EGF-Coupled Gold Nanoparticles Increase the Expression of CNPase and the Myelin-Associated Proteins MAG, MOG, and MBP in the Septal Nucleus Demyelinated by Cuprizone
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cuprizone Administration
2.3. Synthesis of GNPs
2.4. EGF–GNPs Nanocomposite
2.5. UV–Vis Spectroscopy
2.6. Transmission Electron Microscopy (TEM)
2.7. FTIR Analysis
2.8. Administration of EGF–GNPs
2.9. Rotarod Test
2.10. Western Blot
2.11. Statistical Analysis
3. Results
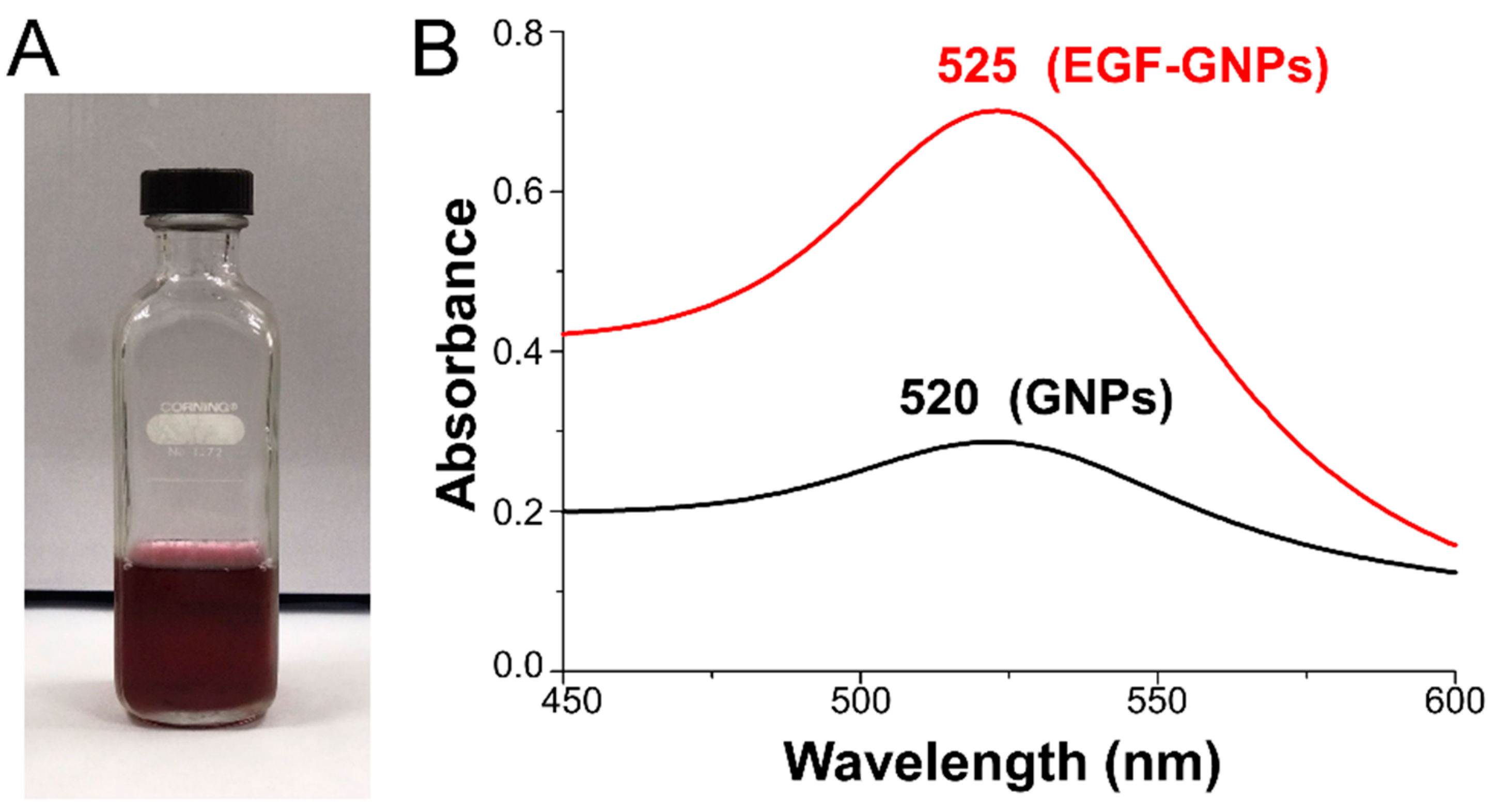
3.1. UV–Vis Analysis of EGF–GNPs
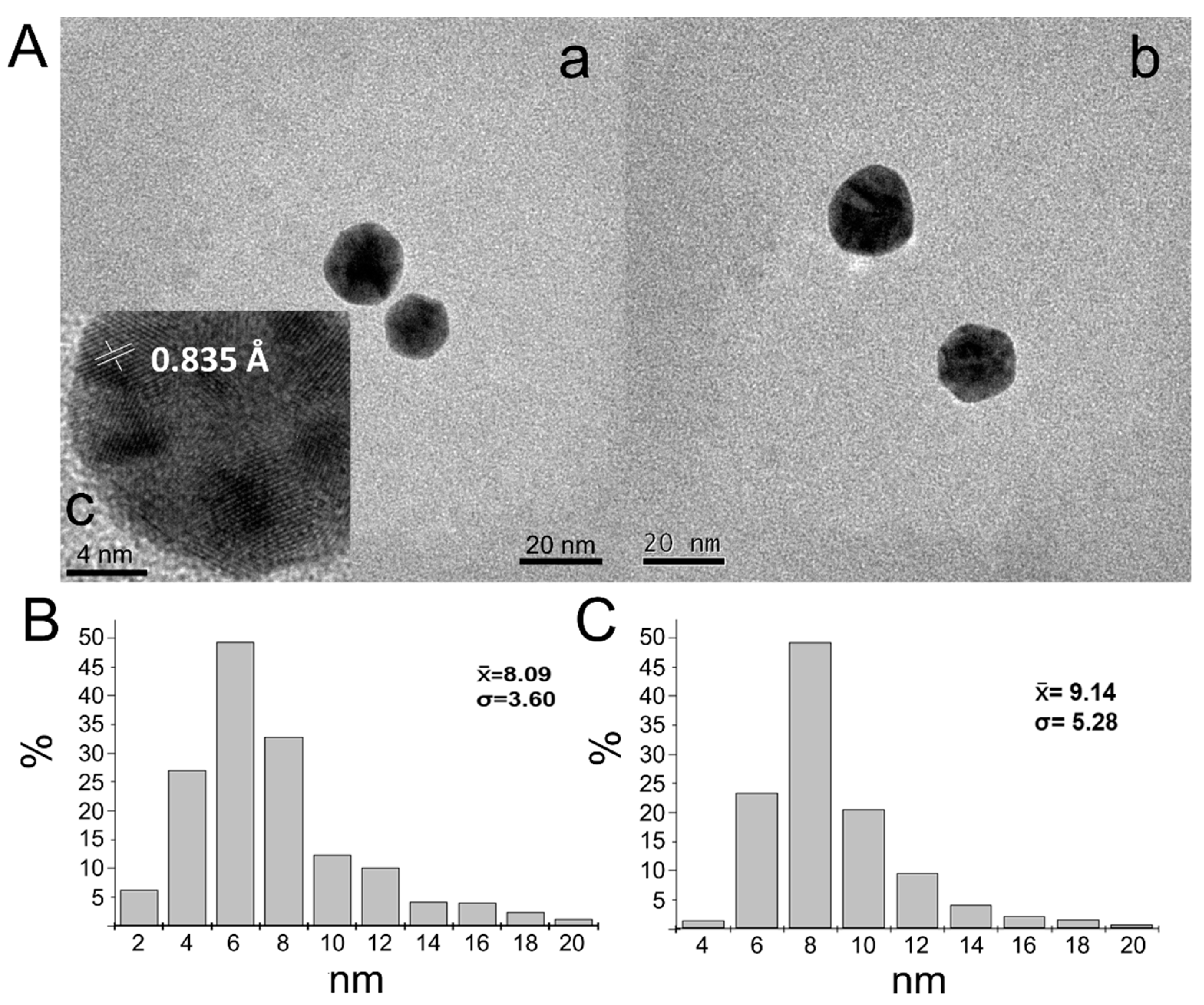
3.2. Structural Analysis of EGF–GNPs
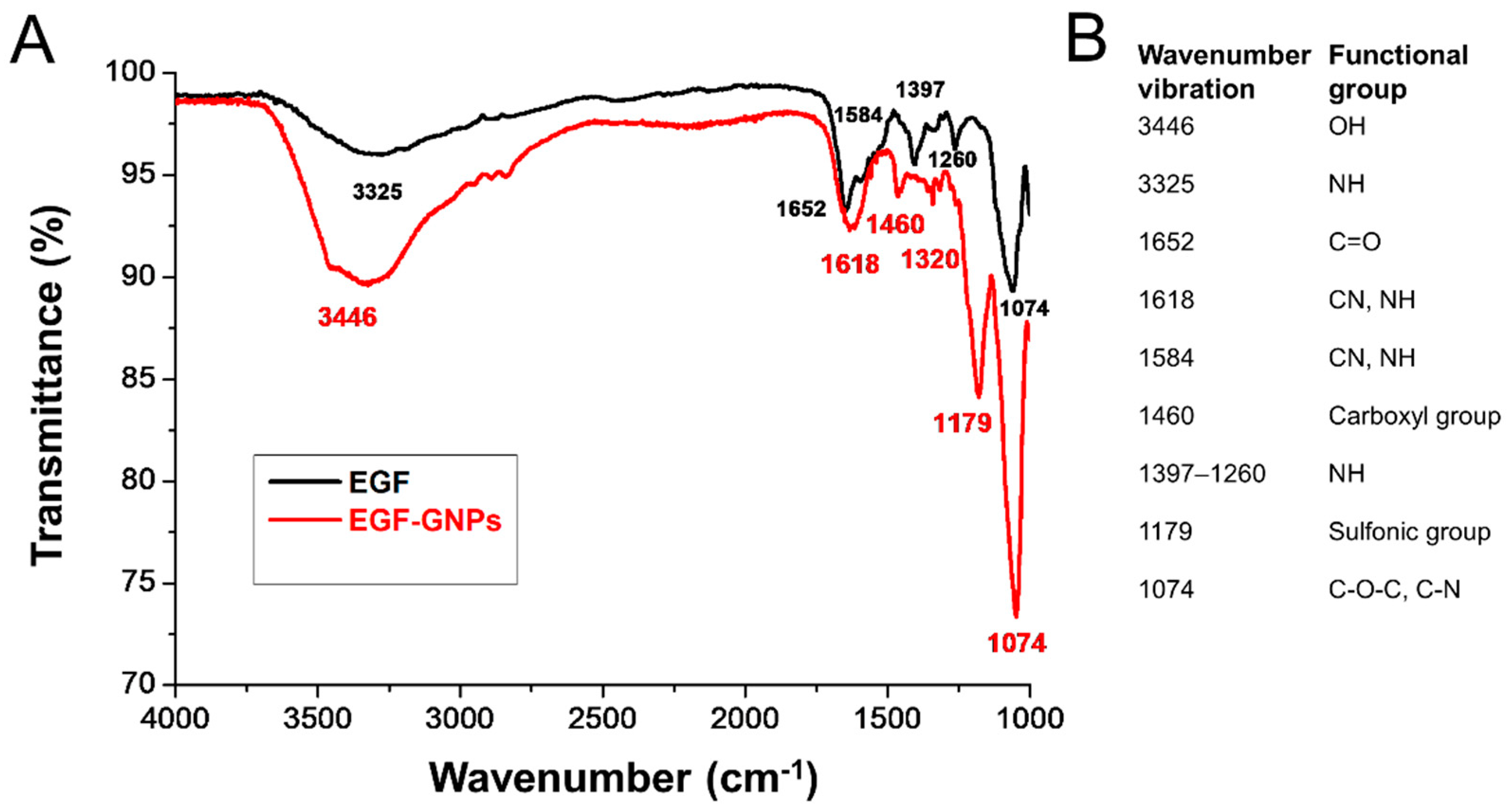
3.3. FTIR Analysis of EGF–GNPs
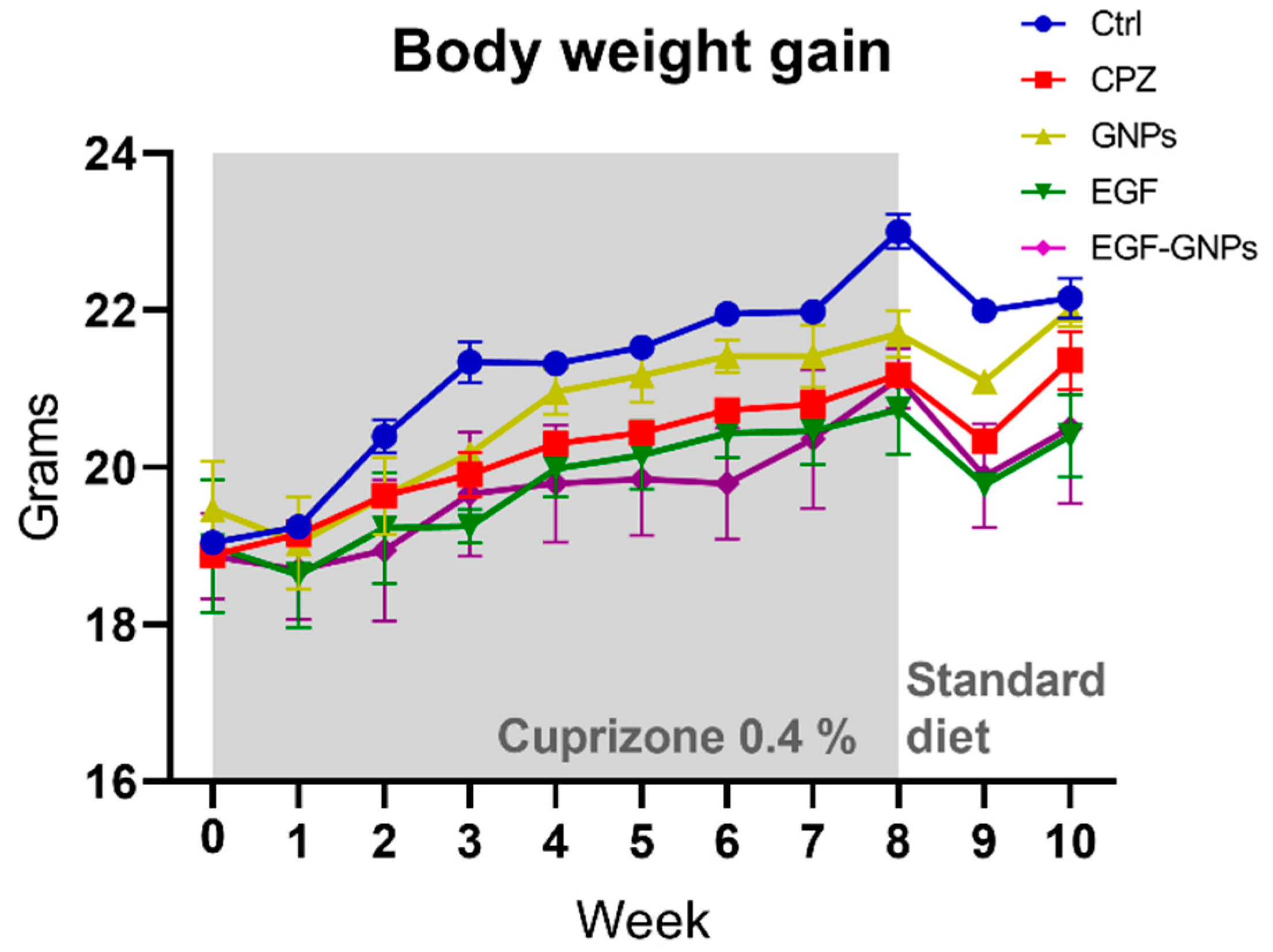
3.4. Body Weight Gain
3.5. EGF–GNPs Improves Motor Coordination
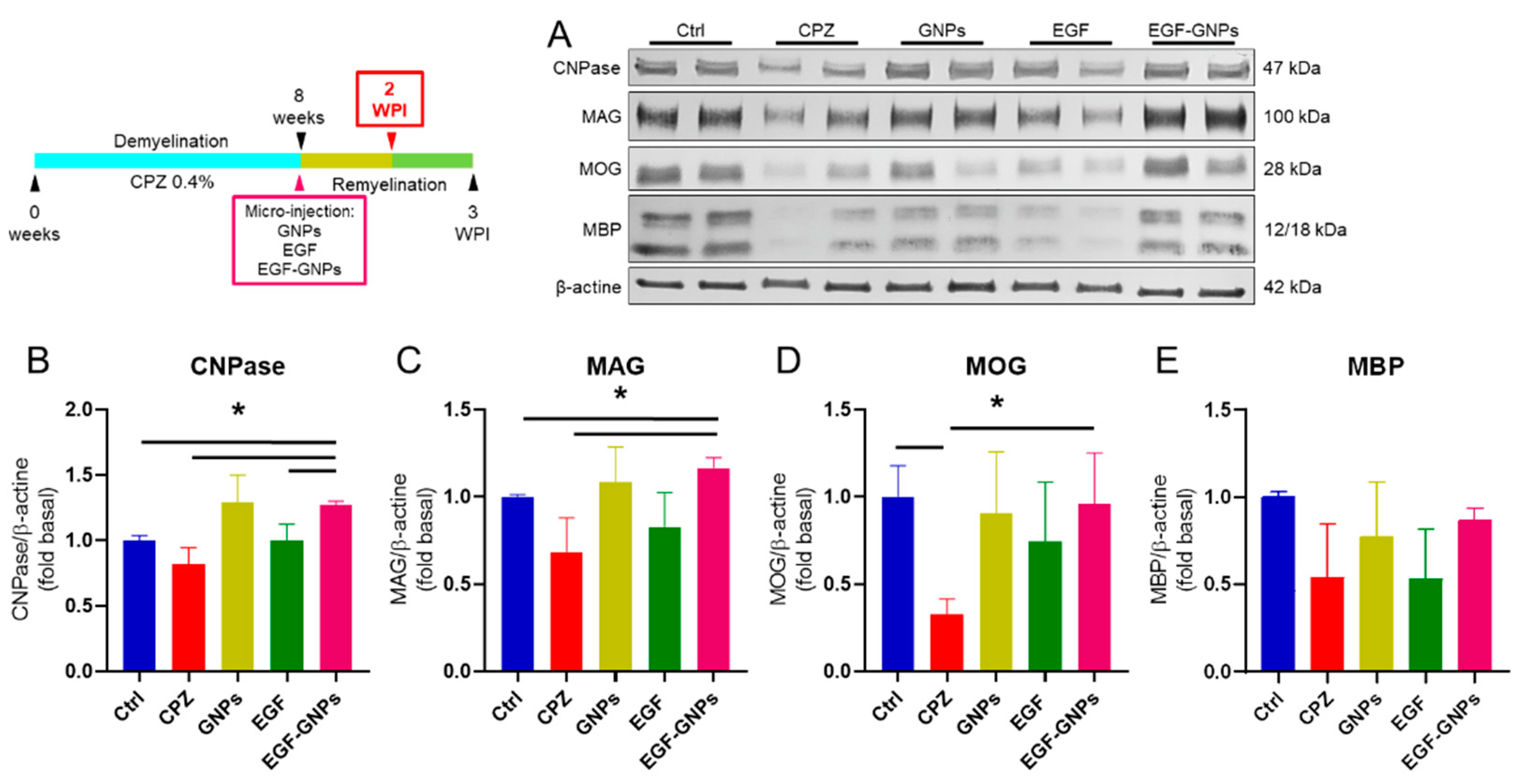
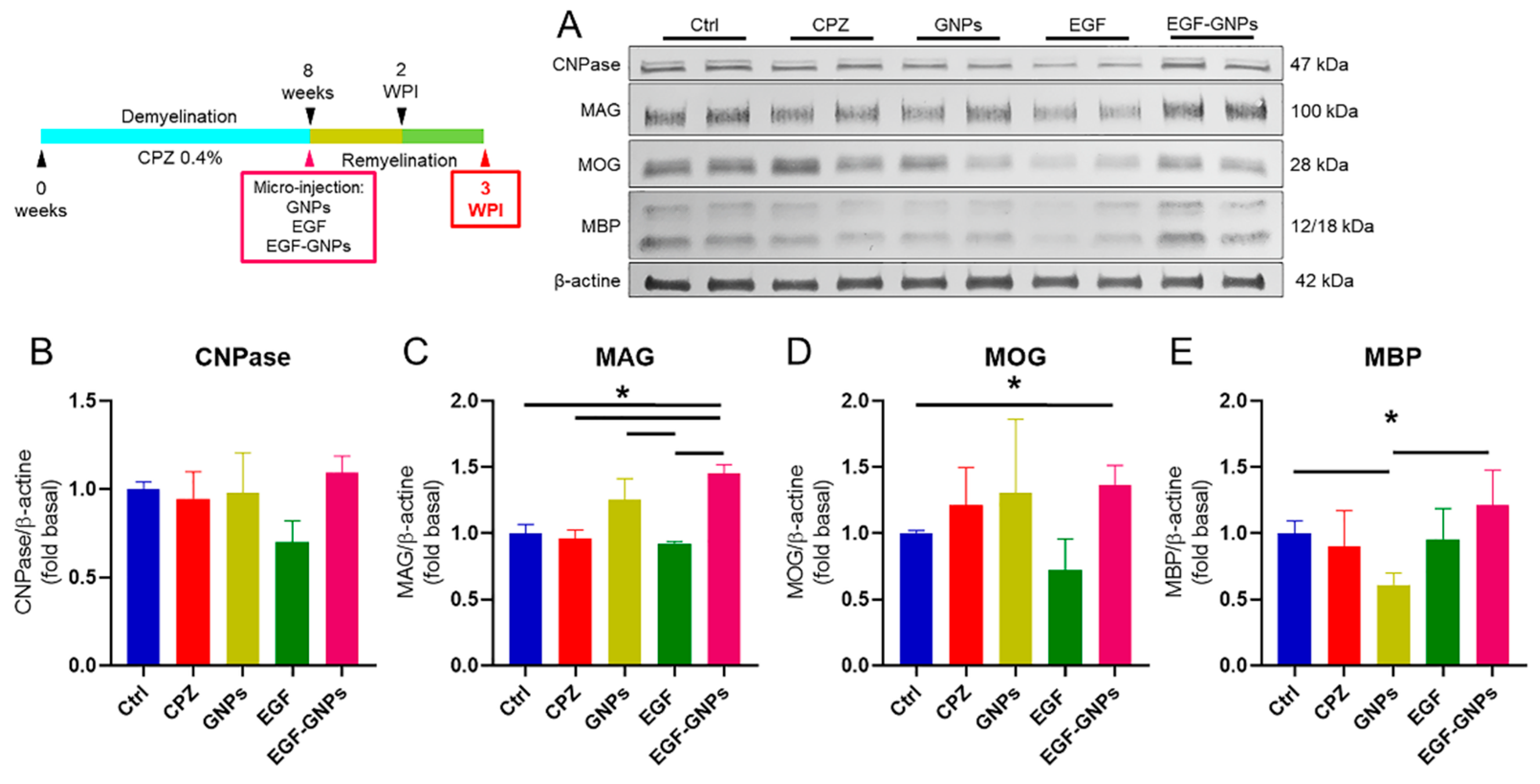
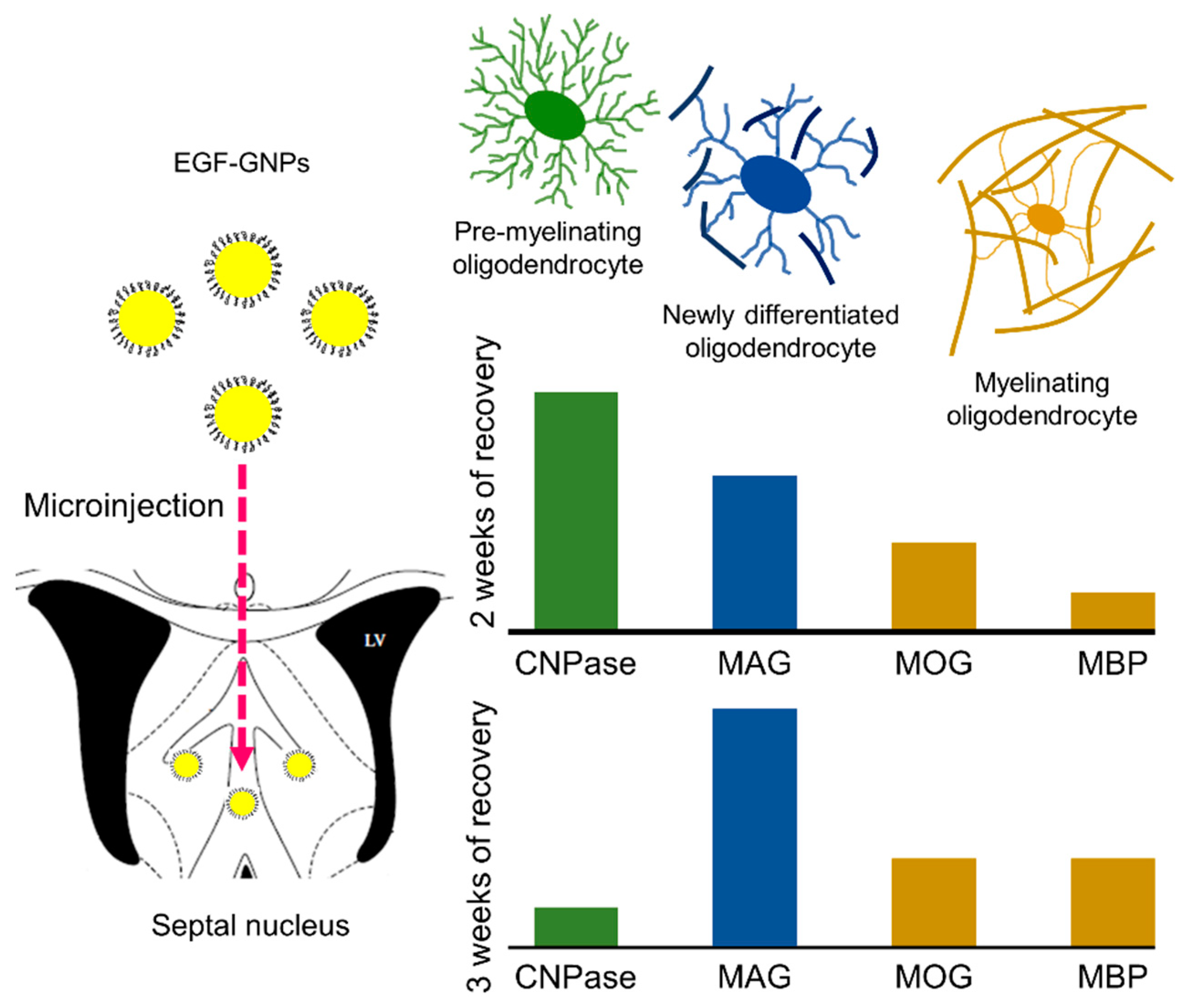
3.6. EGF–GNPs Increase CNPase, MAG, MOG and MBP Proteins
4. Discussion
4.1. Synthesis and Characterization of the Nanocomposite
4.2. Body Weight Gain
4.3. The Nanocomposite EGF–GNPs Improves Motor Coordination at 3 Weeks Post-Injection
4.4. The EGF–GNPs Promotes the Differential Expression of Myelin Proteins
4.5. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gacem, N.; Nait-Oumesmar, B. Oligodendrocyte Development and Regenerative Therapeutics in Multiple Sclerosis. Life 2021, 11, 327. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Gaitán, M.I.; Ysrraelit, M.C.; Fiol, M.P. Progressive multiple sclerosis: From pathogenic mechanisms to treatment. Brain 2017, 140, 527–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Perez, O.; Romero-Rodriguez, R.; Soriano-Navarro, M.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Epidermal growth factor induces the progeny of subventricular zone type B cells to migrate and differentiate into oligodendrocytes. Stem Cells 2009, 27, 2032–2043. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Perez, O.; Quiñones-Hinojosa, A. Dose-dependent effect of EGF on migration and differentiation of adult subventricular zone astrocytes. Glia 2010, 58, 975–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguirre, A.; Dupree, J.L.; Mangin, J.; Gallo, V. A functional role for EGFR signaling in myelination and remyelination. Nat. Neurosci. 2007, 10, 990. [Google Scholar] [CrossRef]
- Galvez-Contreras, A.Y.; Quiñones-Hinojosa, A.; Gonzalez-Perez, O. The role of EGFR and ErbB family related proteins in the oligodendrocyte specification in germinal niches of the adult mammalian brain. Front. Cell. Neurosci. 2013, 7, 258. [Google Scholar] [CrossRef]
- Chen, X.-C.; Wei, X.-T.; Guan, J.-H.; Shu, H.; Chen, D. EGF stimulates glioblastoma metastasis by induction of matrix metalloproteinase-9 in an EGFR-dependent mechanism. Oncotarget 2017, 8, 65969. [Google Scholar] [CrossRef] [Green Version]
- Pudełek, M.; Król, K.; Catapano, J.; Wróbel, T.; Czyż, J.; Ryszawy, D. Epidermal Growth Factor (EGF) Augments the Invasive Potential of Human Glioblastoma Multiforme Cells via the Activation of Collaborative EGFR/ROS-Dependent Signaling. Int. J. Mol. Sci. 2020, 21, 3605. [Google Scholar] [CrossRef]
- Lira-Diaz, E.; Gonzalez-Pedroza, M.G.; Vasquez, C.; Morales-Luckie, R.A.; Gonzalez-Perez, O. Gold nanoparticles produce transient reactive gliosis in the adult brain. Neurosci. Res. 2021, 170, 76–86. [Google Scholar] [CrossRef]
- Vega-Riquer, J.M.; Mendez-Victoriano, G.; Morales-Luckie, R.A.; Gonzalez-Perez, O. Five decades of cuprizone, an updated model to replicate demyelinating diseases. Curr. Neuropharmacol. 2019, 17, 129–141. [Google Scholar] [CrossRef]
- González-Pedroza, M.G.; Sánchez-Mendieta, V.; Morales-Valencia, J.A.; López-Téllez, G.; Argueta-Figueroa, L.; González-Pérez, O.; Morales-Luckie, R.A. X-ray Photoelectron Spectroscopy Study of Interactions Between Gold Nanoparticles and Epidermal Growth Factor for Potential Use in Biomedicine. J. Bionanosci. 2017, 11, 141–147. [Google Scholar] [CrossRef]
- González-Pedroza, M.G.; Argueta-Figueroa, L.; García-Contreras, R.; Jiménez-Martínez, Y.; Martínez-Martínez, E.; Navarro-Marchal, S.A.; Marchal, J.A.; Morales-Luckie, R.A.; Boulaiz, H. Silver Nanoparticles from Annona muricata Peel and Leaf Extracts as a Potential Potent, Biocompatible and Low Cost Antitumor Tool. Nanomaterials 2021, 11, 1273. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Perez, O.; Guerrero-Cazares, H.; Quinones-Hinojosa, A. Targeting of deep brain structures with microinjections for delivery of drugs, viral vectors, or cell transplants. J. Vis. Exp. 2010, 46, e2082. [Google Scholar] [CrossRef] [Green Version]
- Monville, C.; Torres, E.M.; Dunnett, S.B. Comparison of incremental and accelerating protocols of the rotarod test for the assessment of motor deficits in the 6-OHDA model. J. Neurosci. Methods 2006, 158, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Holly, K.S.; Voziyanov, V.; Villalba, S.L.; Tong, R.; Grigsby, H.E.; Glasscock, E.; Szele, F.G.; Vlachos, I.; Murray, T.A. Gradient index microlens implanted in prefrontal cortex of mouse does not affect behavioral test performance over time. PLoS ONE 2016, 11, e0146533. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Hutter, E.; Boridy, S.; Labrecque, S.; Lalancette-Hébert, M.; Kriz, J.; Winnik, F.M.; Maysinger, D. Microglial response to gold nanoparticles. ACS Nano 2010, 4, 2595–2606. [Google Scholar] [CrossRef] [Green Version]
- Tsoli, M.; Kuhn, H.; Brandau, W.; Esche, H.; Schmid, G. Cellular uptake and toxicity of Au55 clusters. Small 2005, 1, 841–844. [Google Scholar] [CrossRef]
- Kong, J.; Yu, S. Fourier transform infrared spectroscopic analysis of protein secondary structures. Acta Biochim. Biophys. Sin. 2007, 39, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Adochitei, A.; Drochioiu, G. Rapid characterization of peptide secondary structure by FT-IR spectroscopy. Rev. Roum. Chim. 2011, 56, 783–791. [Google Scholar]
- Tagge, I.; O’Connor, A.; Chaudhary, P.; Pollaro, J.; Berlow, Y.; Chalupsky, M.; Bourdette, D.; Woltjer, R.; Johnson, M.; Rooney, W. Spatio-temporal patterns of demyelination and remyelination in the cuprizone mouse model. PLoS ONE 2016, 11, e0152480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laflamme, N.; Cisbani, G.; Préfontaine, P.; Srour, Y.; Bernier, J.; St-Pierre, M.-K.; Tremblay, M.-È.; Rivest, S. mCSF-induced microglial activation prevents myelin loss and promotes its repair in a mouse model of multiple sclerosis. Front. Cell. Neurosci. 2018, 12, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stidworthy, M.F.; Genoud, S.; Suter, U.; Mantei, N.; Franklin, R.J. Quantifying the early stages of remyelination following cuprizone-induced demyelination. Brain Pathol. 2003, 13, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Liz-Marzán, L.M. Nanometals. Mater. Today 2004, 7, 26–31. [Google Scholar] [CrossRef]
- Eustis, S.; El-Sayed, M.A. Why gold nanoparticles are more precious than pretty gold: Noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem. Soc. Rev. 2006, 35, 209–217. [Google Scholar] [CrossRef]
- Sarmento, B.; Ferreira, D.; Veiga, F.; Ribeiro, A. Characterization of insulin-loaded alginate nanoparticles produced by ionotropic pre-gelation through DSC and FTIR studies. Carbohydr. Polym. 2006, 66, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, M.A.; Jaafar, J.; Ismail, A.; Othman, M.; Rahman, M. Fourier transform infrared (FTIR) spectroscopy. In Membrane Characterization; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–29. [Google Scholar]
- Franco-Pons, N.; Torrente, M.; Colomina, M.T.; Vilella, E. Behavioral deficits in the cuprizone-induced murine model of demyelination/remyelination. Toxicol. Lett. 2007, 169, 205–213. [Google Scholar] [CrossRef]
- Elbaz, E.M.; Senousy, M.A.; El-Tanbouly, D.M.; Sayed, R.H. Neuroprotective effect of linagliptin against cuprizone-induced demyelination and behavioural dysfunction in mice: A pivotal role of AMPK/SIRT1 and JAK2/STAT3/NF-κB signalling pathway modulation. Toxicol. Appl. Pharmacol. 2018, 352, 153–161. [Google Scholar] [CrossRef]
- Kuhn, S.; Gritti, L.; Crooks, D.; Dombrowski, Y. Oligodendrocytes in development, myelin generation and beyond. Cells 2019, 8, 1424. [Google Scholar] [CrossRef] [Green Version]
- Lappe-Siefke, C.; Goebbels, S.; Gravel, M.; Nicksch, E.; Lee, J.; Braun, P.E.; Griffiths, I.R.; Nave, K.A. Disruption of Cnp1 uncouples oligodendroglial functions in axonal support and myelination. Nat. Genet. 2003, 33, 366–374. [Google Scholar] [CrossRef]
- Snaidero, N.; Möbius, W.; Czopka, T.; Hekking, L.H.; Mathisen, C.; Verkleij, D.; Goebbels, S.; Edgar, J.; Merkler, D.; Lyons, D.A. Myelin membrane wrapping of CNS axons by PI (3, 4, 5) P3-dependent polarized growth at the inner tongue. Cell 2014, 156, 277–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snaidero, N.; Velte, C.; Myllykoski, M.; Raasakka, A.; Ignatev, A.; Werner, H.B.; Erwig, M.S.; Möbius, W.; Kursula, P.; Nave, K.-A. Antagonistic functions of MBP and CNP establish cytosolic channels in CNS myelin. Cell Rep. 2017, 18, 314–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djannatian, M.; Timmler, S.; Arends, M.; Luckner, M.; Weil, M.-T.; Alexopoulos, I.; Snaidero, N.; Schmid, B.; Misgeld, T.; Möbius, W. Two adhesive systems cooperatively regulate axon ensheathment and myelin growth in the CNS. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, E.G.; Stockton, M.E. Premyelinating Oligodendrocytes: Mechanisms Underlying Cell Survival and Integration. Front. Cell Dev. Biol. 2021, 9, 714169. [Google Scholar] [CrossRef] [PubMed]
- Pruvost, M.; Moyon, S. Oligodendroglial Epigenetics, from Lineage Specification to Activity-Dependent Myelination. Life 2021, 11, 62. [Google Scholar] [CrossRef]
- Singh, B.; Carpenter, G.; Coffey, R.J. EGF receptor ligands: Recent advances. F1000Research 2016, 5, 2270. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, R.A.; Luwor, R.B.; Burgess, A.W. The Epidermal Growth Factor Receptor: Structure-Function Informing the Design of Anticancer Therapeutics. Exp. Cell Res. 2018, 371, 1–19. [Google Scholar] [CrossRef]
- Aguirre, A.; Rubio, M.E.; Gallo, V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature 2010, 467, 323. [Google Scholar] [CrossRef] [Green Version]
- Vinukonda, G.; Hu, F.; Mehdizadeh, R.; Dohare, P.; Kidwai, A.; Juneja, A.; Naran, V.; Kierstead, M.; Chawla, R.; Kayton, R. Epidermal growth factor preserves myelin and promotes astrogliosis after intraventricular hemorrhage. Glia 2016, 64, 1987–2004. [Google Scholar] [CrossRef] [Green Version]
- Franklin, R.J.; Goldman, J.E. Remyelination by endogenous glia. In Myelin Biology and Disorders; Elsevier: Amsterdam, The Netherlands, 2004; pp. 173–196. [Google Scholar]
- Almazan, G.; Honegger, P.; Matthieu, J.-M.; Guentert-Lauber, B. Epidermal growth factor and bovine growth hormone stimulate differentiation and myelination of brain cell aggregates in culture. Dev. Brain Res. 1985, 21, 257–264. [Google Scholar] [CrossRef]
- Matthieu, J.-M.; Comte, V.; Tosic, M.; Honegger, P. Myelin gene expression during demyelination and remyelination in aggregating brain cell cultures. J. Neuroimmunol. 1992, 40, 231–234. [Google Scholar] [CrossRef]
- Aguirre, A.; Gallo, V. Reduced EGFR signaling in progenitor cells of the adult subventricular zone attenuates oligodendrogenesis after demyelination. Neuron Glia Biol. 2007, 3, 209–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mangiola, A.; Vigo, V.; Anile, C.; De Bonis, P.; Marziali, G.; Lofrese, G. Role and importance of IGF-1 in traumatic brain injuries. BioMed Res. Int. 2015, 2015, 736104. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lira-Diaz, E.; Monroy-Rodriguez, J.; Gonzalez-Pedroza, M.G.; Morales-Luckie, R.A.; Castro-Sánchez, L.; Gonzalez-Perez, O. EGF-Coupled Gold Nanoparticles Increase the Expression of CNPase and the Myelin-Associated Proteins MAG, MOG, and MBP in the Septal Nucleus Demyelinated by Cuprizone. Life 2022, 12, 333. https://doi.org/10.3390/life12030333
Lira-Diaz E, Monroy-Rodriguez J, Gonzalez-Pedroza MG, Morales-Luckie RA, Castro-Sánchez L, Gonzalez-Perez O. EGF-Coupled Gold Nanoparticles Increase the Expression of CNPase and the Myelin-Associated Proteins MAG, MOG, and MBP in the Septal Nucleus Demyelinated by Cuprizone. Life. 2022; 12(3):333. https://doi.org/10.3390/life12030333
Chicago/Turabian StyleLira-Diaz, Eduardo, Jesus Monroy-Rodriguez, Maria G. Gonzalez-Pedroza, Raul A. Morales-Luckie, Luis Castro-Sánchez, and Oscar Gonzalez-Perez. 2022. "EGF-Coupled Gold Nanoparticles Increase the Expression of CNPase and the Myelin-Associated Proteins MAG, MOG, and MBP in the Septal Nucleus Demyelinated by Cuprizone" Life 12, no. 3: 333. https://doi.org/10.3390/life12030333
APA StyleLira-Diaz, E., Monroy-Rodriguez, J., Gonzalez-Pedroza, M. G., Morales-Luckie, R. A., Castro-Sánchez, L., & Gonzalez-Perez, O. (2022). EGF-Coupled Gold Nanoparticles Increase the Expression of CNPase and the Myelin-Associated Proteins MAG, MOG, and MBP in the Septal Nucleus Demyelinated by Cuprizone. Life, 12(3), 333. https://doi.org/10.3390/life12030333







