Delayed Comprehensive Stroke Unit Care Attributable to the Evolution of Infection Protection Measures across Two Consecutive Waves of the COVID-19 Pandemic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Acquisition
2.3. Performance Indicators of Evidence-Based Stroke Care
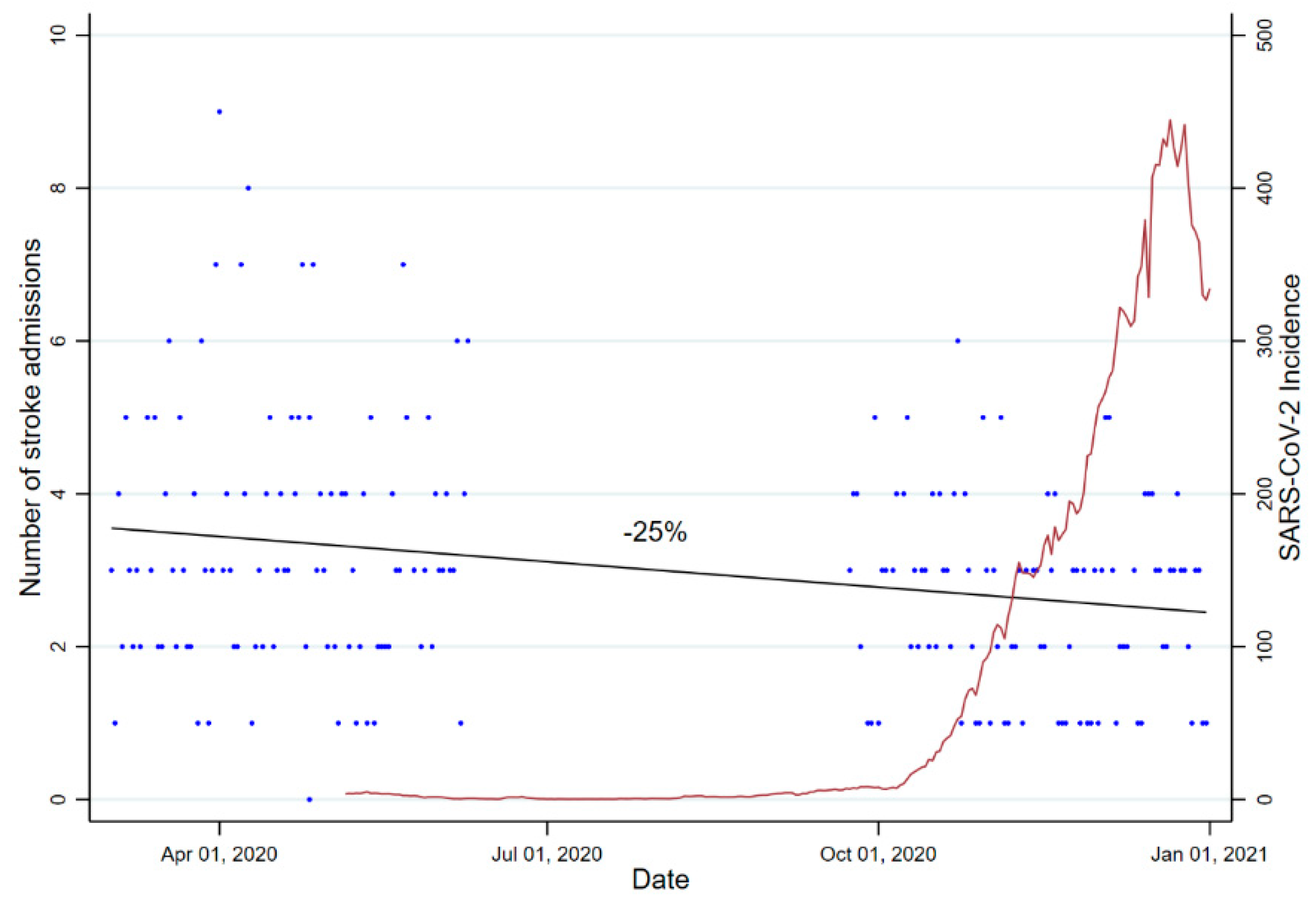
2.4. Statistical Analysis
3. Results
Evidence-Based Stroke Care
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- -
- G45: Transient cerebral ischemic attacks and related syndromes
- -
- G51: Facial nerve disorders
- -
- G81: Hemiplegia and hemiparesis
- -
- G83: Other paralytic syndromes
- -
- H53: Visual disturbances
- -
- I61: Nontraumatic intracerebral hemorrhage
- -
- I62: Other and unspecified nontraumatic intracranial hemorrhage
- -
- I63: Cerebral Infarction
- -
- I64: Stroke, not specified as hemorrhage or infarction
- -
- I65: Occlusion and stenosis of precerebral arteries, not resulting in cerebral infarction
- -
- I66: Occlusion and stenosis of cerebral arteries, not resulting in cerebral infarction
- -
- R20: Disturbances of skin sensation
- -
- R40: Somnolence, stupor, and coma
- -
- R41: Other symptoms and signs involving cognitive functions and awareness
- -
- R42: Dizziness and giddiness
- -
- R47: Speech disturbances, not elsewhere classified
Appendix B
- -
- Cough
- -
- Sore throat/cold symptoms?
- -
- Dyspnea
- -
- Headache/myalgias?
- -
- Diarrhea/emesis?
- -
- Loss of sense of taste and/or smell?
- -
- In quarantine?
- -
- Confirmed SARS-CoV2 Infection?
- -
- Contact with suspected or confirmed SARS-CoV2 Infection during the last 14 days?
- -
- Oxygen saturation level indoor air < 94%?
- -
- Temperature > 38 °C?
Appendix C
| Demographics | Count (n) | Missing Data (%) |
|---|---|---|
| Age total | 0 | 0.0 |
| Female total | 0 | 0.0 |
| Living alone | 62 | 10.6 |
| Nursing home/care facility | 9 | 1.5 |
| Care level/assistance needed | 12 | 2.1 |
| Patient History * | ||
| COVID-19 positive at admission | 6 | 0.0 |
| Diabetes | 6 | 1.0 |
| HbA1c (%) | 49 | 8.4 |
| LDL (mg/dl) | 44 | 7.5 |
| Adipositas | 68 | 11.7 |
| Nicotin | 194 | 33.3 |
| Alcohol | 255 | 43.7 |
| Stroke, radiological | 7 | 1.2 |
| Acute Management | ||
| Acute therapy | 3 | 0.5 |
| Intravenous thrombolysis (intern) | 2 | 0.3 |
| Intravenous thrombolysis (extern) | 5 | 0.9 |
| Endovascular therapy | 0 | 0.0 |
| Carotis-thrombendarterectomy | 1 | 0.2 |
| Carotis-Stent | 1 | 0.2 |
| Temporal Indicators | ||
| Onset-to-admission (total) ** | 24 | 11.2 |
| Door-to-imaging (IVT) | 2 | 2.9 |
| Door-to-needle *** | 5 | 7.1 |
| Onset-to-groin *** | 51 | 39.2 |
| Door-to-groin *** | 9 | 6.9 |
| Case Management | ||
| Admission modality | 0 | 0.0 |
| Discharge modality | 0 | 0.0 |
| Wake-up stroke | 0 | 0.0 |
| Hospitalization duration (total) | 0 | 0.0 |
| No same day certified stroke unit care | 3 | 0.5 |
| Medication at discharge | 0 | 0.0 |
| Stroke Phenotype | ||
| Stroke type | 1 | 0.2 |
| Toast criteria **** | 5 | 1.1 |
| NIHSS | ||
| Admin | 15 | 2.6 |
| Discharge | 8 | 1.4 |
| mRS | ||
| Admin | 6 | 1.0 |
| Discharge | 7 | 1.2 |
References
- Leira, E.C.; Russman, A.N.; Biller, J.; Brown, D.L.; Bushnell, C.D.; Caso, V.; Chamorro, A.; Creutzfeldt, C.J.; Cruz-Flores, S.; Elkind, M.S.V.; et al. Preserving stroke care during the COVID-19 pandemic: Potential issues and solutions. Neurology 2020, 95, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Khosravani, H.; Rajendram, P.; Notario, L.; Chapman, M.G.; Menon, B.K. Protected Code Stroke: Hyperacute Stroke Management during the Coronavirus Disease 2019 (COVID-19) Pandemic. Stroke 2020, 51, 1891–1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qureshi, A.I.; Abd-Allah, F.; Al-Senani, F.; Aytac, E.; Haghighi, A.B.; Ciccone, A.; Gomez, C.R.; Gurkas, E.; Hsu, C.Y.; Jani, V.; et al. Management of acute ischemic stroke in patients with COVID-19 infection: Report of an international panel. Int. J. Stroke 2020, 15, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.G.; Abdalkader, M.; Qureshi, M.M.; Martins, S.O.; Yamagami, H.; Qiu, Z.; Mansour, O.Y.; Sathya, A.; Czlonkowska, A.; Tsivgoulis, G.; et al. Global Impact of COVID-19 on Stroke Care and Intravenous Thrombolysis. Neurology 2021, 96, e2824–e2838. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Gutierrez, S.; Farooqui, M.; Zha, A.; Czap, A.; Sebaugh, J.; Desai, S.; Jadhav, A.; Vora, N.; Rai, V.; Jovin, T.G.; et al. Decline in mild stroke presentations and intravenous thrombolysis during the COVID-19 pandemic: The Society of Vascular and Interventional Neurology Multicenter Collaboration. Clin. Neurol. Neurosurg. 2021, 201, 106436. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, C.; Ebert, A.; Huttner, H.B.; Puetz, V.; Kallmünzer, B.; Barlinn, K.; Haverkamp, C.; Harloff, A.; Brich, J.; Platten, M.; et al. Acute Stroke in Times of the COVID-19 Pandemic: A Multicenter Study. Stroke 2020, 51, 2224–2227. [Google Scholar] [CrossRef] [PubMed]
- Schilling, J.; Lehfeld, A.S.; Schumacher, D.; Diercke, M.; Buda, S.; Haas, W.; RKI COVID-19 Study Group. Krankheitsschwere der ersten COVID-19-Welle in Deutschland basierend auf den Meldungen gemäß Infektionsschutzgesetz. J. Health Monit. 2020, S11, 1–20. [Google Scholar]
- Barlinn, K.; Siepmann, T.; Pallesen, L.P.; Winzer, S.; Sedghi, A.; Schroettner, P.; Hochauf-Stange, K.; Prakapenia, A.; Moustafa, H.; With, K.d.; et al. Universal laboratory testing for SARS-CoV-2 in hyperacute stroke during the COVID-19 pandemic. J. Stroke Cerebrovasc. Dis. 2020, 29, 105061. [Google Scholar] [CrossRef] [PubMed]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline from the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Daten/Fallzahlen_Kum_Tab.html (accessed on 2 June 2021).
- Rinkel, L.A.; Prick, J.C.M.; Slot, R.E.R.; Sombroek, N.M.A.; Burggraaff, J.; Groot, A.E.; Emmer, B.J.; Roos, Y.B.W.E.M.; Brouwer, M.C.; Berg-Vos, R.M.V.D.; et al. Impact of the COVID-19 outbreak on acute stroke care. J. Neurol. 2021, 268, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Uchino, K.; Kolikonda, M.K.; Brown, D.; Kovi, S.; Collins, D.; Khawaja, Z.; Buletko, A.B.; Russman, A.N.; Hussain, M.S. Decline in Stroke Presentations During COVID-19 Surge. Stroke 2020, 51, 2544–2547. [Google Scholar] [CrossRef] [PubMed]
- Richter, D.; Eyding, J.; Weber, R.; Bartig, D.; Grau, A.; Hacke, W.; Krogias, C. Analysis of Nationwide Stroke Patient Care in Times of COVID-19 Pandemic in Germany. Stroke 2021, 52, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Siegler, J.E.; Zha, A.M.; Czap, A.L. Influence of the COVID-19 Pandemic on Treatment Times for Acute Ischemic Stroke: The Society of Vascular and Interventional Neurology Multicenter Collaboration. Stroke 2021, 52, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Langhorne, P.S.; Ramachandra, S.; Stroke Unit Trialists. Organised inpatient (stroke unit) care for stroke: Network meta-analysis. Cochrane Database Syst. Rev. 2020, 4, CD000197. [Google Scholar] [PubMed]
- Rudd, A.G.; Hoffman, A.; Irwin, P.; Lowe, D.; Pearson, M.G. Stroke unit care and outcome: Results from the 2001 National Sentinel Audit of Stroke (England, Wales, and Northern Ireland). Stroke 2005, 36, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Urimubenshi, G.; Langhorne, P.; Cadilhac, D.A.; Kagwiza, J.N.; Wu, O. Association between patient outcomes and key performance indicators of stroke care quality: A systematic review and meta-analysis. Eur. Stroke J. 2017, 2, 287–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biernaskie, J.; Chernenko, G.; Corbett, D. Efficacy of rehabilitative experience declines with time after focal ischemic brain injury. J. Neurosci. 2004, 24, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, J.; Godecke, E.; Johnson, L.; Langhorne, P. Early rehabilitation after stroke. Curr. Opin. Neurol. 2017, 30, 48–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paolucci, S.; Antonucci, G.; Grasso, M.G.; Morelli, D.; Troisi, E.; Coiro, P.; Bragoni, M. Early versus delayed inpatient stroke rehabilitation: A matched comparison conducted in Italy. Arch. Phys. Med. Rehabil. 2000, 81, 695–700. [Google Scholar] [CrossRef]
| Demographics | First Wave (n = 333) | Second Wave (n = 249) | p |
|---|---|---|---|
| Age, years, median (IQR) | 77 (65,83) | 78 (65,84) | 0.65 |
| Female, n (%) | 153 (45.7) | 109 (43.8) | 0.65 |
| Living alone, n (%) | 102 (33.9) | 81 (36.7) | 0.52 |
| Nursing home/care facility, n (%) | 29 (8.1) | 20 (8.1) | 0.88 |
| Care level/assistance needed, n (%) | 109 (33.2) | 71 (29.0) | 0.32 |
| Comorbidities/patient history | |||
| Cardiovascular risk factors, n (%) | |||
| Arterial hypertension | 275 (82.3) | 212 (85.5) | 0.42 |
| Diabetes mellitus | 104 (31.1) | 75 (30.1) | 0.57 |
| HbA1c in DM, median [IQR] | 5.8 (5.5, 6.5) | 5.8 (5.5, 6.6) | 0.58 |
| Hyperlipidemia | 152 (45.5) | 104 (41.9) | 0.35 |
| LDL-C in HLP, mean (±SD) | 2.3 (1.0) | 2.3 (1.1) | 0.70 |
| Obesity | 111 (37.0) | 91 (41.9) | 0.27 |
| Nicotine * | 69 (33.2) | 52 (28.6) | 0.38 |
| Coronary Heart disease | 69 (20.8) | 53 (21.4) | 0.92 |
| Atrial fibrillation | 66 (19.9) | 60 (24.1) | 0.22 |
| Cerebrovascular disease, n (%) | 154 (46.8) | 116 (46.9) | 1.00 |
| Ischemic stroke | 153 (46.7) | 111 (44.6) | |
| Hemorrhagic stroke | 5 (1.5) | 7 (2.8) | |
| Transitory ischemic attack ** | 4 (1.2) | 1 (0.4) | |
| Further comorbidities, n (%) | |||
| Deep venous thrombosis | 9 (2.7) | 7 (2.8) | 1.00 |
| Pulmonary embolism | 7 (2.1) | 5 (2.0) | 1.00 |
| Malignancy | 52 (15.8) | 36 (14.5) | 0.73 |
| Chronic lung disease | 26 (7.9) | 22 (8.8) | 0.76 |
| Dementia | 33 (10.0) | 19 (7.6) | 0.38 |
| Alcohol abuse *** | 49 (26.6) | 45 (31.3) | 0.39 |
| Psychiatric disorder | 33 (10.0) | 24 (9.7) | 1.00 |
| Acute Management | First Wave (n = 333) | Second Wave (n = 249) | p |
|---|---|---|---|
| SARS-CoV-2 positive at admission, n (%) | 0 (0.0) | 11 (4.4) | <0.0001 |
| Delayed stroke unit admission, n (%) | 5 (1.5) | 17 (6.8) | 0.001 |
| Reperfusion therapy *, n (%) | 122 (36.6) | 93 (37.3) | 0.86 |
| Intravenous thrombolysis in-house | 40 (12.1) | 27 (11.0) | |
| Intravenous thrombolysis (outside) | 34 (10.3) | 21 (8.4) | |
| Endovascular therapy, n (%) | 72 (21.7) | 58 (23.4) | |
| Revascularization therapy, n (%) | 25 (7.5) | 13 (5.2) | 0.31 |
| Carotid endarterectomy | 12 (3.6) | 6 (2.4) | |
| Carotid Stenting | 13 (3.9) | 8 (3.2) | |
| Process times ** | |||
| Onset-to-admission (min), median (IQR) | 90 (58.8, 202.8) | 132 (64.8, 247.8) | 0.98 |
| Door-to-imaging (min) (IVT), median (IQR) | 9 (6, 12) | 12 (6, 21) | 0.36 |
| Door-to-needle (min), median (IQR) | 38.4 (24.6, 53.4) | 37.8 (25.8, 66) | 0.47 |
| Onset-to-groin (min), median (IQR) | 234 (162, 300) | 239 (187.2, 292.2) | 0.70 |
| Door-to-groin (min), median (IQR) | 64.8 (51, 85.8) | 70.2 (55.8, 97.8) | 0.22 |
| Case management | |||
| Admission modality, n (%) | 0.82 | ||
| Via emergency medical service | 188 (56.9) | 142 (57.0) | |
| Intra-hospital | 22 (6.7) | 12 (4.9) | |
| Inter-hospital via SOS-NET | 94 (28.5) | 74 (29.7) | |
| Walk-in | 26 (7.9) | 21 (8.4) | |
| Discharge modality, n (%) | 0.42 | ||
| Home | 150 (44.9) | 110 (44.2) | |
| Rehabilitation center | 118 (35.3) | 87 (34.9) | |
| Nursing home | 10 (3.0) | 5 (2.1) | |
| Inter- and intrahospital | 30 (9.0) | 18 (7.2) | |
| Death | 26 (7.8) | 29 (11.6) | |
| Length of hospitalization (rehabilitation), days, median (IQR) | 12 (8, 17) | 20 (11, 27) | <0.0001 |
| Length of hospitalization (excl. rehab), days, median (IQR) | 6 (4, 10) | 7 (5, 12) | 0.01 |
| Medication at discharge, n (%) | 0.57 | ||
| First ever prescription | 129 (39.6) | 87 (34.9) | |
| Dual antiplatelet therapy | 72 (22.9) | 45 (18.1) | |
| Direct oral anticoagulant | 71 (22.6) | 59 (23.7) | |
| Phenprocoumon | 7 (2.2) | 5 (2.0) | |
| LWMH/HWMH (therapeutic) | 11 (3.5) | 6 (2.4) |
| Demographics | First Wave (n = 333) | Second Wave (n = 249) | p |
|---|---|---|---|
| Age, years, median (IQR) | 77 (65,83) | 78 (65,84) | 0.65 |
| Female, n (%) | 153 (45.7) | 109 (43.8) | 0.65 |
| Living alone, n (%) | 102 (33.9) | 81 (36.7) | 0.52 |
| Nursing home/care facility, n (%) | 29 (8.1) | 20 (8.1) | 0.88 |
| Care level/assistance needed, n (%) | 109 (33.2) | 71 (29.0) | 0.32 |
| Comorbidities/patient history | |||
| Cardiovascular risk factors, n (%) | |||
| Arterial hypertension | 275 (82.3) | 212 (85.5) | 0.42 |
| Diabetes mellitus | 104 (31.1) | 75 (30.1) | 0.57 |
| HbA1c in DM, median (IQR) | 5.8 (5.5, 6.5) | 5.8 (5.5, 6.6) | 0.58 |
| Hyperlipidemia | 152 (45.5) | 104 (41.9) | 0.35 |
| LDL-C in HLP, mean (±SD) | 2.3 (1.0) | 2.3 (1.1) | 0.70 |
| Obesity | 111 (37.0) | 91 (41.9) | 0.27 |
| Nicotine * | 69 (33.2) | 52 (28.6) | 0.38 |
| Coronary Heart disease | 69 (20.8) | 53 (21.4) | 0.92 |
| Atrial fibrillation | 66 (19.9) | 60 (24.1) | 0.22 |
| Cerebrovascular disease, n (%) | 154 (46.8) | 116 (46.9) | 1.00 |
| Ischemic stroke | 153 (46.7) | 111 (44.6) | |
| Hemorrhagic stroke | 5 (1.5) | 7 (2.8) | |
| Transitory ischemic attack ** | 4 (1.2) | 1 (0.4) | |
| Further comorbidities, n (%) | |||
| Deep venous thrombosis | 9 (2.7) | 7 (2.8) | 1.00 |
| Pulmonary embolism | 7 (2.1) | 5 (2.0) | 1.00 |
| Malignancy | 52 (15.8) | 36 (14.5) | 0.73 |
| Chronic lung disease | 26 (7.9) | 22 (8.8) | 0.76 |
| Dementia | 33 (10.0) | 19 (7.6) | 0.38 |
| Alcohol abuse *** | 49 (26.6) | 45 (31.3) | 0.39 |
| Psychiatric disorder | 33 (10.0) | 24 (9.7) | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedghi, A.; Siepmann, T.; Pallesen, L.-P.; Reichmann, H.; Puetz, V.; Barlinn, J.; Barlinn, K. Delayed Comprehensive Stroke Unit Care Attributable to the Evolution of Infection Protection Measures across Two Consecutive Waves of the COVID-19 Pandemic. Life 2021, 11, 710. https://doi.org/10.3390/life11070710
Sedghi A, Siepmann T, Pallesen L-P, Reichmann H, Puetz V, Barlinn J, Barlinn K. Delayed Comprehensive Stroke Unit Care Attributable to the Evolution of Infection Protection Measures across Two Consecutive Waves of the COVID-19 Pandemic. Life. 2021; 11(7):710. https://doi.org/10.3390/life11070710
Chicago/Turabian StyleSedghi, Annahita, Timo Siepmann, Lars-Peder Pallesen, Heinz Reichmann, Volker Puetz, Jessica Barlinn, and Kristian Barlinn. 2021. "Delayed Comprehensive Stroke Unit Care Attributable to the Evolution of Infection Protection Measures across Two Consecutive Waves of the COVID-19 Pandemic" Life 11, no. 7: 710. https://doi.org/10.3390/life11070710
APA StyleSedghi, A., Siepmann, T., Pallesen, L.-P., Reichmann, H., Puetz, V., Barlinn, J., & Barlinn, K. (2021). Delayed Comprehensive Stroke Unit Care Attributable to the Evolution of Infection Protection Measures across Two Consecutive Waves of the COVID-19 Pandemic. Life, 11(7), 710. https://doi.org/10.3390/life11070710








