Resveratrol and Reproductive Health
Abstract
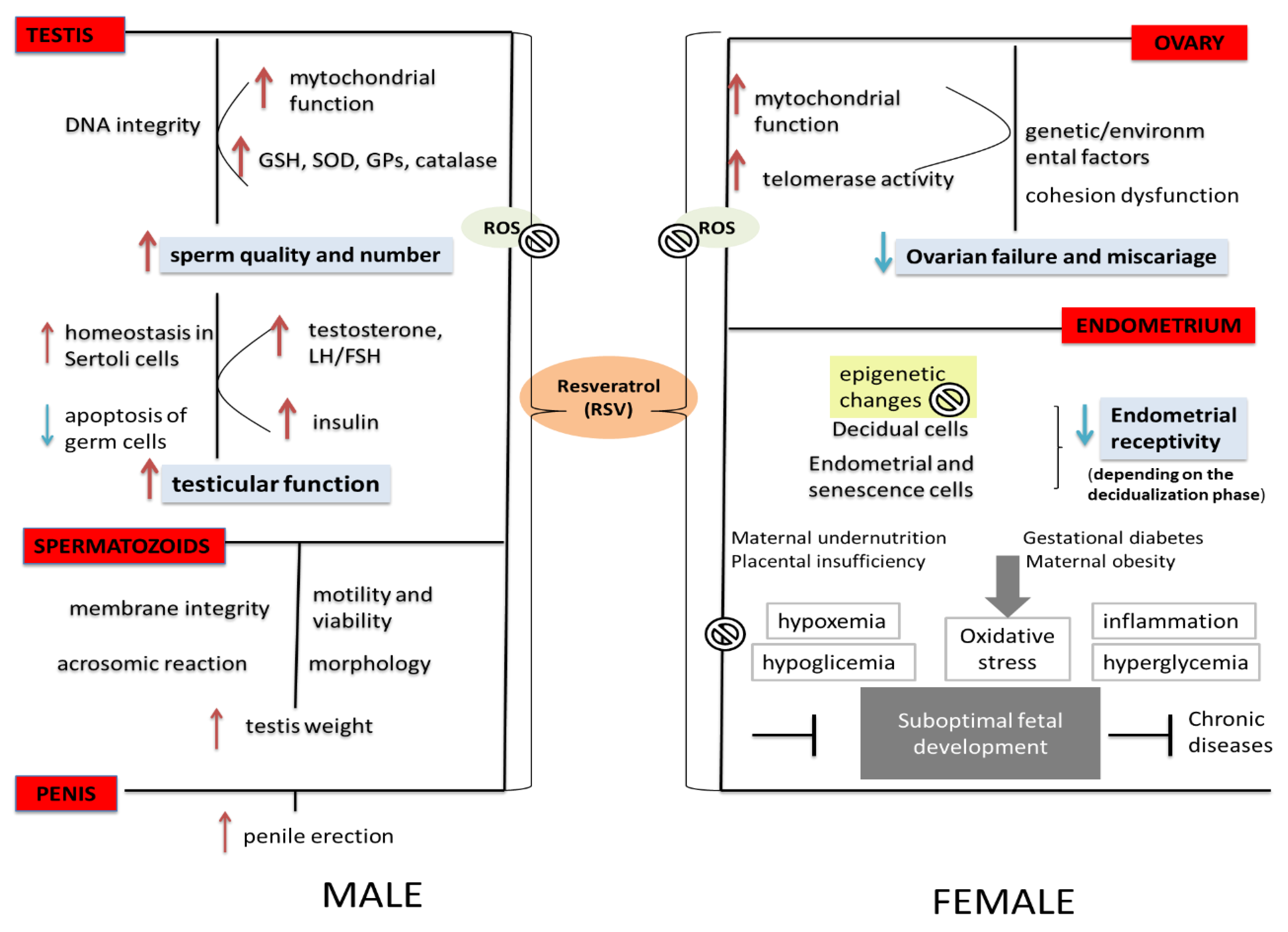
:1. Introduction
2. Methods
3. RSV as a Phytoestrogen
4. RSV—Additional Roles on Overall Reproduction
4.1. The Effects of RSV on the Endometrium
4.2. The Effects of RSV on the Embryogenesis—Preclinical Studies
4.3. The Effects of RSV on the Embryogenesis—Clinical Studies
5. The Effects of RSV on Male Fertility
6. The Effects of RSV on Complications during Pregnancy
7. The Effects of RSV on Women’s Reproductive Health
8. RSV—Future Perspectives
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Xia, N.; Hasselwander, S.; Daiber, A. Resveratrol and Vascular Function. Int. J. Mol. Sci. 2019, 20, 2155. [Google Scholar] [CrossRef] [Green Version]
- Ly, C.; Yockell-Lelièvre, J.; Ferraro, Z.M.; Arnason, J.T.; Ferrier, J.; Gruslin, A. The effects of dietary polyphenols on reproductive health and early development. Hum. Reprod. Updat. 2014, 21, 228–248. [Google Scholar] [CrossRef] [Green Version]
- Gehm, B.D.; McAndrews, J.M.; Chien, P.Y.; Jameson, J.L. Resveratrol, a polyphenolic compound found in grapes and wine, is an agonist for the estrogen receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 14138–14143. [Google Scholar] [CrossRef] [Green Version]
- Mueller, S.O.; Simon, S.; Chae, K.; Metzler, M.; Korach, K. Phytoestrogens and Their Human Metabolites Show Distinct Agonistic and Antagonistic Properties on Estrogen Receptor (ER) and ER in Human Cells. Toxicol. Sci. 2004, 80, 14–25. [Google Scholar] [CrossRef] [Green Version]
- Stahl, S.; Chun, T.-Y.; Gray, W.G. Phytoestrogens Act as Estrogen Agonists in an Estrogen-Responsive Pituitary Cell Line. Toxicol. Appl. Pharmacol. 1998, 152, 41–48. [Google Scholar] [CrossRef]
- Kuiper, G.G.J.M.; Carlsson, B.; Grandien, K.; Enmark, E.; Häggblad, J.; Nilsson, S.; Gustafsson, J.-A. Comparison of the Ligand Binding Specificity and Transcript Tissue Distribution of Estrogen Receptors α and β. Endocrinology 1997, 138, 863–870. [Google Scholar] [CrossRef]
- Kubo, K.; Arai, O.; Omura, M.; Watanabe, R.; Ogata, R.; Aou, S. Low dose effects of bisphenol A on sexual differentiation of the brain and behavior in rats. Neurosci. Res. 2003, 45, 345–356. [Google Scholar] [CrossRef]
- Pasquariello, R.; Verdile, N.; Brevini, T.A.L.; Gandolfi, F.; Boiti, C.; Zerani, M.; Maranesi, M. The role of resveratrol in mammalian re-production. Molecules 2020, 25, 4554. [Google Scholar] [CrossRef]
- Nikaido, Y.; Yoshizawa, K.; Danbara, N.; Tsujita-Kyutoku, M.; Yuri, T.; Uehara, N.; Tsubura, A. Effects of maternal xenoestrogen exposure on development of the reproductive tract and mammary gland in female CD-1 mouse offspring. Reprod. Toxicol. 2004, 18, 803–811. [Google Scholar] [CrossRef]
- Nikaido, Y.; Yoshizawa, K.; Pei, R.-J.; Yuri, T.; Danbara, N.; Hatano, T.; Tsubura, A. Prepubertal Zearalenone Exposure Suppresses N-Methyl-N-nitrosourea-Induced Mammary Tumorigenesis but Causes Severe Endocrine Disruption in Female Sprague-Dawley Rats. Nutr. Cancer 2003, 47, 164–170. [Google Scholar] [CrossRef]
- Bowers, J.L.; Tyulmenkov, V.V.; Jernigan, S.C.; Klinge, C.M. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology 2000, 141, 3657–3667. [Google Scholar] [CrossRef]
- Henry, L.A.; Witt, D.M. Resveratrol: Phytoestrogen Effects on Reproductive Physiology and Behavior in Female Rats. Horm. Behav. 2002, 41, 220–228. [Google Scholar] [CrossRef]
- Sato, M.; Pei, R.-J.; Yuri, T.; Danbara, N.; Nakane, Y.; Tsubura, A. Prepubertal resveratrol exposure accelerates N-methyl-N-nitrosourea-induced mammary carcinoma in female Sprague–Dawley rats. Cancer Lett. 2003, 202, 137–145. [Google Scholar] [CrossRef]
- Pan, W.; Yu, H.; Huang, S.; Zhu, P. Resveratrol Protects against TNF-α-Induced Injury in Human Umbilical Endothelial Cells through Promoting Sirtuin-1-Induced Repression of NF-KB and p38 MAPK. PLoS ONE 2016, 11, e0147034. [Google Scholar] [CrossRef] [Green Version]
- Klinge, C.M.; Blankenship, K.A.; Risinger, K.E.; Bhatnagar, S.; Noisin, E.L.; Sumanasekera, W.K.; Zhao, L.; Brey, D.M.; Keynton, R.S. Resveratrol and estradiol rapidly activate mapk signaling through estrogen receptors alpha and beta in endothelial cells. J. Biol. Chem. 2005, 280, 7460–7468. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.-L.; Yi, L.; Jin, X.; Xie, Q.; Zhang, T.; Zhou, X.; Chang, H.; Fu, Y.-J.; Zhu, J.-D.; Zhang, Q.-Y.; et al. Absorption of resveratrol by vascular endothelial cells through passive diffusion and an SGLT1-mediated pathway. J. Nutr. Biochem. 2013, 24, 1823–1829. [Google Scholar] [CrossRef]
- Mestre Citrinovitz, A.C.; Langer, L.; Strowitzki, T.; Germeyer, A. Resveratrol enhances decidualization of human endometrial stromal cells. Reproduction. 2020, 159, 453–463. [Google Scholar] [CrossRef]
- Morita, Y.; Wada-Hiraike, O.; Yano, T.; Shirane, A.; Hirano, M.; Hiraike, H.; Koyama, S.; Oishi, H.; Yoshino, O.; Miyamoto, Y.; et al. Resveratrol promotes expression of SIRT1 and StAR in rat ovarian granulosa cells: An implicative role of SIRT1 in the ovary. Reprod. Biol. Endocrinol. 2012, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Dumollard, R.; Duchen, M.; Carroll, J. The Role of Mitochondrial Function in the Oocyte and Embryo. Curr. Top. Dev. Biol. 2007, 77, 21–49. [Google Scholar] [CrossRef]
- Gellersen, B.; Brosens, J. Cyclic Decidualization of the Human Endometrium in Reproductive Health and Failure. Endocr. Rev. 2014, 35, 851–905. [Google Scholar] [CrossRef]
- Yoon, J.; Juhn, K.M.; Jung, E.H.; Park, H.J.; Yoon, S.H.; Ko, Y.; Hur, C.Y.; Lim, J.H. Effects of resveratrol, granulocyte-macrophage colony-stimulating factor or dichloroacetic acid in the culture media on embryonic development and pregnancy rates in aged mice. Aging Albany NY 2020, 12, 2659–2669. [Google Scholar]
- Lee, S.; Jin, J.-X.; Taweechaipaisankul, A.; Kim, G.A.; Lee, B.C. Synergistic effects of resveratrol and melatonin on in vitro maturation of porcine oocytes and subsequent embryo development. Theriogenology 2018, 114, 191–198. [Google Scholar] [CrossRef]
- Zheng, S.; Feng, Q.; Cheng, J.; Zheng, J. Maternal resveratrol consumption and its programming effects on metabolic health in offspring mechanisms and potential implications. Biosci. Rep. 2018, 38. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Ibarra, J.L.; Espinoza-Mendoza, E.A.; Rangel-Santos, R.; Ambriz-García, D.A.; Navarro-Maldonado, M.D.C. Effect of resveratrol on the in vitro maturation of ovine (Ovis aries) oocytes and the subsequent development of handmade cloned embryos. Vet. México 2019, 5. [Google Scholar] [CrossRef]
- Darby, J.R.T.; Mohd Dollah, M.H.B.; Regnault, T.R.H.; Williams, M.T.; Morrison, J.L. Systematic review: Impact of resveratrol exposure during pregnancy on maternal and fetal outcomes in animal models of human pregnancy complications-Are we ready for the clinic? Pharmacol. Res. 2019, 144, 264–278. [Google Scholar] [CrossRef]
- Bourque, S.; Dolinsky, V.; Dyck, J.; Davidge, S. Maternal resveratrol treatment during pregnancy improves adverse fetal outcomes in a rat model of severe hypoxia. Placenta 2012, 33, 449–452. [Google Scholar] [CrossRef]
- Ochiai, A.; Kuroda, K. Preconception resveratrol intake against infertility: Friend or foe? Reprod. Med. Biol. 2019, 19, 107–113. [Google Scholar] [CrossRef]
- Ochiai, A.; Kuroda, K.; Ozaki, R.; Ikemoto, Y.; Murakami, K.; Muter, J.; Matsumoto, A.; Itakura, A.; Brosens, J.J.; Takeda, S. Resveratrol inhibits decidualization by accelerating downregulation of the CRABP2-RAR pathway in differentiating human endometrial stromal cells. Cell Death Dis. 2019, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ochiai, A.; Kuroda, K.; Ikemoto, Y.; Ozaki, R.; Nakagawa, K.; Nojiri, S.; Takeda, S.; Sugiyama, R. Influence of resveratrol supplemen-tation on IVF-embryo transfer cycle outcomes. Reprod. Biomed. Online 2019, 39, 205–210. [Google Scholar] [CrossRef]
- Bódis, J.; Sulyok, E.; Kőszegi, T.; Gödöny, K.; Prémusz, V.; Várnagy, A. Serum and follicular fluid levels of sirtuin 1, sirtuin 6, and resveratrol in women undergoing in vitro fertilization: An observational, clinical study. J. Int. Med. Res. 2018, 47, 772–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zielinsky, P.; Busato, S. Prenatal effects of maternal consumption of polyphenol-rich foods in late pregnancy upon fetal ductus arteriosus. Birth Defects Res. Part C Embryo Today Rev. 2013, 99, 256–274. [Google Scholar] [CrossRef] [Green Version]
- Vian, I.; Zielinsky, P.; Zílio, A.M.; Schaun, M.I.; Brum, C.; Lampert, K.V.; De Ávila, N.; Baldissera, G.; Klanovicz, T.M.; Zenki, K.; et al. Increase of prostaglandin E2 in the reversal of fetal ductal constriction after polyphenol restriction. Ultrasound Obstet. Gynecol. 2017, 52, 617–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jozkowiak, M.; Hutchings, G.; Jankowski, M.; Kulcenty, K.; Mozdziak, P.; Kempisty, B.; Spaczynski, R.Z.; Piotrowska-Kempisty, H. The Stemness of Human Ovarian Granulosa Cells and the Role of Resveratrol in the Differentiation of MSCs—A Review Based on Cellular and Molecular Knowledge. Cells 2020, 9, 1418. [Google Scholar] [CrossRef] [PubMed]
- Gerli, S.; Della Morte, C.; Ceccobelli, M.; Mariani, M.; Favilli, A.; Leonardi, L.; Lanti, A.; Iannitti, R.G.; Fioretti, B. Biological and clinical effects of a resveratrol-based multivitamin supplement on intracytoplasmic sperm injection cycles: A single-center, randomized controlled trial. J. Matern. Neonatal Med. 2021, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Ochiai, A.; Brosens, J.J. The actions of resveratrol in decidualizing endometrium: Acceleration or inhibition? Biol. Reprod. 2020, 103, 1152–1156. [Google Scholar] [CrossRef]
- Amaral, A.; Lourenço, B.; Marques, M.; Ramalho-Santos, J. Mitochondria functionality and sperm quality. Reproduction 2013, 146, R163–R174. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Jing, X.; Wu, X.; Yan, M. Protective effect of resveratrol on spermatozoa function in male infertility induced by excess weight and obesity. Mol. Med. Rep. 2016, 14, 4659–4665. [Google Scholar] [CrossRef] [Green Version]
- Martins, L.A.; Coelho, B.P.; Behr, G.; Pettenuzzo, L.F.; Souza, I.C.; Moreira, J.C.; Borojevic, R.; Gottfried, C.; Guma, F.C. Resveratrol induces pro-oxidant effects and time-dependent resistance to cytotoxicity in activated hepatic stellate cells. Cell Biochem. Biophys. 2014, 68, 247–257. [Google Scholar] [CrossRef]
- Garcez, M.E.; dos Santos Branco, C.; Lara, L.V.; Pasqualotto, F.F.; Salvador, M. Effects of resveratrol supplementation on cryopres-ervation medium of human semen. Fertil. Steril. 2010, 94, 2118–2121. [Google Scholar] [CrossRef]
- Shahidi, M.; Parhizkary, F.; Sharifi, R.; Ghotaslou, A.; Barati, M. Effects of resveratrol on coagulative, fibrinolytic, and inflammatory marker expression and secretion by endothelial cells (human umbilical vein endothelial cells). Blood Coagul. Fibrinolysis 2020, 31, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Li, X.; Zhao, X.; Du, K.; Li, X.; Wang, B. Resveratrol attenuates hydrogen peroxide-induced apoptosis, reactive oxygen spe-cies generation, and psgl-1 and vwf activation in human umbilical vein endothelial cells, potentially via mapk signalling pathways. Mol. Med. Rep. 2018, 17, 2479–2487. [Google Scholar] [PubMed]
- Liu, Y.; Chen, X.; Li, J. Resveratrol protects against oxidized low-density lipoprotein-induced human umbilical vein endothelial cell apoptosis via inhibition of mitochondrial-derived oxidative stress. Mol. Med. Rep. 2017, 15, 2457–2464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zhou, X.; Zeng, X.; Hu, O.; Yi, L.; Mi, M. Resveratrol attenuates oxidative injury in human umbilical vein endothelial cells through regulating mitochondrial fusion via tyrrs-parp1 pathway. Nutr. Metab. 2019, 16, 9. [Google Scholar] [CrossRef]
- Hannan, N.; Brownfoot, F.C.; Cannon, P.; Deo, M.; Beard, S.; Nguyen, T.V.; Palmer, K.; Tong, S.; Kaitu’U-Lino, T.J. Resveratrol inhibits release of soluble fms-like tyrosine kinase (sFlt-1) and soluble endoglin and improves vascular dysfunction—Implications as a preeclampsia treatment. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sridharan, K.; Sequeira, R.P. Drugs for treating severe hypertension in pregnancy: A network meta-analysis and trial sequential analysis of randomized clinical trials. Br. J. Clin. Pharmacol. 2018, 84, 1906–1916. [Google Scholar] [CrossRef] [Green Version]
- Singh, C.K.; Kumar, A.; Lavoie, H.A.; Dipette, D.J.; Singh, U.S. Diabetic complications in pregnancy: Is resveratrol a solution? Exp. Biol. Med. 2013, 238, 482–490. [Google Scholar] [CrossRef] [Green Version]
- Singh, C.K.; Kumar, A.; Hitchcock, D.B.; Fan, D.; Goodwin, R.; LaVoie, H.A.; Nagarkatti, P.; DiPette, D.J.; Singh, U.S. Resveratrol pre-vents embryonic oxidative stress and apoptosis associated with diabetic embryopathy and improves glucose and lipid profile of diabetic dam. Mol. Nutr. Food Res. 2011, 55, 1186–1196. [Google Scholar] [CrossRef] [Green Version]
- Aujla, T.; Darby, J.R.T.; Saini, B.S.; Lock, M.C.; Holman, S.L.; Bradshaw, E.L.; Perumal, S.R.; McInnes, S.J.P.; Voelcker, N.H.; Wiese, M.D.; et al. Impact of resveratrol-mediated increase in uterine artery blood flow on fetal haemo-dynamics, blood pressure and oxygenation in sheep. Exp. Physiol. 2021, 106, 1166–1180. [Google Scholar] [CrossRef]
- Darby, J.R.T.; Saini, B.; Soo, J.Y.; Lock, M.C.; Holman, S.; Bradshaw, E.L.; McInnes, S.J.P.; Voelcker, N.H.; Macgowan, C.K.; Seed, M.; et al. Subcutaneous maternal resveratrol treatment increases uterine artery blood flow in the pregnant ewe and increases fetal but not cardiac growth. J. Physiol. 2019, 597, 5063–5077. [Google Scholar] [CrossRef]
- Protic, D.; Beleslin-Cokic, B.; Spremovic-Radjenovic, S.; Radunovic, N.; Heinle, H.; Scepanovic, R.; Gojkovic Bukarica, L. The different effects of resveratrol and naringenin on isolated human umbilical vein: The role of atp-sensitive k+ channels. Phytother Res. 2014, 28, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Protic, D.; Radunovic, N.; Spremovic-Radjenovic, S.; Zivanovic, V.; Heinle, H.; Petrovic, A.; Gojkovic-Bukarica, L. The role of potassium channels in the vasodilatation induced by resveratrol and naringenin in isolated human umbilical vein. Drug Dev. Res. 2015, 76, 17–23. [Google Scholar] [CrossRef]
- Farquhar, C. Endometriosis. BMJ 2007, 334, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Lousse, J.C.; Van Langendonckt, A.; Defrere, S.; Ramos, R.G.; Colette, S.; Donnez, J. Peritoneal endometriosis is an inflammatory disease. Front. Biosci. (Elite Ed.). 2012, 4, 23–40. [Google Scholar] [CrossRef] [PubMed]
- Bruner-Tran, K.L.; Osteen, K.G.; Taylor, H.S.; Sokalska, A.; Haines, K.; Duleba, A.J. Resveratrol inhibits development of experi-mental endometriosis in vivo and reduces endometrial stromal cell invasiveness in vitro. Biol. Reprod. 2011, 84, 106–112. [Google Scholar] [CrossRef]
- Ricci, A.G.; Olivares, C.N.; Bilotas, M.A.; Baston, J.I.; Singla, J.J.; Meresman, G.F.; Barañao, R.I. Natural therapies assessment for the treatment of endometriosis. Hum Reprod. 2013, 28, 178–188. [Google Scholar] [CrossRef] [Green Version]
- Yavuz, S.; Aydin, N.E.; Celik, O.; Yilmaz, E.; Ozerol, E.; Tanbek, K. Resveratrol successfully treats experimental endometriosis through modulation of oxidative stress and lipid peroxidation. J. Cancer Res. Ther. 2014, 10, 324–329. [Google Scholar] [CrossRef]
- Tekin, S.; Hansen, P.J. Natural killer-like cells in the sheep: Functional characterization and regulation by pregnancy-associated proteins. Exp. Biol. Med. 2002, 227, 803–811. [Google Scholar] [CrossRef]
- Taguchi, A.; Koga, K.; Kawana, K.; Makabe, T.; Sue, F.; Miyashita, M.; Yoshida, M.; Urata, Y.; Izumi, G.; Tkamura, M.; et al. Resveratrol Enhances Apoptosis in Endometriotic Stromal Cells. Am. J. Reprod. Immunol. 2016, 75, 486–492. [Google Scholar] [CrossRef]
- Cenksoy, P.O.; Fıcıcıoglu, C.; Yesiladali, M.; Akcin, O.A.; Kaspar, C. The importance of the length of uterine cavity, the position of the tip of the inner catheter and the distance between the fundal endometrial surface and the air bubbles as determinants of the pregnancy rate in IVF cycles. Eur. J. Obstet. Gynecol. Reprod. Biol. 2014, 172, 46–50. [Google Scholar] [CrossRef]
- Arablou, T.; Delbandi, A.; Khodaverdi, S.; Arefi, S.; Kolahdouz-Mohammadi, R.; Heidari, S.; Mohammadi, T.; Aryaeian, N. Resveratrol reduces the expression of insulin-like growth factor-1 and hepatocyte growth factor in stromal cells of women with endometriosis compared with nonendometriotic women. Phytotherapy Res. 2019, 33, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Maia, H., Jr.; Haddad, C.; Pinheiro, N.; Casoy, J. Advantages of the association of resveratrol with oral contraceptives for man-agement of endometriosis-related pain. Int. J. Womens Health. 2012, 4, 543–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferries-Rowe, E.; Corey, E.; Archer, J.S. Primary Dysmenorrhea: Diagnosis and Therapy. Obstet Gynecol. 2020, 136, 1047–1058. [Google Scholar] [CrossRef]
- Burnett, M.; Lemyre, M. No. 345-Primary Dysmenorrhea Consensus Guideline. J. Obstet. Gynaecol. Can. 2017, 39, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Hsia, S.M.; Wang, K.L.; Wang, P.S. Effects of resveratrol, a grape polyphenol, on uterine contraction and Ca2+ mobilization in rats in vivo and in vitro. Endocrinology. 2011, 152, 2090–2099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novakovic, R.; Ilic, B.; Beleslin-Cokic, B.; Radunovic, N.; Heinle, H.; Scepanovic, R.; Gojkovic-Bukarica, L. The effect of resveratrol on contractility of non-pregnant rat uterus: The contribution of K(+) channels. J. Physiol. Pharmacol. 2013, 64, 795–805. [Google Scholar] [PubMed]
- Carreiro, J.N.; Magnani, M.; Jobling, P.; van Helden, D.F.; Nalivaiko, E.; Braga, V.A. Resveratrol restores uterine contractions during hypoxia by blockade of ATP-sensitive potassium channels. J. Funct. Foods. 2017, 33, 307–313. [Google Scholar] [CrossRef]
- Ferrero, S.; Barra, F.; Vigliercio, G.M.; Scala, C. The efficacy of oral administration of resveratrol in association with feverfew (RevifastDol®) for the treatment of primary dysmenorrhea: A retrospective cohort study. Women’s Health Res. 2018, 2, 1–13. [Google Scholar]
- da Silva, D.M.; Gross, L.A.; Guedes Neto, E.P.; Lessey, B.A.; Savaris, R.F. The Use of Resveratrol as an Adjuvant Treatment of Pain in Endometriosis: A Randomized Clinical Trial. J. Endocr. Soc. 2017, 1, 359–369. [Google Scholar] [CrossRef]
- Banaszewska, B.; Wrotynska-Barczynska, J.; Spaczynski, R.Z.; Pawelczyk, L.; Duleba, A.J. Effects of resveratrol on polycystic ovary syndrome: A double-blind, randomized, placebo-controlled trial. J. Clin. Endocrinol. Metab. 2016, 101, 4322–4328. [Google Scholar] [CrossRef]
- Abbott, D.H.; Bacha, F. Ontogeny of polycystic ovary syndrome and insulin resistance in utero and early childhood. Fertil. Steril. 2013, 100, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Wild, S.; Pierpoint, T.; McKeigue, P.; Jacobs, H. Cardiovascular disease in women with polycystic ovary syndrome at long-term follow-up: A retrospective cohort study. Clin. Endocrinol. 2000, 52, 595–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridker, P.M.; Buring, J.E.; Cook, N.R.; Rifai, N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: An 8-year follow-up of 14 719 initially healthy American women. Circulation 2003, 107, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Shojaei-Zarghani, S.; Rafraf, M. Resveratrol and Markers of Polycystic Ovary Syndrome: A Systematic Review of Animal and Clinical Studies. Reprod. Sci. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Furat Rencber, S.; Kurnaz Ozbek, S.; Eraldemır, C.; Sezer, Z.; Kum, T.; Ceylan, S.; Guzel, E. Effect of resveratrol and metformin on ovarian reserve and ultrastructure in PCOS: An experimental study. J. Ovarian Res. 2018, 11, 55. [Google Scholar] [CrossRef] [Green Version]
- Rotches-Ribalta, M.; Andres-Lacueva, C.; Estruch, R.; Escribano, E.; Urpi-Sarda, M. Pharmacokinetics of resveratrol metabolic pro-file in healthy humans after moderate consumption of red wine and grape extract tablets. Pharmacol. Research. 2012, 66, 375–382. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Yan, J.; Soleas, G.J. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin. Biochem. 2003, 36, 79–87. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E., Jr.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in hu-mans. Drug Metab. Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [Green Version]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef]
- Cottart, C.-H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.-L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2009, 54, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Burkon, A.; Somoza, V. Quantification of free and protein-bound trans-resveratrol metabolites and identification of trans-resveratrol-C/O-conjugated diglucuronides—Two novel resveratrol metabolites in human plasma. Mol. Nutr. Food Res. 2008, 52, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I ran-domized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases--safety, pharmaco-kinetics, and pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Novakovic, R.; Rajkovic, J.; Gostimirovic, M.; Gojkovic-Bukarica, L.; Radunovic, N. Resveratrol and Reproductive Health. Life 2022, 12, 294. https://doi.org/10.3390/life12020294
Novakovic R, Rajkovic J, Gostimirovic M, Gojkovic-Bukarica L, Radunovic N. Resveratrol and Reproductive Health. Life. 2022; 12(2):294. https://doi.org/10.3390/life12020294
Chicago/Turabian StyleNovakovic, Radmila, Jovana Rajkovic, Milos Gostimirovic, Ljiljana Gojkovic-Bukarica, and Nebojsa Radunovic. 2022. "Resveratrol and Reproductive Health" Life 12, no. 2: 294. https://doi.org/10.3390/life12020294
APA StyleNovakovic, R., Rajkovic, J., Gostimirovic, M., Gojkovic-Bukarica, L., & Radunovic, N. (2022). Resveratrol and Reproductive Health. Life, 12(2), 294. https://doi.org/10.3390/life12020294





