Case Report of Cerebral Sinus Thrombosis Related to Immune Thrombotic Thrombocytopenia Following Administration of ChAdOx1 nCoV-19 for Vaccination against COVID-19
Abstract
:1. Introduction
2. The Case
3. Discussion
3.1. Pathophysiology
3.2. Prevalence and Mortality
3.3. Risk Factors
3.4. Symptomatology
3.5. Imaging Studies
3.6. Laboratory Tests
3.7. Treatment
3.8. Prognosis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schultz, N.H.; Sørvoll, I.H.; Michelsen, A.E.; Munthe, L.A.; Lund-Johansen, F.; Ahlen, M.T.; Wiedmann, M.; Aamodt, A.-H.; Skattør, T.H.; Tjønnfjord, G.E.; et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2124–2130. [Google Scholar] [CrossRef] [PubMed]
- Wiedmann, M.; Skattør, T.; Stray-Pedersen, A.; Romundstad, L.; Antal, E.-A.; Marthinsen, P.B.; Sørvoll, I.H.; Ernstsen, S.L.; Lund, C.G.; Holme, P.A.; et al. Vaccine Induced Immune Thrombotic Thrombocytopenia Causing a Severe Form of Cerebral Venous Thrombosis with High Fatality Rate: A Case Series. Front. Neurol. 2021, 12, 721146. [Google Scholar] [CrossRef] [PubMed]
- Pomara, C.; Sessa, F.; Ciaccio, M.; Dieli, F.; Esposito, M.; Garozzo, S.F.; Giarratano, A.; Prati, D.; Rappa, F.; Salerno, M.; et al. Post-mortem findings in vaccine-induced thrombotic thombocytopenia. Haematologica 2021, 106, 2291–2293. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Singh, D.; Lown, R.; Poles, A.; Solomon, T.; Levi, M.; Goldblatt, D.; Kotoucek, P.; Thomas, W.; Lester, W. Pathologic Antibodies to Platelet Factor 4 after ChAdOx1 nCoV-19 Vaccination. N. Engl. J. Med. 2021, 384, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Greinacher, A.; Thiele, T.; Warkentin, T.E.; Weisser, K.; Kyrle, P.A.; Eichinger, S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N. Engl. J. Med. 2021, 384, 2092–2101. [Google Scholar] [CrossRef]
- Global Advisory Committee on Vaccine Safety (GACVS) Review of Latest Evidence of Rare Adverse Blood Coagulation Events with AstraZeneca COVID-19 Vaccine (Vaxzevria and Covishield). Available online: https://www.who.int/news/item/16-04-2021-global-advisory-committee-on-vaccine-safety-(gacvs)-review-of-latest-evidence-of-rare-adverse-blood-coagulation-events-with-astrazeneca-covid-19-vaccine-(vaxzevria-and-covishield) (accessed on 1 October 2021).
- Statement of the COVID-19 Subcommittee of the WHO Global Advisory Committee on Vaccine Safety (GACVS) on Safety Signals Related to the Johnson & Johnson/Janssen COVID-19 Vaccine. Available online: https://www.who.int/news/item/19-05-2021-statement-gacvs-safety-johnson-johnson-janssen-covid-19-vaccine (accessed on 1 October 2021).
- Coronavirus Vaccine—Weekly Summary of Yellow Card Reporting. Available online: https://www.gov.uk/government/publications/coronavirus-covid-19-vaccine-adverse-reactions/coronavirus-vaccine-summary-of-yellow-card-reporting (accessed on 1 October 2021).
- Mahase, E. AstraZeneca vaccine: Blood clots are “extremely rare” and benefits outweigh risks, regulators conclude. BMJ 2021, 373, n931. [Google Scholar] [CrossRef]
- Coronavirus Disease (COVID-19): Vaccines Safety. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-(covid-19)-vaccines-safety (accessed on 1 October 2021).
- Taquet, M.; Husain, M.; Geddes, J.R.; Luciano, S.; Harrison, P.J. Cerebral venous thrombosis and portal vein thrombosis: A retrospective cohort study of 537,913 COVID-19 cases. EClinicalMedicine 2021, 39, 101061. [Google Scholar] [CrossRef]
- Krzywicka, K.; Heldner, M.R.; van Kammen, M.S.; van Haaps, T.; Hiltunen, S.; Silvis, S.M.; Levi, M.; Hovinga, J.A.K.; Jood, K.; Lindgren, E.; et al. Post-SARS-CoV-2-vaccination cerebral venous sinus thrombosis: An analysis of cases notified to the European Medicines Agency. Eur. J. Neurol. 2021, 28, 3656–3662. [Google Scholar] [CrossRef]
- Haghighi, A.B.; Edgell, R.C.; Cruz-Flores, S.; Feen, E.; Piriyawat, P.; Vora, N.; Callison, R.C.; Alshekhlee, A. Mortality of Cerebral Venous–Sinus Thrombosis in a Large National Sample. Stroke 2012, 43, 262–264. [Google Scholar] [CrossRef] [Green Version]
- See, I.; Su, J.R.; Lale, A.; Woo, E.J.; Guh, A.Y.; Shimabukuro, T.T.; Streiff, M.B.; Rao, A.K.; Wheeler, A.P.; Beavers, S.F.; et al. US Case Reports of Cerebral Venous Sinus Thrombosis with Thrombocytopenia after Ad26.COV2.S Vaccination, March 2 to April 21, 2021. JAMA J. Am. Med. Assoc. 2021, 325, 2448–2456. [Google Scholar] [CrossRef]
- Suresh, P.; Petchey, W. ChAdOx1 nCOV-19 vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis (CVST). BMJ Case Rep. 2021, 14, e243931. [Google Scholar] [CrossRef] [PubMed]
- Röttger, C.; Bachmann, G.; Gerriets, T.; Kaps, M.; Kuchelmeister, K.; Schachenmayr, W.; Walberer, M.; Wessels, T.; Stolz, E. A New Model of Reversible Sinus Sagittalis Superior Thrombosis in the Rat: Magnetic Resonance Imaging Changes. Neurosurgery 2005, 57, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Stam, J. Thrombosis of the Cerebral Veins and Sinuses. N. Engl. J. Med. 2005, 352, 1791–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferro, J.M.; Canhão, P.; de Sousa, D.A. Cerebral venous thrombosis. La Presse Médicale 2016, 45, e429–e450. [Google Scholar] [CrossRef] [PubMed]
- Pavord, S.; Scully, M.; Hunt, B.J.; Lester, W.; Bagot, C.; Craven, B.; Rampotas, A.; Ambler, G.; Makris, M. Clinical Features of Vaccine-Induced Immune Thrombocytopenia and Thrombosis. N. Engl. J. Med. 2021, 385, 1680–1689. [Google Scholar] [CrossRef] [PubMed]
- Sharifian-Dorche, M.; Bahmanyar, M.; Sharifian-Dorche, A.; Mohammadi, P.; Nomovi, M.; Mowla, A. Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review. J. Neurol. Sci. 2021, 428, 117607. [Google Scholar] [CrossRef]
- Ferro, J.M.; Bousser, M.; Canhão, P.; Coutinho, J.M.; Crassard, I.; Dentali, F.; di Minno, M.; Maino, A.; Martinelli, I.; Masuhr, F.; et al. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis—Endorsed by the European Academy of Neurology. Eur. J. Neurol. 2017, 24, 1203–1213. [Google Scholar] [CrossRef] [Green Version]
- Ikenberg, B.; Demleitner, A.F.; Thiele, T.; Wiestler, B.; Götze, K.; Mößmer, G.; Lingor, P. Cerebral venous sinus thrombosis after ChAdOx1 nCov-19 vaccination with a misleading first cerebral MRI scan. Stroke Vasc. Neurol. 2021, 6, 668–670. [Google Scholar] [CrossRef] [PubMed]
- Tadi, P.; Behgam, B.; Baruffi, S. Cerebral Venous Thrombosis. [Updated 2021 September 29]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459315/ (accessed on 1 October 2021).
- Azeemuddin, M.; Awais, M.; Mubarak, F.; Rehman, A.; Baloch, H.N.U.A. Prevalence of subarachnoid haemorrhage among patients with cranial venous sinus thrombosis in the presence and absence of venous infarcts. Neuroradiol. J. 2018, 31, 496–503. [Google Scholar] [CrossRef]
- Sørvoll, I.H.; Horvei, K.D.; Ernstsen, S.L.; Lægreid, I.J.; Lund, S.; Grønli, R.H.; Olsen, M.K.; Jacobsen, H.K.; Eriksson, A.; Halstensen, A.M.; et al. An observational study to identify the prevalence of thrombocytopenia and anti-PF4/polyanion antibodies in Norwegian health care workers after COVID-19 vaccination. J. Thromb. Haemost. 2021, 19, 1813–1818. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Causality Assessment of an Adverse Event following Immunization (AEFI): User Manual for the Revised WHO Classification, 2nd ed.; 2019 Update; World Health Organization: Geneva, Switzerland, 2019; Available online: https://apps.who.int/iris/handle/10665/340802 (accessed on 1 October 2021).
- Fanni, D.; Saba, L.; Demontis, R.; Gerosa, C.; Chighine, A.; Nioi, M.; Suri, J.S.; Ravarino, A.; Cau, F.; Barcellona, D.; et al. Vaccine-induced severe thrombotic thrombocytopenia following COVID-19 vaccination: A report of an autoptic case and review of the literature. Rev. Med. Pharm. Sci. 2021, 25, 5063–5069. [Google Scholar]
- Pomara, C.; Sessa, F.; Ciaccio, M.; Dieli, F.; Esposito, M.; Giammanco, G.; Garozzo, S.; Giarratano, A.; Prati, D.; Rappa, F.; et al. COVID-19 Vaccine and Death: Causality Algorithm According to the WHO Eligibility Diagnosis. Diagnostics 2021, 11, 955. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, A.; Chen, C.-J.; Raper, D.M.; Ding, D.; Buell, T.; Mastorakos, P.; Liu, K.C. Endovascular mechanical thrombectomy for cerebral venous sinus thrombosis: A systematic review. J. NeuroInterventional Surg. 2017, 9, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, F.M.; Dandapat, S.; Banerjee, C.; Zuurbier, S.M.; Johnson, M.; Stam, J.; Coutinho, J.M. Mechanical Thrombectomy in Cerebral Venous Thrombosis. Stroke 2015, 46, 1263–1268. [Google Scholar] [CrossRef]
- Liao, C.-H.; Liao, N.-C.; Chen, W.-H.; Chen, H.-C.; Shen, C.-C.; Yang, S.-F.; Tsuei, Y.-S. Endovascular Mechanical Thrombectomy and On-Site Chemical Thrombolysis for Severe Cerebral Venous Sinus Thrombosis. Sci. Rep. 2020, 10, 4937. [Google Scholar] [CrossRef] [Green Version]
- Coutinho, J.M.; Zuurbier, S.M.; Bousser, M.-G.; Ji, X.; Canhão, P.; Roos, Y.B.; Crassard, I.; Nunes, A.P.; Uyttenboogaart, M.; Chen, J.; et al. Effect of Endovascular Treatment with Medical Management vs Standard Care on Severe Cerebral Venous Thrombosis. JAMA Neurol. 2020, 77, 966–973. [Google Scholar] [CrossRef]
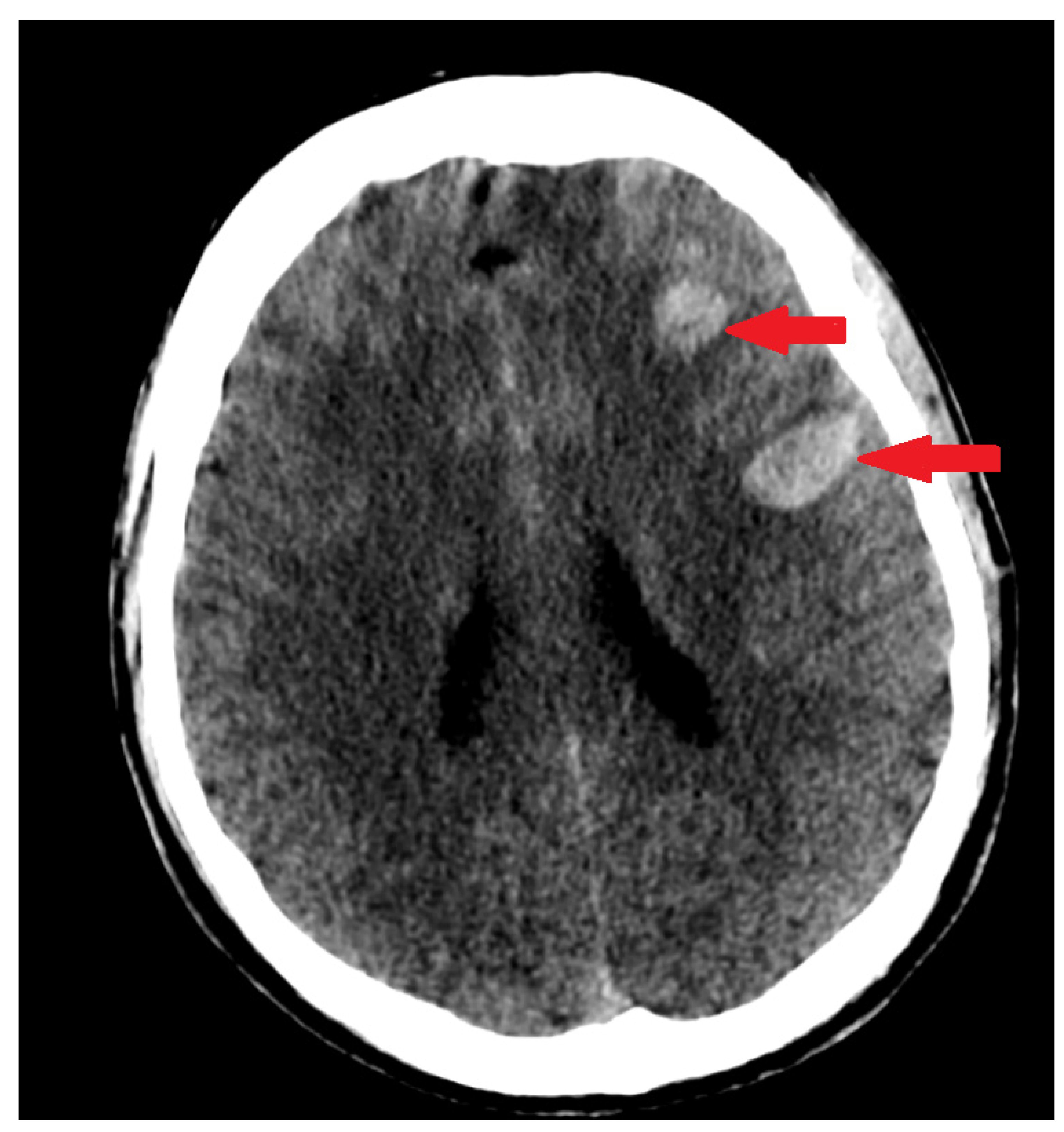
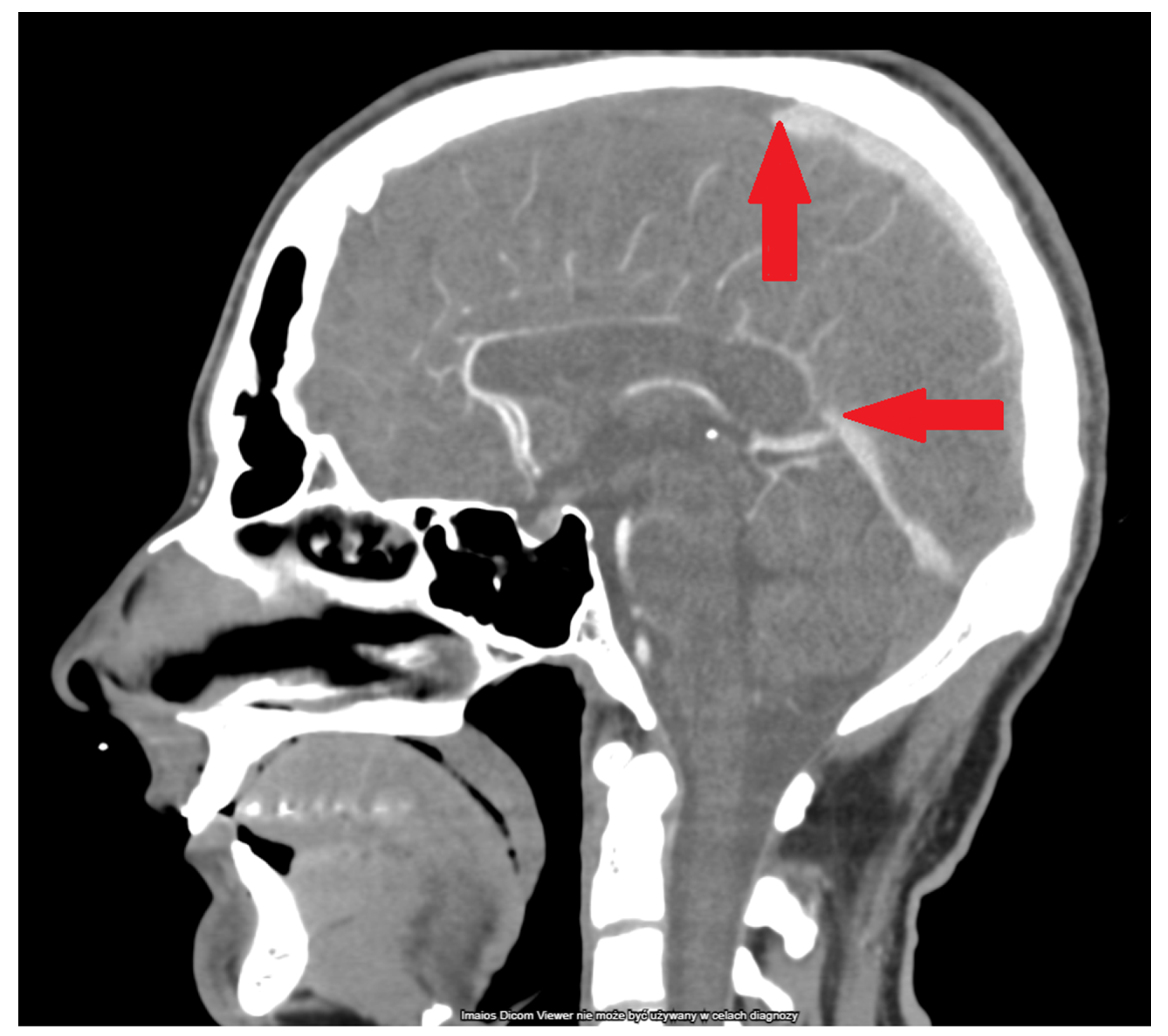
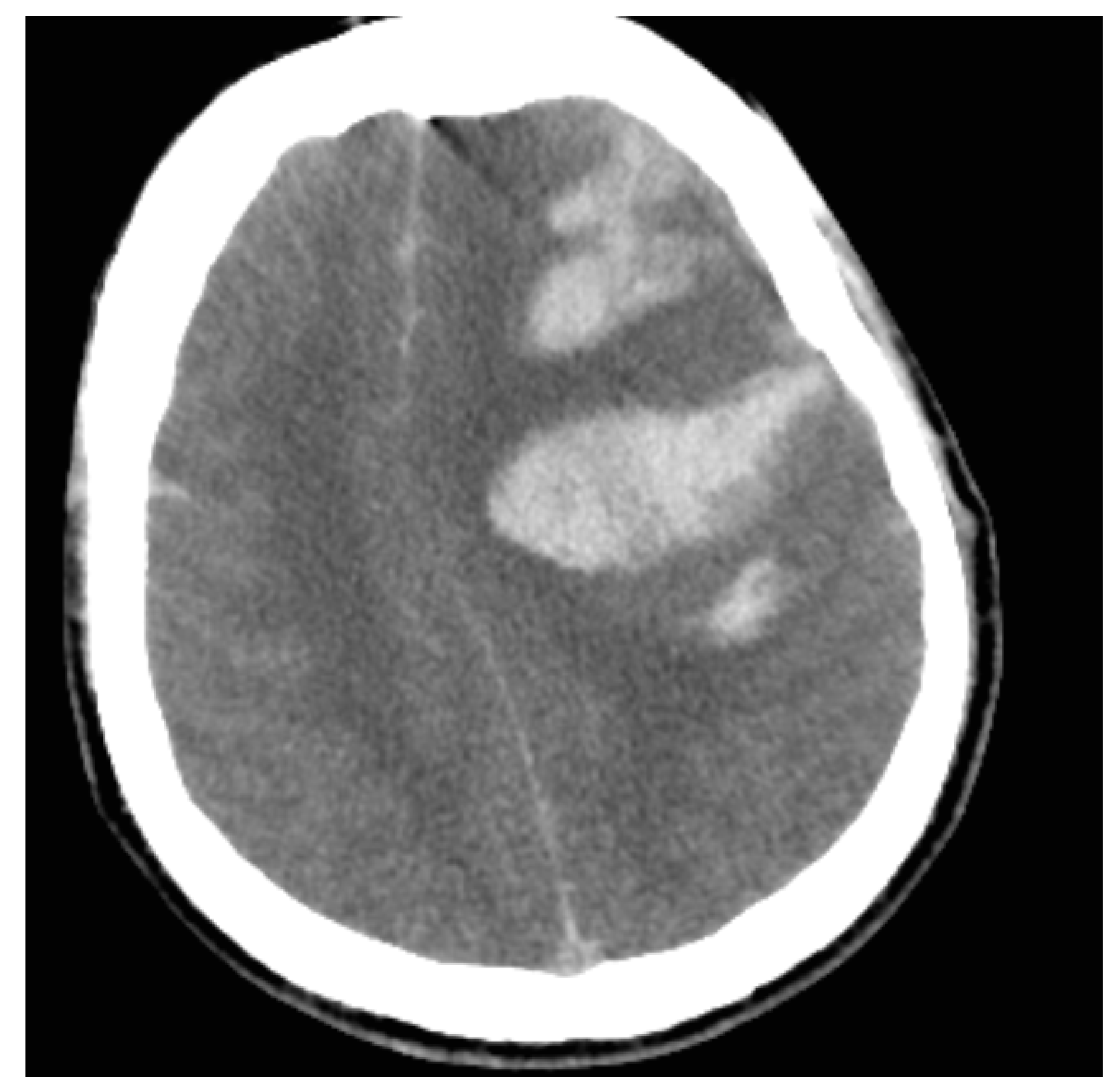
| Lab Finding | Admission | After 6 h | After 12 h |
|---|---|---|---|
| Platelet count (×109/L) | 39 | 92 | 65 |
| Hemoglobin (g/dL) | 14.7 | 15.5 | 16.8 |
| WBC (×109/L) | 8.18 | 13.44 | 23.13 |
| APTT (s) | 42.1 | 61.6 | 51.2 |
| PT (s) | 12.9 | 12.9 | 13.6 |
| INR | 1.14 | 1.14 | 1.21 |
| D-Dimer (µgFEU/L) | 125,780 | 153,660 | 157,660 |
| Fibrinogen (mg/dL) | - | 183 | 314 |
| AST | - | 38 | 99 |
| ALT | - | 34 | 71 |
| Creatinine (mg/dL) | 1.0 | 1.3 | 2.0 |
| eGFR (ml/min/1.73 m2) | >90 | >90 | 42 |
| Urea (mg/dL) | 33 | 32 | 54 |
| CRP (mg/dL) | 2.3 | 5.2 | - |
| Type of VITT | Description |
|---|---|
| Definite VITT | All five of the following criteria:
|
| Probable VITT |
|
| Possible VITT |
|
| Unlikely VITT |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szypowski, W.; Dębiec, A.; Świstak, J.; Nowocień, M.; Rzepecki, P.; Możański, M.; Staszewski, J.; Stępień, A. Case Report of Cerebral Sinus Thrombosis Related to Immune Thrombotic Thrombocytopenia Following Administration of ChAdOx1 nCoV-19 for Vaccination against COVID-19. Life 2022, 12, 168. https://doi.org/10.3390/life12020168
Szypowski W, Dębiec A, Świstak J, Nowocień M, Rzepecki P, Możański M, Staszewski J, Stępień A. Case Report of Cerebral Sinus Thrombosis Related to Immune Thrombotic Thrombocytopenia Following Administration of ChAdOx1 nCoV-19 for Vaccination against COVID-19. Life. 2022; 12(2):168. https://doi.org/10.3390/life12020168
Chicago/Turabian StyleSzypowski, Wojciech, Aleksander Dębiec, Jarosław Świstak, Maciej Nowocień, Piotr Rzepecki, Marcin Możański, Jacek Staszewski, and Adam Stępień. 2022. "Case Report of Cerebral Sinus Thrombosis Related to Immune Thrombotic Thrombocytopenia Following Administration of ChAdOx1 nCoV-19 for Vaccination against COVID-19" Life 12, no. 2: 168. https://doi.org/10.3390/life12020168
APA StyleSzypowski, W., Dębiec, A., Świstak, J., Nowocień, M., Rzepecki, P., Możański, M., Staszewski, J., & Stępień, A. (2022). Case Report of Cerebral Sinus Thrombosis Related to Immune Thrombotic Thrombocytopenia Following Administration of ChAdOx1 nCoV-19 for Vaccination against COVID-19. Life, 12(2), 168. https://doi.org/10.3390/life12020168






