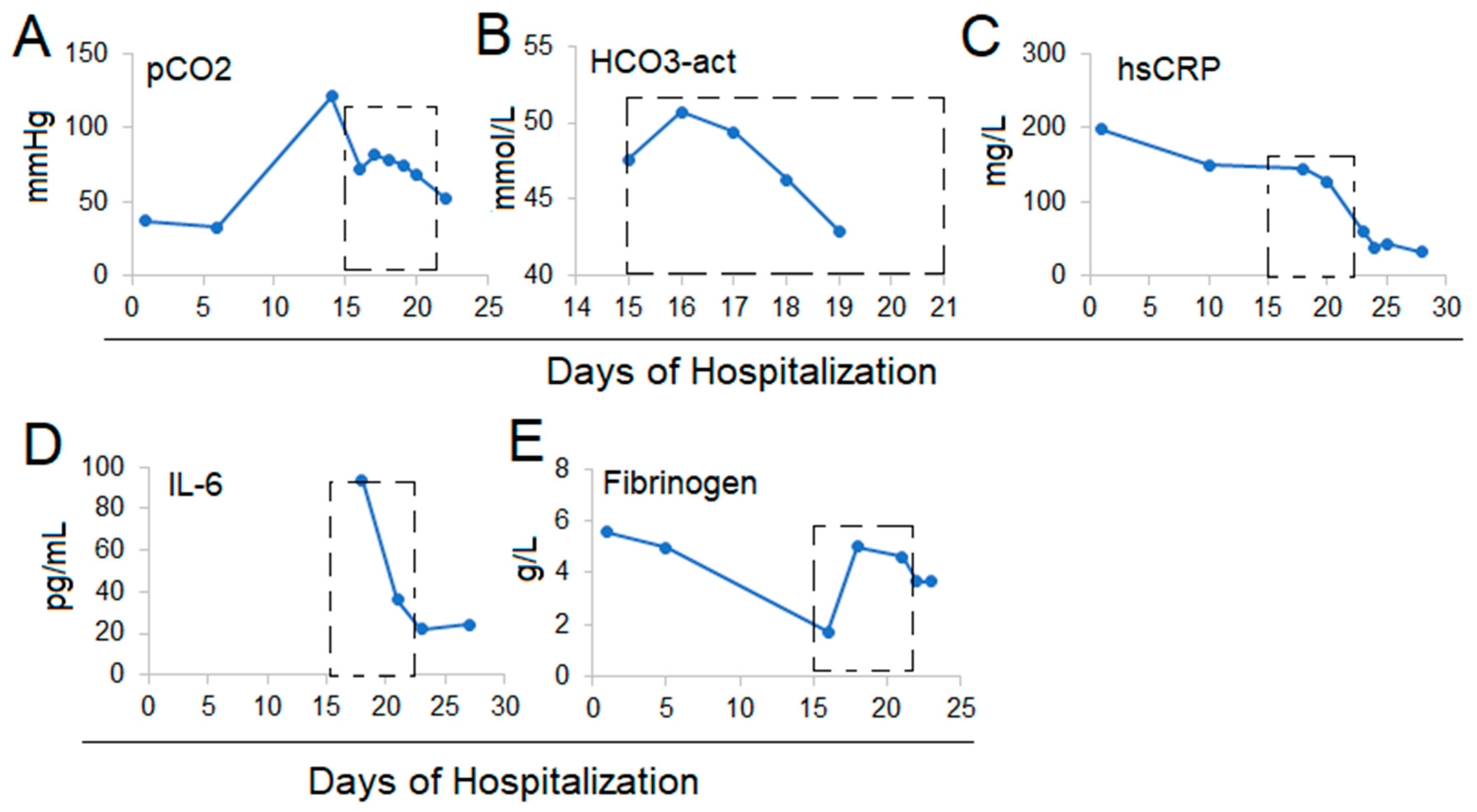
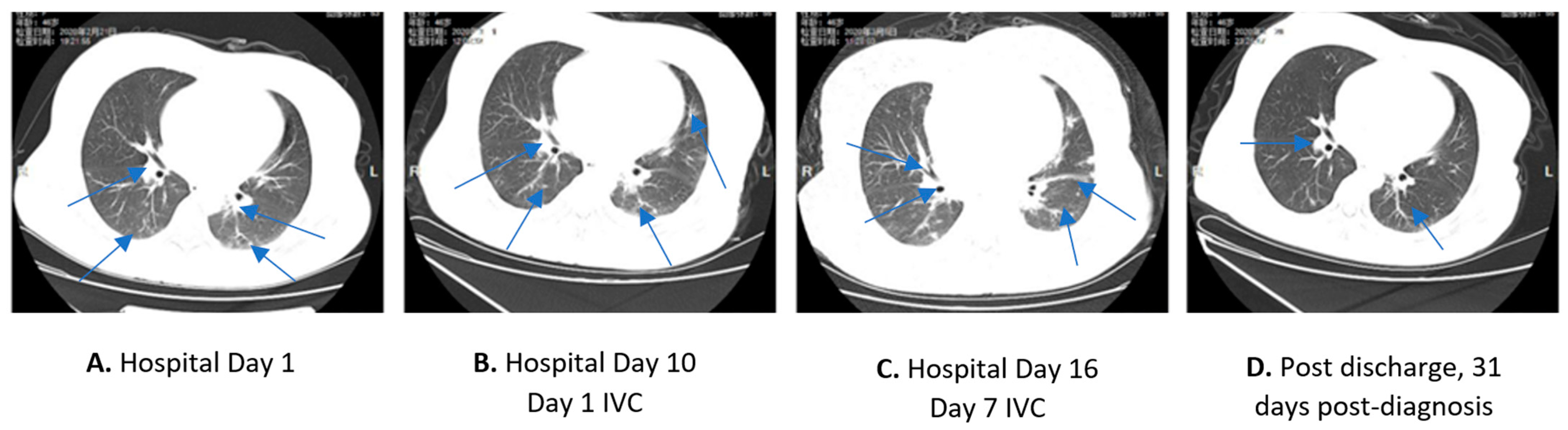
2.1. Case 1
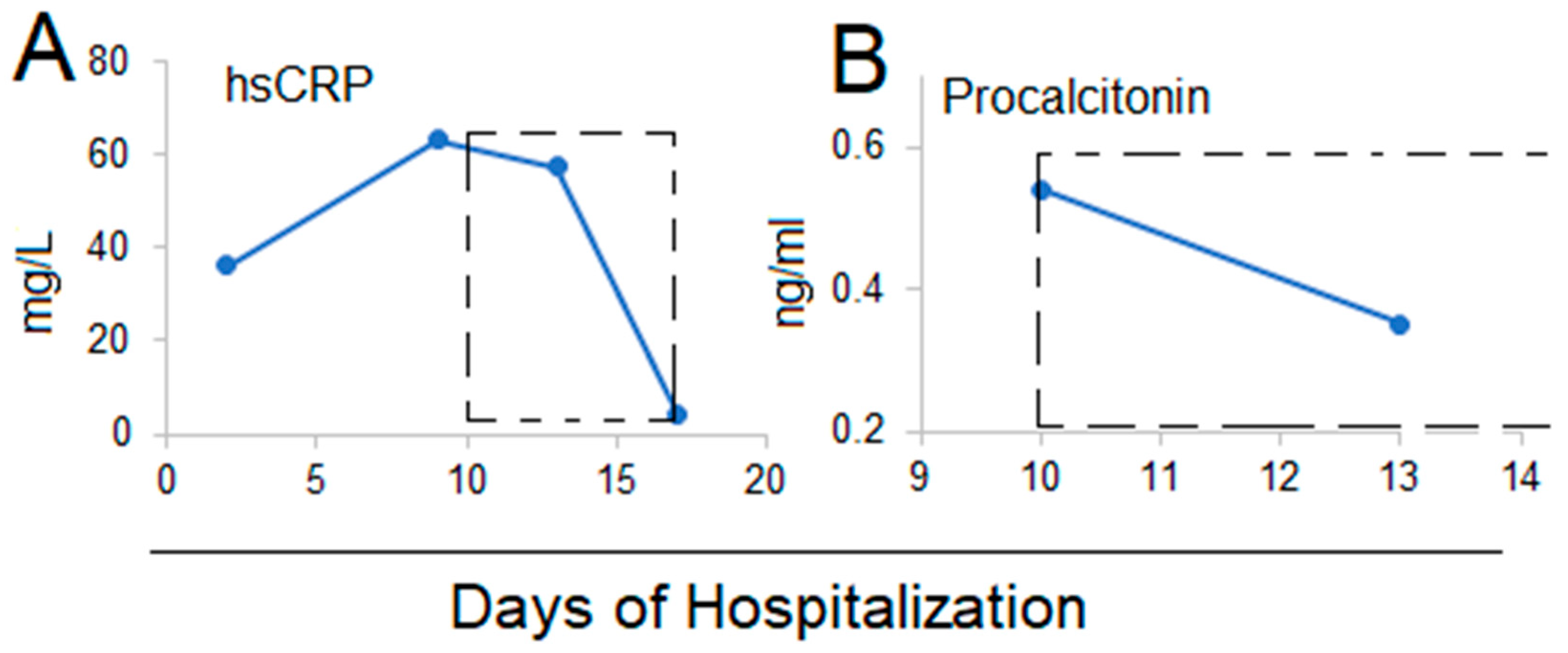
2.2. Case 2
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Coronavirus Disease (COVID-2019) Situation Reports. 2022. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (accessed on 2 February 2022).
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1406–1407. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef]
- Kupferschmidt, K.; Cohen, J. Race to find COVID-19 treatments accelerates. Science 2020, 367, 1412–1413. [Google Scholar] [CrossRef] [Green Version]
- Cheng, R.Z. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Med. Drug Discov. 2020, 5, 100028. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.A.; Kim, C.K.; Lepler, L.; Malhotra, R.; Debesa, O.; Natarajan, R.; Fisher, B.; Syed, A.; DeWilde, C.; Priday, A.; et al. Intravenous vitamin C as adjunctive therapy for enterovirus/rhinovirus induced acute respiratory distress syndrome. World J. Cri. Care Med. 2017, 6, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.A., 3rd; Truwit, J.D.; Hite, R.D.; Morris, P.E.; De Wilde, C.; Priday, A.; Fisher, B.; Thacker, L.R., 2nd; Natarajan, R.; Brophy, D.F.; et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients with Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. JAMA 2019, 322, 1261–1270. [Google Scholar] [CrossRef]
- Marik, P.E.; Khangoora, V.; Rivera, R.; Hooper, M.H.; Catravas, J. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest 2017, 151, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chapman, J.; Levine, M.; Polireddy, K.; Drisko, J.; Chen, Q. High-dose parenteral ascorbate enhanced chemosensitivity of ovarian cancer and reduced toxicity of chemotherapy. Sci. Transl. Med. 2014, 6, 222ra218. [Google Scholar] [CrossRef] [PubMed]
- Polireddy, K.; Dong, R.; Reed, G.; Yu, J.; Chen, P.; Williamson, S.; Violet, P.C.; Pessetto, Z.; Godwin, A.K.; Fan, F.; et al. High Dose Parenteral Ascorbate Inhibited Pancreatic Cancer Growth and Metastasis: Mechanisms and a Phase I/IIa study. Sci. Rep. 2017, 7, 17188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, A.A., 3rd; Syed, A.A.; Knowlson, S.; Sculthorpe, R.; Farthing, D.; De Wilde, C.; Farthing, C.A.; Larus, T.L.; Martin, E.; Brophy, D.F.; et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J. Transl. Med. 2014, 12, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Polireddy, K.; Chen, P.; Dong, R. The unpaved journey of vitamin C in cancer treatment. Can. J. Physiol. Pharmacol. 2015, 93, 1055–1063. [Google Scholar] [CrossRef]
- Ngo, B.; Van Riper, J.M.; Cantley, L.C.; Yun, J. Targeting cancer vulnerabilities with high-dose vitamin C. Nat. Rev. Cancer 2019, 19, 271–282. [Google Scholar] [CrossRef]
- Waqas Khan, H.M.; Parikh, N.; Megala, S.M.; Predeteanu, G.S. Unusual Early Recovery of a Critical COVID-19 Patient after Administration of Intravenous Vitamin C. Am. J. Case Rep. 2020, 21, e925521. [Google Scholar] [CrossRef]
- Hoang, B.X.; Shaw, G.; Fang, W.; Han, B. Possible application of high-dose vitamin C in the prevention and therapy of coronavirus infection. J. Glob. Antimicrob. Resist. 2020, 23, 256–262. [Google Scholar] [CrossRef]
- Cheng, R.Z.; Kogan, M.; Davis, D. Ascorbate as Prophylaxis and Therapy for COVID-19—Update from Shanghai and U.S. Medical Institutions. Glob. Adv. Health Med. 2020, 9, 2164956120934768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, F.; Xie, L.; Wang, C.; Wang, J.; Chen, R.; Jia, P.; Guan, H.Q.; Peng, L.; Chen, Y.; et al. Clinical characteristics of COVID-19-infected cancer patients: A retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020, 31, 894–901. [Google Scholar] [CrossRef]
- Dai, M.; Liu, D.; Liu, M.; Zhou, F.; Li, G.; Chen, Z.; Zhang, Z.; You, H.; Wu, M.; Zheng, Q.; et al. Patients with cancer appear more vulnerable to SARS-CoV-2: A multi-center study during the COVID-19 outbreak. Cancer Discov. 2020, 10, 783–791. [Google Scholar]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Lee, J.-H.; Krishna, M.C.; Shacter, E.; Choyke, P.L.; Pooput, C.; Kirk, K.L.; Buettner, G.R.; et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc. Natl. Acad. Sci. USA 2007, 104, 8749–8754. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Espey, M.G.; Sun, A.Y.; Pooput, C.; Kirk, K.L.; Krishna, M.C.; Khosh, D.S.; Drisko, J.; Levine, M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc. Natl. Acad. Sci. USA 2008, 105, 11105–11109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agathocleous, M.; Meacham, C.E.; Burgess, R.J.; Piskounova, E.; Zhao, Z.; Crane, G.M.; Cowin, B.L.; Bruner, E.; Murphy, M.M.; Chen, W.; et al. Ascorbate regulates haematopoietic stem cell function and leukaemogenesis. Nature 2017, 549, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Magri, A.; Germano, G.; Lorenzato, A.; Lamba, S.; Chila, R.; Montone, M.; Amodio, V.; Ceruti, T.; Sassi, F.; Arena, S.; et al. High-dose vitamin C enhances cancer immunotherapy. Sci. Transl. Med. 2020, 12, eaay8707. [Google Scholar] [CrossRef] [PubMed]
- Luchtel, R.A.; Bhagat, T.; Pradhan, K.; Jacobs, W.R., Jr.; Levine, M.; Verma, A.; Shenoy, N. High-dose ascorbic acid synergizes with anti-PD1 in a lymphoma mouse model. Proc. Natl. Acad. Sci. USA 2020, 117, 1666–1677. [Google Scholar] [CrossRef] [Green Version]
- Cimmino, L.; Dolgalev, I.; Wang, Y.; Yoshimi, A.; Martin, G.H.; Wang, J.; Ng, V.; Xia, B.; Witkowski, M.T.; Mitchell-Flack, M.; et al. Restoration of TET2 Function Blocks Aberrant Self-Renewal and Leukemia Progression. Cell 2017, 170, 1079–1095.e20. [Google Scholar] [CrossRef] [Green Version]
- Kuck, J.L.; Bastarache, J.A.; Shaver, C.M.; Fessel, J.P.; Dikalov, S.I.; May, J.M.; Ware, L.B. Ascorbic acid attenuates endothelial permeability triggered by cell-free hemoglobin. Biochem. Biophys. Res. Commun. 2018, 495, 433–437. [Google Scholar] [CrossRef]
- May, J.M.; Harrison, F.E. Role of vitamin C in the function of the vascular endothelium. Antioxid. Redox. Signal. 2013, 19, 2068–2083. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.X. Mechanism of action of vitamin C in sepsis: Ascorbate modulates redox signaling in endothelium. Biofactors 2009, 35, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Cheng, R. Hospital Treatment of Serious and Critical COVID-19 Infection with High-Dose Vitamin C. Available online: http://www.drwlc.com/blog/2020/03/18/hospital-treatment-of-serious-and-critical-covid-19-infection-with-high-dose-vitamin-c/ (accessed on 20 April 2020).
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef] [Green Version]

| Reference Range | IVC Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 8 | Day 13 | Day 15 | Day 18 | Day 20 | Day 22 | Day 24 | ||
| Pulse | 60–100 BPM | 81 | 114 | 127 | 99 | 89 | 99 | 101 | |
| Respiratory rate | 12–20 BPM | 15 | 28–43 | 31 | 28 | 25 | 32 | 22 | |
| Blood pressure | 90/60–120/80 mmHg | 86/54 | 117/62 | 115/59 | 144/67 | 105/56 | 127/76 | 117/63 | |
| pO2 arterial | 80–100 mmHg | 65 | 192.2 | 80.7 | 71 | 91.2 | 82.3 | ||
| pCO2 arterial | 35–45 mmHg | 37 | 120.7 | 72 | 78.4 | 67.6 | 52.2 | ||
| pH arterial | 7.35–7.45 | 7.48 | 7.21 | 7.44 | 7.41 | 7.42 | 7.46 | ||
| WBC | 3.5–9.5 G/L | 16.39 | 7.56 | 5.59 | 6.04 | ||||
| Lymphocyte | 1.1–3.2 G/L | 0.44 | 0.13 | 0.37 | 0.34 | 0.25 | 0.27 | 0.46 | |
| Neutrophil | 1.8–7.5 G/L | 5.04 | 16.23 | 8.27 | 5.1 | 3.42 | 5.18 | 5.33 | |
| MPV | 8.2–12.5 fL | 8 | 8 | 9.7 | 11.2 | 11.7 | |||
| Creatinine | 44–120 umol/L | 42.1 | 28.9 | 39.3 | 55.8 | 56.9 | 41 | ||
| Phosphors | 0.85–1.51 mmol/L | 0.73 | 0.82 | 0.95 | 0.87 | ||||
| hsCRP | 0–5 mg/L | 198 | 100 | 150 | 144 | 127 | 61 | 38 | |
| Amyloid A | 0–8 mg/L | 512 | 447 | 25 | |||||
| IL-6 | 0–16.4 pg/mL | 94 | 36 | 22 | |||||
| Prealbumin determination | 170–420 mg/L | 16 | 93 | 10 | 16 | 41 | 58 | 102 | |
| ALT | 0–50 U/L | 55 | 8 | 12 | 9 | ||||
| AST | 0–40 U/L | 150 | 13 | 31 | 27 | ||||
| BNP | <100 ng/L | 362 | 1411 | 765 | 1114 | 1475 | 1539 | ||
| Prothrombin time | 9–13 s | 16 | 14.4 | 14.1 | 14.3 | 12.7 | 14 | 14 | |
| PT ratio | 0.85–1.15 | 1.47 | 1.32 | 1.29 | 1.31 | 1.17 | 1.28 | ||
| Fibrinogen | 2–4 g/L | 5.55 | 4.98 | 1.72 | 5.01 | 4.62 | 3.68 | 3.67 | |
| D-dimer | 0–0.25 mg/L | 0.74 | 0.84 | 1.1 | 0.94 | 0.94 | 2.46 | ||
| Fibrinogen degradation products | 0–5 mg/L | 5.13 | 4.19 | 3.03 | 5.61 | ||||
| Reference Range | IVC Treatment | ||||||
|---|---|---|---|---|---|---|---|
| Day 2 | Day 9 | Day 10 | Day 13 | Day 16 | Day 17 | ||
| Pulse | 60–100 BPM | 88 | 74 | 70 | 81 | 72 | 89 |
| Respiratory rate | 12–20 BPM | 17 | 21 | 18 | 17 | 19 | 20 |
| Blood pressure | 90/60–120/80 mmHg | 110/59 | 124/70 | 119/69 | 124/64 | 118/72 | 123/43 |
| pO2 arterial | 80–100 mmHg | 74.5 | 79.5 | ||||
| pCO2 arterial | 35–45 mmHg | 31.6 | 29.3 | ||||
| HCO3 artierial | 22–26 mmol/L | 19.3 | 22.9 | ||||
| Platelet | 125–350 G/L | 79 | 105 | 115 | 129 | 138 | |
| Bilirubin | <5.1 µmol/L | 11.2 | 21.4 | 15.6 | 4.9 | ||
| Glucose | 3.9–6.1 mmol/L | 8.01 | 6.49 | 8.58 | |||
| Creatinine | 44–120 umol/L | 71.8 | 64.7 | 53.1 | |||
| Phosphors | 0.85–1.51 mmol/L | 1.08 | 1.07 | 1 | |||
| hsCRP | 0–5 mg/L | 36.25 | 63 | 57.43 | <5 | ||
| Procalcitonin | 0–0.5 ng/mL | 0.54 | 0.35 | ||||
| Prealbumin determination | 170–420 mg/L | 134.7 | 84.7 | ||||
| Prothrombin time | 9–13 s | 14.6 | 15.5 | 17.6 | 16.5 | ||
| PT ratio | 0.85–1.15 | 1.34 | 1.42 | 1.61 | 1.51 | ||
| Fibrinogen | 2–4 g/L | 5.45 | 4.99 | 7.95 | 4.87 | ||
| D-dimer | 0–0.25 mg/L | 0.63 | 0.86 | 1.38 | 1.11 | ||
| Fibrinogen degradation products | 0–5 mg/L | 6.12 | 5.49 | ||||
| INR | <1.5 | 1.59 | 1.5 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, G.; Chen, Q.; Luo, G.; Meng, Z.; Lei, P.; Chen, P.; Drisko, J.A. High Dose Intravenous Vitamin C as Adjunctive Therapy for COVID-19 Patients with Cancer: Two Cases. Life 2022, 12, 335. https://doi.org/10.3390/life12030335
Guo G, Chen Q, Luo G, Meng Z, Lei P, Chen P, Drisko JA. High Dose Intravenous Vitamin C as Adjunctive Therapy for COVID-19 Patients with Cancer: Two Cases. Life. 2022; 12(3):335. https://doi.org/10.3390/life12030335
Chicago/Turabian StyleGuo, Guangling, Qi Chen, Guoshi Luo, Zhongji Meng, Pan Lei, Ping Chen, and Jeanne A. Drisko. 2022. "High Dose Intravenous Vitamin C as Adjunctive Therapy for COVID-19 Patients with Cancer: Two Cases" Life 12, no. 3: 335. https://doi.org/10.3390/life12030335
APA StyleGuo, G., Chen, Q., Luo, G., Meng, Z., Lei, P., Chen, P., & Drisko, J. A. (2022). High Dose Intravenous Vitamin C as Adjunctive Therapy for COVID-19 Patients with Cancer: Two Cases. Life, 12(3), 335. https://doi.org/10.3390/life12030335








