A 90-Day Safety Study of Meat from MSTN and FGF5 Double-Knockout Sheep in Wistar Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sources of Sheep Meat
2.2. Diet Composition
2.3. Animals and Feeding Doses
2.4. Indicator Testing
2.4.1. Appearance, Weight, Food Intake, and Ocular Examinations
2.4.2. Routine Blood Tests and Blood Biochemistry
2.4.3. Routine Urine Tests
2.4.4. Weighing of Organs
2.4.5. Histopathology
2.5. Statistical Analysis
3. Results
3.1. Clinical Signs
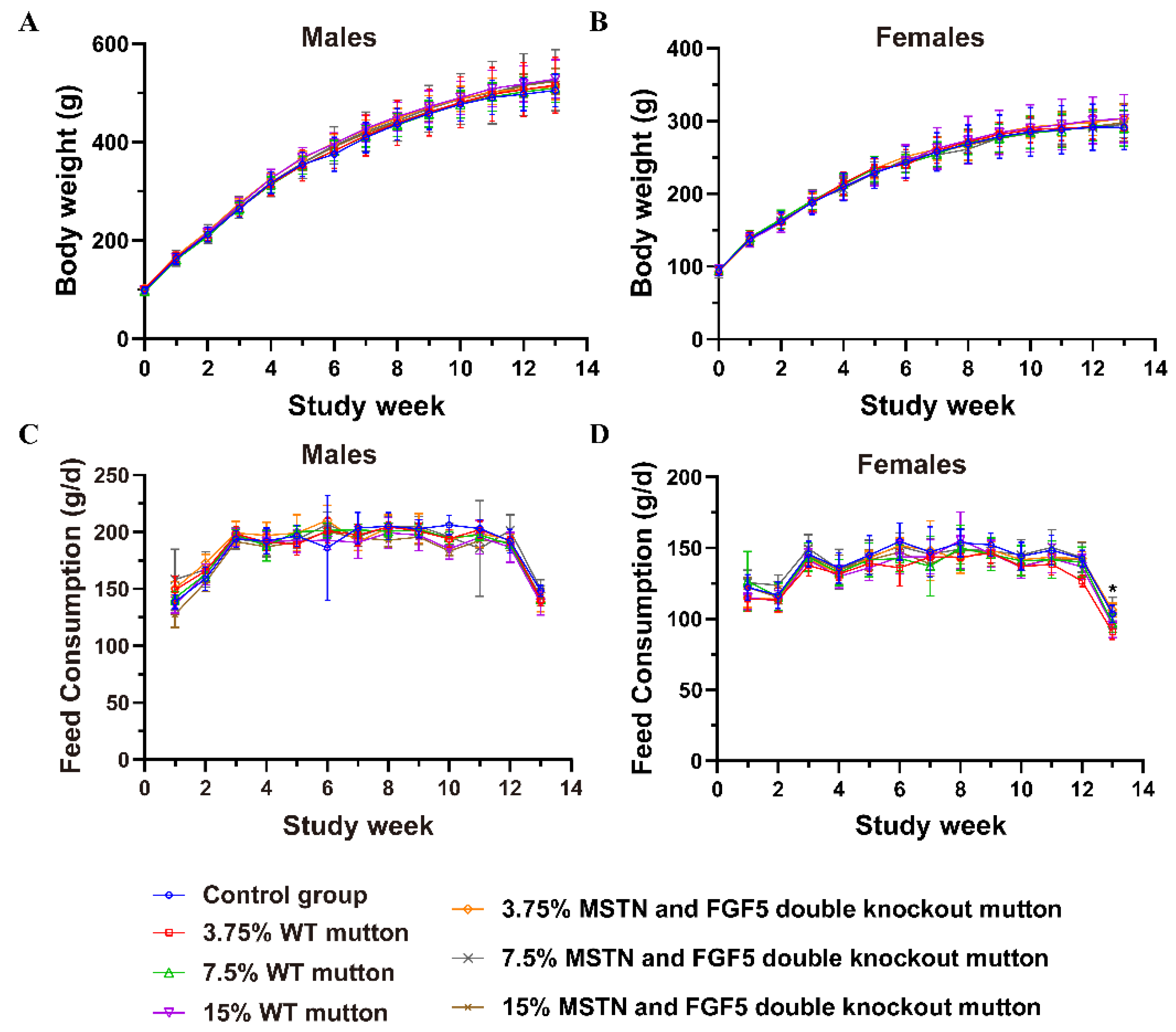
3.2. Body Weight and Food Consumption
3.3. Routine Blood Tests
3.4. Blood Biochemistry
3.5. Routine Urine Tests
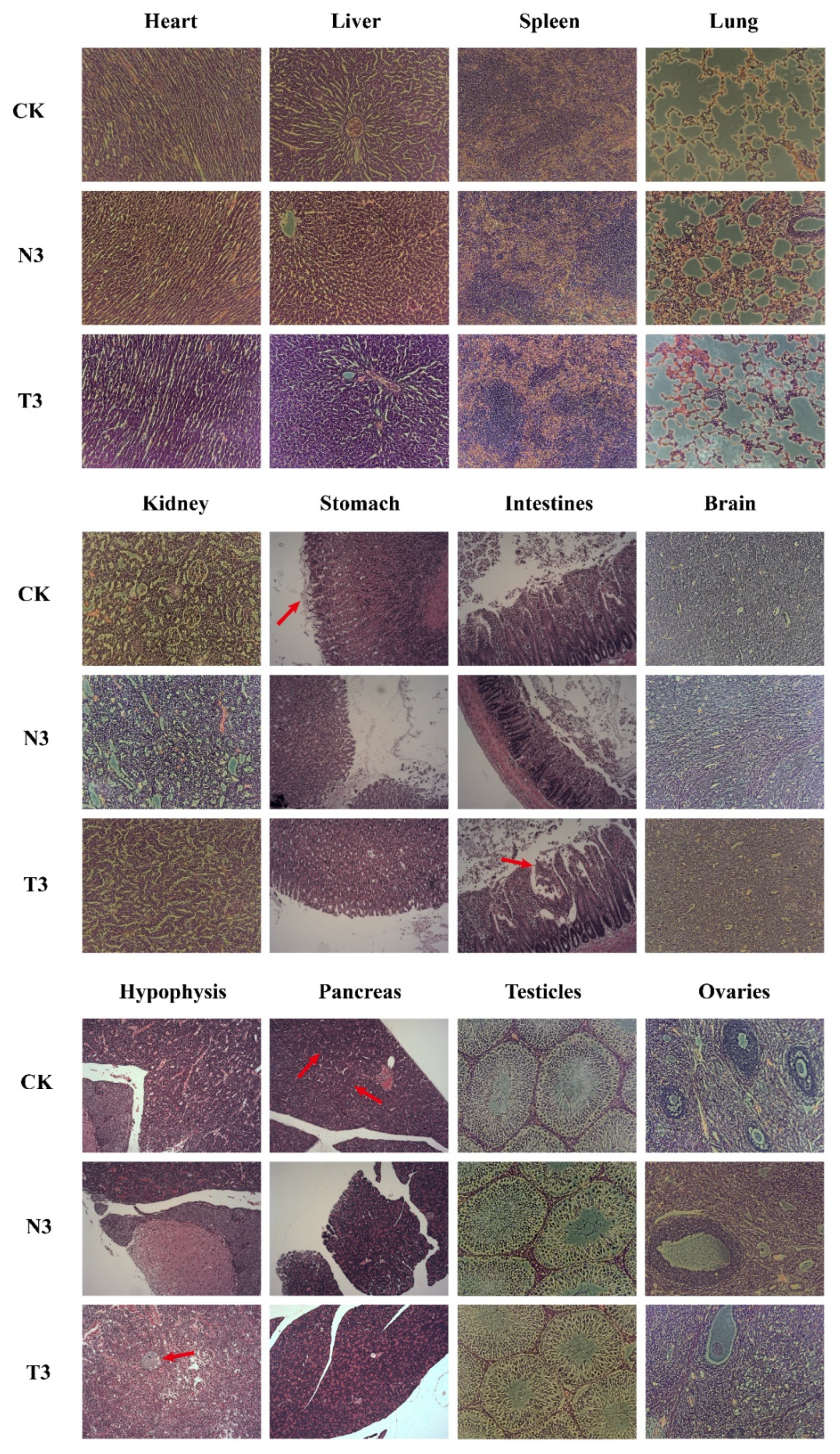
3.6. Organ Weights and Histopathology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Grobet, L.; Martin, L.J.R.; Poncelet, D.; Pirottin, D.; Brouwers, B.; Riquet, J.; Schoeberlein, A.; Dunner, S.; Ménissier, F.; Massabanda, J.; et al. A deletion in the bovine myostatin gene causes the double–muscled phenotype in cattle. Nat. Genet. 1997, 17, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Ma, Y.; Wang, T.; Lian, L.; Tian, X.; Hu, R.; Deng, S.; Li, K.; Wang, F.; Li, N.; et al. One-step generation of myostatin gene knockout sheep via the CRISPR/Cas9 system. Front. Agric. Sci. Eng. 2014, 1, 2–5. [Google Scholar]
- Luo, J.; Song, Z.; Yu, S.; Cui, D.; Wang, B.; Ding, F.; Li, S.; Dai, Y.; Li, N. Efficient Generation of Myostatin (MSTN) Biallelic Mutations in Cattle Using Zinc Finger Nucleases. PLoS ONE 2014, 9, e95225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McPherron, A.C.; Lee, S.-J. Double muscling in cattle due to mutations in the myostatin gene. Proc. Natl. Acad. Sci. USA 1997, 94, 12457–12461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, L.; Tang, M.; Yang, J.; Wang, Q.; Cai, C.; Jiang, S.; Li, H.; Jiang, K.; Gao, P.; Ma, D.; et al. Targeted mutations in myostatin by zinc-finger nucleases result in double-muscled phenotype in Meishan pigs. Sci. Rep. 2015, 5, 14435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hébert, J.M.; Rosenquist, T.; Götz, J.; Martin, G.R. FGF5 as a regulator of the hair growth cycle: Evidence from targeted and spontaneous mutations. Cell 1994, 78, 1017–1025. [Google Scholar] [CrossRef]
- Higgins, C.; Petukhova, L.; Harel, S.; Ho, Y.Y.; Drill, E.; Shapiro, L.; Wajid, M.; Christiano, A.M. FGF5 is a crucial regulator of hair length in humans. Proc. Natl. Acad. Sci. USA 2014, 111, 10648–10653. [Google Scholar] [CrossRef] [Green Version]
- Dierks, C.; Mömke, S.; Philipp, U.; Distl, O. Allelic heterogeneity ofFGF5mutations causes the long-hair phenotype in dogs. Anim. Genet. 2013, 44, 425–431. [Google Scholar] [CrossRef]
- Kehler, J.S.; David, V.A.; Schäffer, A.A.; Bajema, K.; Eizirik, E.; Ryugo, D.K.; Hannah, S.S.; O’Brien, S.J.; Menotti-Raymond, M. Four independent mutations in the feline fibroblast growth factor 5 gene determine the long-haired phenotype in domestic cats. J. Hered. 2007, 98, 555–566. [Google Scholar] [CrossRef]
- Hu, R.; Fan, Z.Y.; Wang, B.Y.; Deng, S.L.; Zhang, X.S.; Zhang, J.L.; Han, H.B.; Lian, Z.X. RAPID COMMUNICATION: Generation of FGF5 knockout sheep via the CRISPR/Cas9 system12. J. Anim. Sci. 2017, 95, 2019–2024. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Li, Y.; Jia, K.; Xu, X.; Li, Y.; Zhao, Y.; Zhang, X.; Zhang, J.; Liu, G.; Deng, S.; et al. Crosstalk between androgen and Wnt/beta-catenin leads to changes of wool density in FGF5-knockout sheep. Cell Death Dis. 2020, 11, 407. [Google Scholar] [CrossRef]
- Efsa Gmo Panel Working Group on Animal Feeding Trials. Safety and nutritional assessment of GM plants and derived food and feed: The role of animal feeding trials. Food Chem Toxicol. 2008, 46 (Suppl. 1), S2–S70. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, C.-X.; Feng, X.-L.; Wang, H.-L.; Liu, H.-B.; Zhi, Y.; Geng, G.-Y.; Zhao, J.; Xu, H.-B. Safety assessment of meat from transgenic cattle by 90-day feeding study in rats. Food Chem. Toxicol. 2013, 57, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Ito, Y.; Takahashi, S. Fourteen-week feeding test of meat and milk derived from cloned cattle in the rat. Theriogenology 2007, 67, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gao, M.-Q.; Liang, D.; Yin, S.; Yao, K.; Zhang, Y. Safety assessment of genetically modified milk containing human beta-defensin-3 on rats by a 90-day feeding study. Food Chem. Toxicol. 2017, 100, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, S.; Zhou, Q.; Li, S.; Zhang, J.; Zhang, L.; Jiang, S.; Zhang, Q.; Zhou, X.; Wu, C.; et al. Subchronic feeding toxicity studies of drought-tolerant transgenic wheat MGX11-10 in Wistar Han RCC rats. Food Chem. Toxicol. 2020, 137, 111129. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Zheng, J.; Zhou, Y.; Jia, J.; Ye, X.; Zhou, B.; Chen, X.; Mo, Y.; Wang, J. Ferroptosis is Involved in Hyperoxic Lung Injury in Neonatal Rats. J. Inflamm. Res. 2021, 14, 5393–5401. [Google Scholar] [CrossRef] [PubMed]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Hihara, E. Human body surface area: A theoretical approach. Eur. J. Appl. Physiol. 2004, 91, 425–428. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, W.; Xu, X. China’s meat industry revolution: Challenges and opportunities for the future. Meat Sci. 2012, 92, 188–196. [Google Scholar] [CrossRef]
- Bai, H.; Wang, Z.; Hu, R.; Kan, T.; Li, Y.; Zhang, X.; Zhang, J.; Lian, L.; Han, H.; Lian, Z. A 90-Day Toxicology Study of Meat from Genetically Modified Sheep Overexpressing TLR4 in Sprague-Dawley Rats. PLoS ONE 2015, 10, e0121636. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.-J.; Jiang, S.-W.; Qian, L.-L.; Cai, C.-B.; Wang, Q.-Q.; Ma, D.-Z.; Li, B.; Xie, S.-S.; Cui, W.-T.; Li, K. A 90-Day Feeding Study in Rats to Assess the Safety of Genetically Engineered Pork. PLoS ONE 2016, 11, e0165843. [Google Scholar] [CrossRef] [PubMed]
- Palmer, B.F.; Deborah, J.C. Starvation Ketosis and the Kidney. Am. J. Nephrol. 2021, 52, 467–478. [Google Scholar] [CrossRef]
- Mishra, P.K. Why the diabetic heart is energy inefficient: A ketogenesis and ketolysis perspective. Am. J. Physiol.-Heart Circ. Physiol. 2021, 321, H751–H755. [Google Scholar] [CrossRef] [PubMed]
- Teymourian, H.; Moonla, C.; Tehrani, F.; Vargas, E.; Aghavali, R.; Barfidokht, A.; Tangkuaram, T.; Mercier, P.P.; Dassau, E.; Wang, J. Microneedle-Based Detection of Ketone Bodies along with Glucose and Lactate: Toward Real-Time Continuous Interstitial Fluid Monitoring of Diabetic Ketosis and Ketoacidosis. Anal. Chem. 2019, 92, 2291–2300. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, G.; Upadhyay, A. Assessment of Proteinuria. Adv. Chronic Kidney Dis. 2011, 18, 243–248. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, R.; Hojs, R.; Trevisani, F.; Morales, E.; Fernández, G.; Bevc, S.; Corona, C.M.C.; Cruzado, J.M.; Quero, M.; Díaz, M.N.; et al. The Role of Vascular Lesions in Diabetes Across a Spectrum of Clinical Kidney Disease. Kidney Int. Rep. 2021, 6, 2392–2403. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Y.; Li, Y.; Huang, J.; Yu, H.; Wang, Z. Accelerating peroxidase-like activity of gold nanozymes using purine derivatives and its application for monitoring of occult blood in urine. Sens. Actuators B Chem. 2018, 270, 443–451. [Google Scholar] [CrossRef]
| Ingredients | Wild-Type Mutton | MSTN and FGF5 Double Knock-Out Mutton | ||||
|---|---|---|---|---|---|---|
| 3.75% | 7.5% | 15% | 3.75% | 7.5% | 15% | |
| Water content (g/100 g) | 6.54 | 6.01 | 4.97 | 6.49 | 5.72 | 6.24 |
| Ash content (g/100 g) | 5.8 | 5.9 | 5.9 | 5.8 | 5.8 | 5.8 |
| Crude protein (g/100 g) | 20.5 | 18.4 | 19.1 | 20.3 | 19.5 | 19.1 |
| Crude fat (g/100 g) | 4.7 | 5.0 | 5.4 | 4.7 | 5.2 | 5.1 |
| Crude fiber (g/100 g) | 4.3 | 4.0 | 4.3 | 4.2 | 3.9 | 4.0 |
| Ca (g/100 g) | 1.14 | 1.11 | 1.11 | 1.13 | 1.10 | 1.04 |
| p (g/100 g) | 0.86 | 0.78 | 0.79 | 0.83 | 0.73 | 0.74 |
| Group | Diet | Number of Animals/Group | |
|---|---|---|---|
| Male | Female | ||
| CK | commercially available diet | 10 | 10 |
| N1 | 3.75% wild-type control mutton | 10 | 10 |
| N2 | 7.5% wild-type control mutton | 10 | 10 |
| N3 | 15% wild-type control mutton | 10 | 10 |
| T1 | 3.75% MSTN and FGF5 double-knockout mutton | 10 | 10 |
| T2 | 7.5% MSTN and FGF5 double-knockout mutton | 10 | 10 |
| T3 | 15% MSTN and FGF5 double-knockout mutton | 10 | 10 |
| Sex | Group | Body Weight Gain (g, n = 10) | Total Food Consumption (g, n = 10) | Total Food Utilization (%, n = 10) |
|---|---|---|---|---|
| Male rats | CK | 406.9 ± 29.7 | 2426.8 ± 101.3 | 16.8 ± 1.0 |
| N1 | 413.8 ± 54.4 | 2425.1 ± 47.1 | 17.1 ± 2.1 | |
| N2 | 413.3 ± 26.0 | 2417.7 ± 55.1 | 17.1 ± 0.8 | |
| N3 | 429.0 ± 38.7 | 2362.2 ± 112.6 | 18.2 ± 1.4 | |
| T1 | 410.8 ± 25.3 | 2450.9 ± 117.5 | 16.8 ± 1.0 | |
| T2 | 426.5 ± 59.7 | 2446.9 ± 130.2 | 17.4 ± 2.0 | |
| T3 | 425.1 ± 25.0 | 2346.4 ± 56.2 | 18.1 ± 1.0 | |
| Female rats | CK | 196.9 ± 29.7 | 1811.9 ± 112.5 | 10.8 ± 1.4 |
| N1 | 197.3 ± 13.8 | 1698.3 ± 57.2 | 11.6 ± 0.8 | |
| N2 | 195.4 ± 28.2 | 1755.2 ± 102.5 | 11.1 ± 1.2 | |
| N3 | 207.6 ± 32.0 | 1736.0 ± 114.9 | 12.0 ± 1.6 | |
| T1 | 210.8 ± 18.6 | 1770.3 ± 52.0 | 11.9 ± 1.2 | |
| T2 | 202.0 ± 18.6 | 1814.0 ± 99.2 | 11.1 ± 1.1 | |
| T3 | 204.6 ± 25.0 | 1764.1 ± 73.1 | 11.6 ± 1.4 |
| Sex | Group | WBC (109/L) | RBC (1012/L) | HCT (%) | HGB (g/L) | PLT (109/L) | PT (s) | APTT (s) | NE (109/L) | LY (109/L) | EO (109/L) | MO (109/L) | BA (109/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male rats | CK | 9.22 ± 2.31 | 7.17 ± 0.40 | 135 ± 6 | 44.0 ± 1.7 | 702 ± 80 | 9.2 ± 0.1 | 20.7 ± 1.4 | 3.41 ± 0.75 | 4.74 ± 1.37 | 0.02 ± 0.01 | 1.04 ± 0.25 | 0.00 ± 0.00 |
| N1 | 7.18 ± 1.37 | 7.17 ± 0.47 | 129 ± 7 | 42.0 ± 2.3 | 661 ± 84 | 9.3 ± 0.4 | 23.8 ± 5.2 | 2.84 ± 0.33 | 3.60 ± 1.20 | 0.05 ± 0.02 | 0.68 ± 0.20 * | 0.00 ± 0.00 | |
| N2 | 7.98 ± 3.22 | 7.44 ± 0.46 | 136 ± 8 | 45.0 ± 2.6 | 637 ± 55 | 9.4 ± 0.2 | 26.0 ± 3.6 * | 3.10 ± 0.98 | 3.96 ± 1.96 | 0.06 ± 0.06 | 0.85 ± 0.39 | 0.01 ± 0.02 | |
| N3 | 7.06 ± 2.18 | 7.14 ± 0.29 | 129 ± 8 | 42.2 ± 2.1 | 692 ± 50 | 9.6 ± 0.3 * | 24.6 ± 2.4 * | 2.61 ± 0.75 | 3.62 ± 1.46 | 0.03 ± 0.01 | 0.80 ± 0.25 | 0.00 ± 0.01 | |
| T1 | 8.28 ± 2.11 | 7.06 ± 0.52 | 129 ± 10 | 41.9 ± 3.3 | 680 ± 80 | 9.7 ± 0.6 | 25.7 ± 3.6 * | 3.23 ± 0.86 | 4.22 ± 1.30 | 0.04 ± 0.02 | 0.78 ± 0.26 | 0.00 ± 0.01 | |
| T2 | 9.49 ± 2.04 | 7.56 ± 0.57 | 138 ± 11 | 44.9 ± 3.8 | 613 ± 75 | 9.7 ± 0.4 * | 24.6 ± 2.4 * | 3.41 ± 0.83 | 5.13 ± 1.23 | 0.06 ± 0.05 | 0.89 ± 0.17 | 0.01 ± 0.02 | |
| T3 | 8.99 ± 1.78 | 7.53 ± 0.44 | 138 ± 9 | 44.5 ± 2.6 | 673 ± 72 | 9.7 ± 0.4 * | 26.5 ± 2.5 * | 3.55 ± 0.85 | 4.70 ± 1.01 | 0.06 ± 0.05 | 0.67 ± 0.20 * | 0.01 ± 0.03 | |
| Female rats | CK | 5.73 ± 1.89 | 6.81 ± 0.45 | 129 ± 11 | 43.4 ± 3.6 | 637 ± 65 | 9.6 ± 0.5 | 24.6 ± 5.1 | 1.56 ± 0.29 | 3.77 ± 1.72 | 0.01 ± 0.01 | 0.39 ± 0.11 | 0.00 ± 0.00 |
| N1 | 5.65 ± 1.53 | 6.63 ± 0.74 | 129 ± 17 | 42.3 ± 5.4 | 686 ± 95 | 9.4 ± 0.6 | 22.5 ± 4.3 | 1.77 ± 0.44 | 3.43 ± 1.03 | 0.03 ± 0.02 | 0.42 ± 0.15 | 0.00 ± 0.00 | |
| N2 | 6.43 ± 1.50 | 6.43 ± 0.31 | 123 ± 9 | 41.7 ± 2.9 | 712 ± 86 | 9.6 ± 0.9 | 23.8 ± 3.6 | 1.90 ± 0.35 | 4.07 ± 1.18 | 0.02 ± 0.02 | 0.43 ± 0.15 | 0.00 ± 0.00 | |
| N3 | 5.87 ± 1.85 | 6.62 ± 0.47 | 131 ± 9 | 43.1 ± 3.2 | 652 ± 76 | 9.7 ± 0.5 | 25.2 ± 5.1 | 1.90 ± 0.53 | 3.50 ± 1.30 | 0.02 ± 0.02 | 0.45 ± 0.13 | 0.01 ± 0.02 | |
| T1 | 5.35 ± 1.37 | 6.32 ± 0.49 | 120 ± 11 | 40.6 ± 3.7 | 699 ± 70 | 9.6 ± 0.5 | 25.2 ± 4.3 | 1.47 ± 0.42 | 3.49 ± 1.13 | 0.02 ± 0.02 | 0.37 ± 0.11 | 0.01 ± 0.02 | |
| T2 | 5.83 ± 1.30 | 6.56 ± 0.42 | 124 ± 6 | 42.0 ± 2.3 | 654 ± 42 | 9.7 ± 0.7 | 24.1 ± 2.7 | 1.78 ± 0.37 | 3.62 ± 1.02 | 0.02 ± 0.01 | 0.41 ± 0.06 | 0.00 ± 0.00 | |
| T3 | 5.43 ± 0.87 | 6.16 ± 0.49 | 123 ± 9 | 39.7 ± 3.0 | 689 ± 74 | 9.9 ± 0.5 | 24.4 ± 2.6 | 1.72 ± 0.34 | 3.28 ± 0.72 | 0.02 ± 0.01 | 0.42 ± 0.10 | 0.00 ± 0.00 |
| Gender | Group | ALT | AST | TP | Alb | ALP | Glu | Urea | Cr | TC | TG | GGT | LDH | K | Na | Cl |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (U/L) | (U/L) | (g/L) | (g/L) | (U/L) | (mmol/L) | (mmol/L) | (μmol/L) | (mmol/L) | (mmol/L) | (U/L) | (U/L) | (mmol/L) | (mmol/L) | (mmol/L) | ||
| Male rats | CK | 58.9 ± 9.0 | 118 ± 16 | 61.2 ± 5.0 | 23.4 ± 2.0 | 138 ± 35 | 11.83 ± 1.76 | 9.37 ± 1.95 | 50.3 ± 8.7 | 3.07 ± 0.75 | 0.75 ± 0.24 | 1.89 ± 0.96 | 1349 ± 203 | 5.03 ± 0.39 | 135.7 ± 4.8 | 97.8 ± 4.5 |
| N1 | 59.4 ± 11.8 | 111 ± 16 | 63.9 ± 3.6 | 23.4 ± 1.9 | 128 ± 31 | 12.96 ± 3.41 | 7.79 ± 1.36 | 51.9 ± 9.3 | 2.96 ± 0.45 | 1.02 ± 0.47 | 1.43 ± 1.52 | 1044 ± 364 | 5.37 ± 1.13 | 136.1 ± 10.0 | 98.2 ± 9.8 | |
| N2 | 61.9 ± 13.2 | 141 ± 22 | 64.8 ± 7.9 | 24.7 ± 2.4 | 129 ± 34 | 13.53 ± 2.99 | 8.90 ± 2.02 | 53.3 ± 12.0 | 2.88 ± 0.60 | 0.80 ± 0.33 | 1.07 ± 1.86 | 1363 ± 197 | 4.73 ± 0.32 | 135.9 ± 8.2 | 96.6 ± 7.5 | |
| N3 | 45.0 ± 7.9 | 111 ± 15 | 54.4 ± 7.6 | 19.5 ± 1.8 * | 106 ± 11 | 10.98 ± 2.18 | 7.66 ± 1.62 | 41.1 ± 4.7 | 2.18 ± 0.24 | 0.77 ± 0.42 | 2.70 ± 1.49 | 1274 ± 326 | 5.16 ± 0.38 | 139.6 ± 1.7 | 101.3 ± 2.7 | |
| T1 | 44.7 ± 7.0 | 94 ± 13 | 56.3 ± 3.6 | 19.1 ± 2.1 *# | 112 ± 26 | 11.01 ± 1.22 | 7.14 ± 0.89 | 40.9 ± 2.6 | 2.51 ± 0.50 | 0.54 ± 0.16 | 2.81 ± 0.69 | 945 ± 250 * | 4.85 ± 0.34 | 140.3 ± 1.6 | 102.6 ± 2.5 | |
| T2 | 49.8 ± 12.2 | 91 ± 14 # | 57.8 ± 6.5 | 21.5 ± 1.6 # | 122 ± 24 | 8.83 ± 1.21 *# | 7.78 ± 1.22 | 41.1 ± 5.7 | 2.62 ± 0.26 | 0.65 ± 0.19 | 1.48 ± 1.67 | 981 ± 314 # | 4.97 ± 0.30 | 141.3 ± 1.7 | 103.9 ± 3.5 | |
| T3 | 57.9 ± 24.3 | 116 ± 46 | 59.3 ± 2.7 | 18.5 ± 2.8 * | 106 ± 21 | 9.76 ± 1.75 | 7.40 ± 0.75 | 41.6 ± 5.9 | 2.66 ± 0.34 # | 0.70 ± 0.22 | 1.09 ± 1.23 | 951 ± 249 * | 5.31 ± 0.34 | 143.2 ± 11.3 | 105.7 ± 5.2 * | |
| Female rats | CK | 43.0 ± 6.5 | 89 ± 19 | 65.9 ± 10.5 | 26.6 ± 4.1 | 71 ± 17 | 8.42 ± 1.71 | 9.00 ± 0.90 | 47.6 ± 11.6 | 2.77 ± 0.79 | 0.57 ± 0.25 | 3.04 ± 0.93 | 968 ± 333 | 4.49 ± 0.29 | 139.9 ± 1.5 | 107.7 ± 3.2 |
| N1 | 44.1 ± 8.4 | 92 ± 18 | 57.7 ± 13.7 | 24.8 ± 4.0 | 79 ± 22 | 9.01 ± 2.91 | 8.79 ± 2.58 | 46.0 ± 11.6 | 2.70 ± 0.47 | 0.38 ± 0.08 | 1.70 ± 1.23 | 995 ± 404 | 4.47 ± 0.18 | 141.0 ± 2.0 | 107.5 ± 4.7 | |
| N2 | 46.1 ± 6.8 | 93 ± 19 | 57.5 ± 6.8 | 23.7 ± 2.0 | 75 ± 20 | 9.32 ± 2.30 | 7.97 ± 0.85 | 40.8 ± 4.0 | 2.50 ± 0.57 | 0.50 ± 0.14 | 1.23 ± 1.09 * | 994 ± 324 | 4.58 ± 0.34 | 141.6 ± 1.4 | 108.6 ± 3.4 | |
| N3 | 57.3 ± 12.1 | 119 ± 29 | 73.9 ± 15.1 | 31.6 ± 4.8 * | 89 ± 19 | 11.09 ± 1.71 | 9.20 ± 1.73 | 58.4 ± 10.7 | 3.69 ± 1.02 * | 0.73 ± 0.26 | 2.67 ± 1.21 | 1473 ± 540 | 4.43 ± 0.28 | 140.6 ± 2.1 | 108.1 ± 4.3 | |
| T1 | 58.9 ± 12.9 * | 121 ± 39 | 76.1 ± 10.5 # | 29.0 ± 3.8 | 98 ± 30 | 12.00 ± 2.70 * | 9.27 ± 2.41 | 54.3 ± 9.3 | 2.84 ± 0.62 | 0.45 ± 0.10 | 1.61 ± 0.64 | 1209 ± 412 | 4.97 ± 0.61 | 140.4 ± 1.3 | 109.3 ± 2.1 | |
| T2 | 51.1 ± 15.4 | 89 ± 18 | 58.5 ± 7.9 | 22.9 ± 3.0 | 67 ± 12 | 10.92 ± 2.76 | 8.15 ± 0.91 | 41.8 ± 6.5 | 2.35 ± 0.42 | 0.36 ± 0.07 | 2.08 ± 0.98 | 708 ± 122 | 4.59 ± 0.27 | 146.5 ± 12.4 | 113.9 ± 12.7 | |
| T3 | 44.0 ± 12.4 | 80 ± 10 # | 57.0 ± 8.2 # | 21.9 ± 2.9 # | 76 ± 32 | 8.90 ± 1.12 | 7.47 ± 1.18 | 43.0 ± 6.9 # | 2.30 ± 0.59 # | 0.52 ± 0.13 | 2.66 ± 1.28 | 919 ± 152 | 4.23 ± 0.33 | 140.5 ± 4.3 | 107.2 ± 5.4 |
| Sex | Group | Ketone Body (mmol/L) | Protein (g/L) | Glucose (mmol/L) | Urine Specific Gravity | Occult Blood (cell/µL) | pH |
|---|---|---|---|---|---|---|---|
| Male rats | CK | 0.2 ± 0.2 | 0.22 ± 0.30 | 0 ± 0 | 1.017 ± 0.003 | 0.0 ± 0.0 | 7.8 ± 0.3 |
| N1 | 0.1 ± 0.2 | 0.03 ± 0.06 | 0 ± 0 | 1.013 ± 0.003 | 2.5 ± 7.9 | 7.7 ± 0.3 | |
| N2 | 0.2 ± 0.2 | 0.39 ± 0.43 | 0 ± 0 | 1.014 ± 0.003 | 0.0 ± 0.0 | 7.8 ± 0.4 | |
| N3 | 0.0 ± 0.0 | 0.02 ± 0.05 | 0 ± 0 | 1.013 ± 0.003 | 1.0 ± 3.2 | 7.8 ± 0.3 | |
| T1 | 0.2 ± 0.3 | 0.09 ± 0.13 | 0 ± 0 | 1.015 ± 0.003 | 9.0 ± 25.1 | 7.8 ± 0.3 | |
| T2 | 0.2 ± 0.3 | 0.11 ± 0.14 | 0 ± 0 | 1.013 ± 0.003 | 0.0 ± 0.0 | 7.5 ± 0.3 | |
| T3 | 0.1 ± 0.2 | 0.16 ± 0.32 | 0 ± 0 | 1.015 ± 0.003 | 0.0 ± 0.0 | 7.7 ± 0.3 | |
| Female rats | CK | 0.0 ± 0.0 | 0.36 ± 0.94 | 0 ± 0 | 1.018 ± 0.005 | 0.0 ± 0.0 | 7.5 ± 0.9 |
| N1 | 0.0 ± 0.0 | 0.15 ± 0.32 | 0 ± 0 | 1.014 ± 0.004 | 0.0 ± 0.0 | 7.5 ± 0.4 | |
| N2 | 0.0 ± 0.0 | 0.02 ± 0.05 | 0 ± 0 | 1.014 ± 0.003 | 0.0 ± 0.0 | 7.4 ± 0.3 | |
| N3 | 0.0 ± 0.0 | 0.20 ± 0.42 | 0 ± 0 | 1.015 ± 0.004 | 0.0 ± 0.0 | 7.3 ± 0.4 | |
| T1 | 0.0 ± 0.0 | 0.08 ± 0.13 | 0 ± 0 | 1.014 ± 0.003 | 0.0 ± 0.0 | 7.3 ± 0.4 | |
| T2 | 0.0 ± 0.0 | 0.03 ± 0.09 | 0 ± 0 | 1.013 ± 0.003 | 0.0 ± 0.0 | 7.4 ± 0.3 | |
| T3 | 0.0 ± 0.0 | 0.12 ± 0.31 | 0 ± 0 | 1.014 ± 0.004 | 0.0 ± 0.0 | 7.2 ± 0.3 |
| Sex | Group (g) | Brain (g) | Liver (g) | Spleen (g) | Heart (g) | Thymus (g) | Kidney (g) | Adrenal (g) | Testicles (Male) Ovaries (Female) (g) | Epididymis (Male) Uterus (Female) (g) |
|---|---|---|---|---|---|---|---|---|---|---|
| Male rats | CK | 2.09 ± 0.10 | 13.51 ± 1.69 | 0.95 ± 0.16 | 1.33 ± 0.10 | 0.79 ± 0.27 | 3.22 ± 0.35 | 0.065 ± 0.010 | 3.90 ± 0.52 | 1.49 ± 0.24 |
| N1 | 2.09 ± 0.08 | 15.31 ± 2.59 | 0.88 ± 0.12 | 1.49 ± 0.19 | 0.82 ± 0.20 | 3.97 ± 0.30 * | 0.068 ± 0.006 | 3.60 ± 0.50 | 1.41 ± 0.13 | |
| N2 | 2.11 ± 0.08 | 13.56 ± 1.68 | 1.00 ± 0.23 | 1.28 ± 0.13 | 0.62 ± 0.24 | 3.81 ± 0.38 * | 0.069 ± 0.013 | 3.71 ± 0.32 | 1.44 ± 0.13 | |
| N3 | 2.13 ± 0.06 | 14.59 ± 1.49 | 0.90 ± 0.11 | 1.41 ± 0.14 | 0.76 ± 0.12 | 3.67 ± 0.35 | 0.064 ± 0.019 | 3.78 ± 0.40 | 1.31 ± 0.13 | |
| T1 | 2.22 ± 0.24 | 14.14 ± 1.23 | 0.98 ± 0.12 | 1.52 ± 0.11 | 0.52 ± 0.10 *# | 3.69 ± 0.35 * | 0.070 ± 0.009 | 3.91 ± 0.22 | 1.42 ± 0.11 | |
| T2 | 2.12 ± 0.14 | 14.45 ± 1.84 | 1.00 ± 0.19 | 1.43 ± 0.19 | 0.60 ± 0.15 | 3.28 ± 0.30 # | 0.067 ± 0.014 | 3.59 ± 0.34 | 1.31 ± 0.19 | |
| T3 | 2.15 ± 0.08 | 14.32 ± 1.02 | 0.92 ± 0.12 | 1.34 ± 0.08 | 0.92 ± 0.17 | 3.33 ± 0.36 | 0.061 ± 0.014 | 3.93 ± 0.50 | 1.38 ± 0.12 | |
| Female rats | CK | 1.88 ± 0.09 | 7.67 ± 1.15 | 0.60 ± 0.08 | 0.88 ± 0.12 | 0.33 ± 0.09 | 1.91 ± 0.23 | 0.085 ± 0.012 | 0.171 ± 0.033 | 0.52 ± 0.10 |
| N1 | 1.93 ± 0.11 | 8.35 ± 0.91 | 0.61 ± 0.11 | 0.91 ± 0.09 | 0.36 ± 0.06 | 2.05 ± 0.17 | 0.097 ± 0.009 | 0.149 ± 0.030 | 0.52 ± 0.20 | |
| N2 | 1.92 ± 0.09 | 8.01 ± 0.50 | 0.60 ± 0.09 | 0.99 ± 0.08 | 0.41 ± 0.11 | 2.02 ± 0.13 | 0.082 ± 0.016 | 0.173 ± 0.048 | 0.54 ± 0.26 | |
| N3 | 1.94 ± 0.07 | 7.67 ± 1.13 | 0.58 ± 0.05 | 0.97 ± 0.15 | 0.33 ± 0.10 | 1.88 ± 0.19 | 0.079 ± 0.014 | 0.143 ± 0.038 | 0.52 ± 0.10 | |
| T1 | 1.93 ± 0.10 | 8.03 ± 0.68 | 0.61 ± 0.06 | 0.92 ± 0.06 | 0.38 ± 0.07 | 1.96 ± 0.12 | 0.081 ± 0.013 | 0.159 ± 0.038 | 0.51 ± 0.11 | |
| T2 | 1.93 ± 0.11 | 8.27 ± 1.03 | 0.62 ± 0.09 | 0.91 ± 0.07 | 0.37 ± 0.10 | 1.97 ± 0.09 | 0.086 ± 0.015 | 0.169 ± 0.023 | 0.57 ± 0.15 | |
| T3 | 1.93 ± 0.08 | 7.64 ± 0.73 | 0.62 ± 0.06 | 0.97 ± 0.14 | 0.38 ± 0.07 | 2.02 ± 0.15 | 0.085 ± 0.008 | 0.163 ± 0.041 | 0.54 ± 0.14 |
| Gender | Group | Brain (%) | Liver (%) | Spleen (%) | Heart (%) | Thymus (%) | Kidney (%) | Adrenal (%) | Testicles (Male) Ovaries (Female) (%) | Epididymis (Male) Uterus (Female) (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Male rats | CK | 0.41 ± 0.02 | 2.67 ± 0.29 | 0.19 ± 0.03 | 0.26 ± 0.02 | 0.15 ± 0.05 | 0.64 ± 0.06 | 0.013 ± 0.002 | 0.77 ± 0.09 | 0.30 ± 0.06 |
| N1 | 0.41 ± 0.04 | 2.97 ± 0.40 | 0.17 ± 0.01 | 0.29 ± 0.02 | 0.16 ± 0.03 | 0.78 ± 0.11 * | 0.013 ± 0.002 | 0.70 ± 0.10 | 0.28 ± 0.03 | |
| N2 | 0.42 ± 0.02 | 2.65 ± 0.20 | 0.20 ± 0.04 | 0.25 ± 0.01 | 0.11 ± 0.06 | 0.75 ± 0.07 * | 0.014 ± 0.002 | 0.73 ± 0.08 | 0.28 ± 0.03 | |
| N3 | 0.41 ± 0.03 | 2.76 ± 0.22 | 0.17 ± 0.02 | 0.27 ± 0.02 | 0.14 ± 0.02 | 0.70 ± 0.05 | 0.012 ± 0.003 | 0.72 ± 0.08 | 0.25 ± 0.03 | |
| T1 | 0.43 ± 0.05 | 2.76 ± 0.29 | 0.19 ± 0.02 | 0.30 ± 0.02 * | 0.10 ± 0.02 *# | 0.72 ± 0.06 | 0.014 ± 0.002 | 0.77 ± 0.07 | 0.28 ± 0.02 | |
| T2 | 0.41 ± 0.04 | 2.75 ± 0.19 | 0.19 ± 0.03 | 0.27 ± 0.03 | 0.11 ± 0.03 | 0.63 ± 0.04 # | 0.013 ± 0.002 | 0.69 ± 0.09 | 0.25 ± 0.04 | |
| T3 | 0.41 ± 0.02 | 2.74 ± 0.28 | 0.18 ± 0.02 | 0.26 ± 0.02 | 0.18 ± 0.03 | 0.64 ± 0.07 | 0.012 ± 0.003 | 0.75 ± 0.09 | 0.26 ± 0.02 | |
| Female rats | CK | 0.65 ± 0.07 | 2.64 ± 0.35 | 0.21 ± 0.05 | 0.30 ± 0.02 | 0.11 ± 0.03 | 0.66 ± 0.05 | 0.029 ± 0.005 | 0.059 ± 0.013 | 0.18 ± 0.04 |
| N1 | 0.67 ± 0.04 | 2.87 ± 0.30 | 0.21 ± 0.03 | 0.31 ± 0.03 | 0.12 ± 0.02 | 0.71 ± 0.07 | 0.033 ± 0.003 | 0.051 ± 0.012 | 0.16 ± 0.10 | |
| N2 | 0.66 ± 0.05 | 2.77 ± 0.18 | 0.21 ± 0.02 | 0.34 ± 0.02 * | 0.14 ± 0.04 | 0.70 ± 0.06 | 0.028 ± 0.006 | 0.060 ± 0.019 | 0.19 ± 0.09 | |
| N3 | 0.65 ± 0.07 | 2.53 ± 0.25 | 0.19 ± 0.02 | 0.32 ± 0.04 | 0.11 ± 0.03 | 0.62 ± 0.03 | 0.026 ± 0.005 | 0.048 ± 0.016 | 0.18 ± 0.05 | |
| T1 | 0.64 ± 0.04 | 2.65 ± 0.13 | 0.20 ± 0.02 | 0.30 ± 0.01 | 0.13 ± 0.02 | 0.65 ± 0.05 | 0.027 ± 0.004 # | 0.052 ± 0.010 | 0.17 ± 0.03 | |
| T2 | 0.65 ± 0.04 | 2.80 ± 0.33 | 0.21 ± 0.03 | 0.31 ± 0.01 | 0.12 ± 0.03 | 0.67 ± 0.05 | 0.029 ± 0.006 | 0.057 ± 0.008 | 0.19 ± 0.04 | |
| T3 | 0.65 ± 0.04 | 2.57 ± 0.17 | 0.21 ± 0.02 | 0.32 ± 0.03 | 0.13 ± 0.02 | 0.68 ± 0.04 | 0.029 ± 0.003 | 0.055 ± 0.017 | 0.18 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Chen, M.; Li, Y.; Xu, X.; Wu, S.; Liu, Z.; Qi, S.; Yi, G.; Zhang, X.; Zhang, J.; et al. A 90-Day Safety Study of Meat from MSTN and FGF5 Double-Knockout Sheep in Wistar Rats. Life 2022, 12, 204. https://doi.org/10.3390/life12020204
Zhao Y, Chen M, Li Y, Xu X, Wu S, Liu Z, Qi S, Yi G, Zhang X, Zhang J, et al. A 90-Day Safety Study of Meat from MSTN and FGF5 Double-Knockout Sheep in Wistar Rats. Life. 2022; 12(2):204. https://doi.org/10.3390/life12020204
Chicago/Turabian StyleZhao, Yue, Mingming Chen, Yao Li, Xueling Xu, Sujun Wu, Zhimei Liu, Shiyu Qi, Guang Yi, Xiaosheng Zhang, Jinlong Zhang, and et al. 2022. "A 90-Day Safety Study of Meat from MSTN and FGF5 Double-Knockout Sheep in Wistar Rats" Life 12, no. 2: 204. https://doi.org/10.3390/life12020204
APA StyleZhao, Y., Chen, M., Li, Y., Xu, X., Wu, S., Liu, Z., Qi, S., Yi, G., Zhang, X., Zhang, J., Guo, X., Yu, K., Deng, S., Li, Y., & Lian, Z. (2022). A 90-Day Safety Study of Meat from MSTN and FGF5 Double-Knockout Sheep in Wistar Rats. Life, 12(2), 204. https://doi.org/10.3390/life12020204








