Isoprostanoid Plasma Levels Are Relevant to Cerebral Adrenoleukodystrophy Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Plasma Sample Preparation
2.3. Sample Preparation and Analysis
2.4. Chemical Synthesis of 4-F4t-NeuroP, 10-F4t-NeuroP, and F2t-dihomo-IsoPs Reference Molecules
2.5. Data Analysis
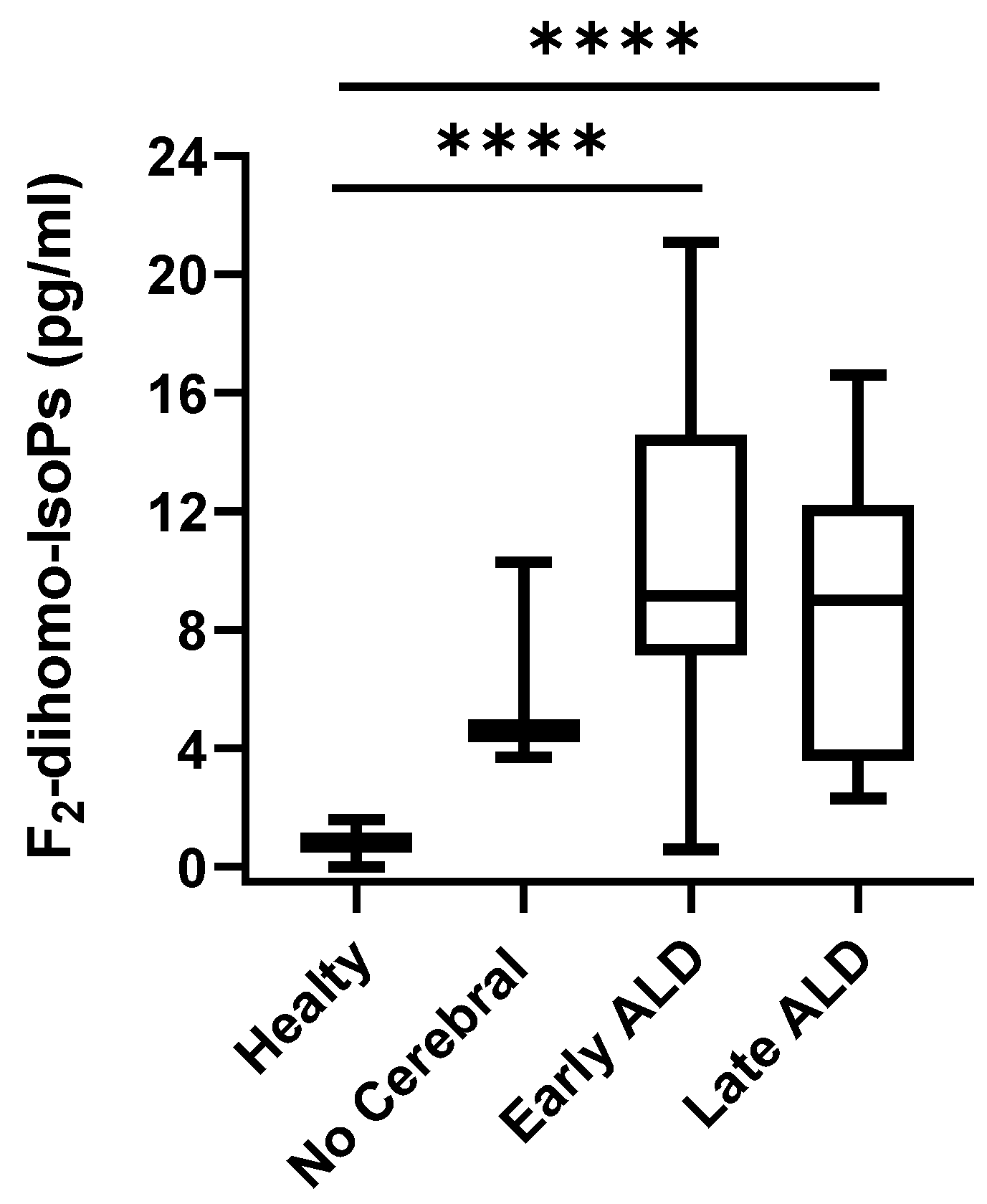
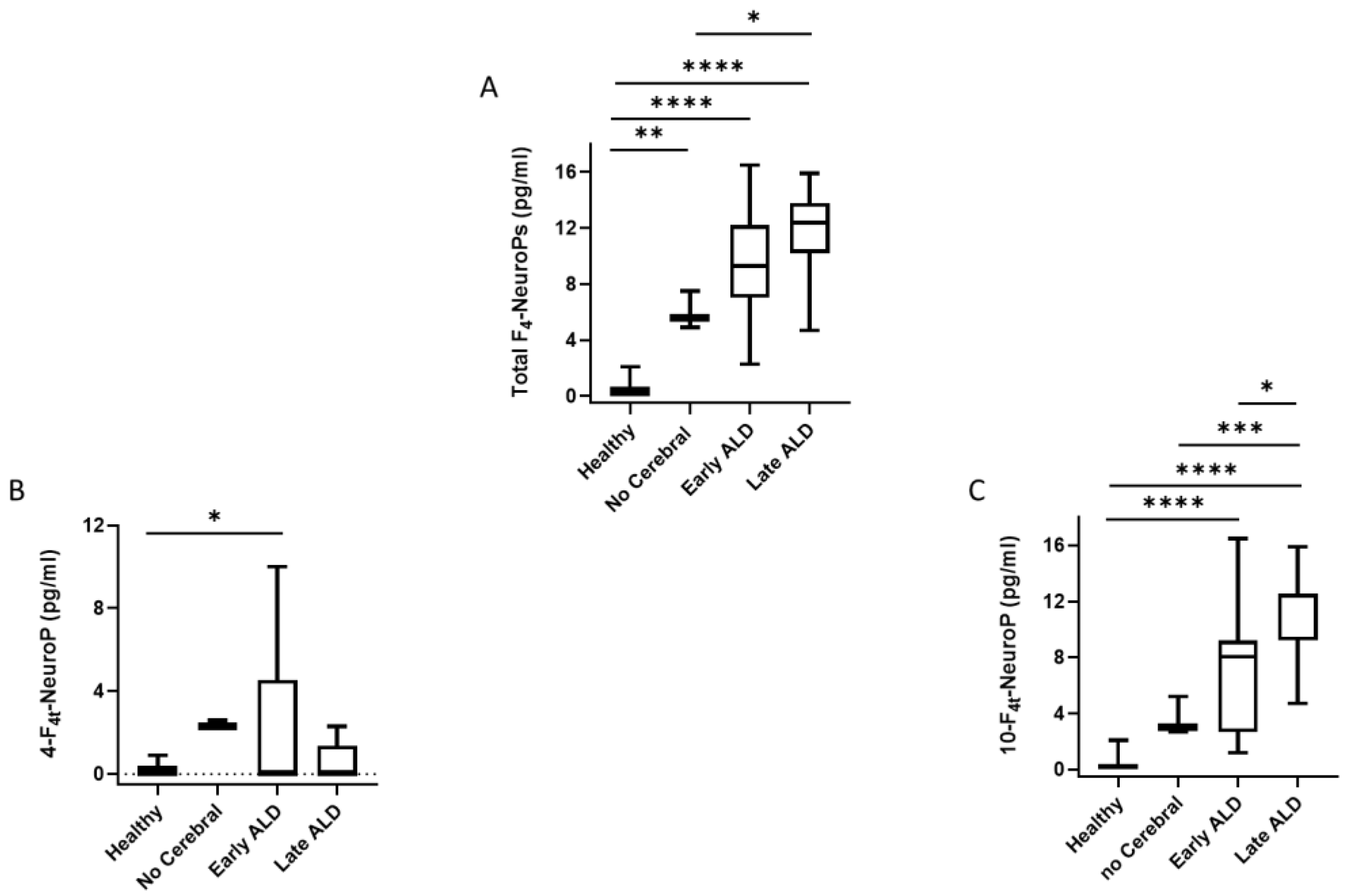
3. Results
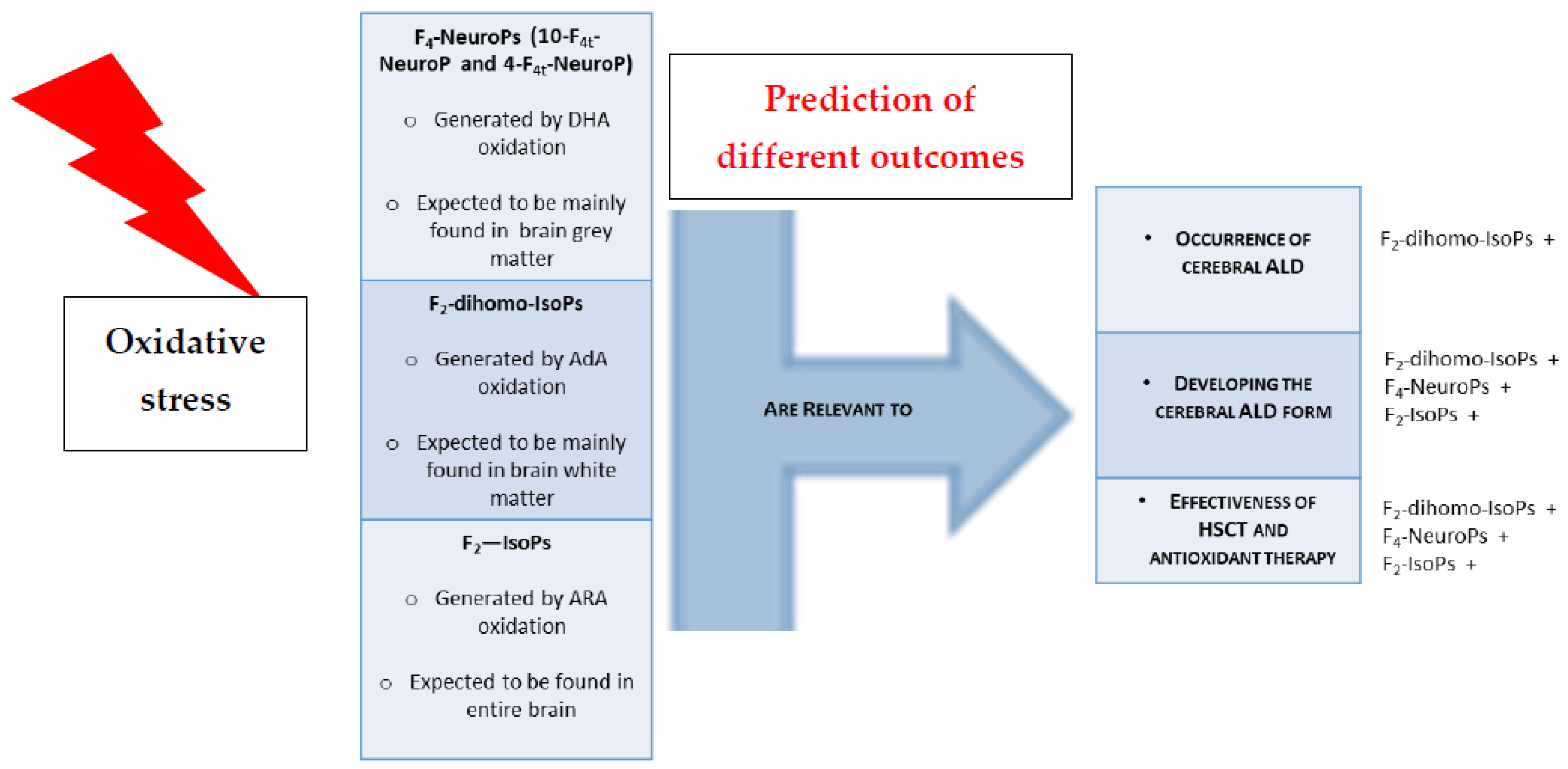
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bezman, L.; Moser, A.B.; Raymond, G.V.; Rinaldo, P.; Watkins, P.A.; Smith, K.D.; Kass, N.E.; Moser, H.W. Adrenoleukodystrophy: Incidence, new mutation rate, and results of extended family screening. Ann. Neurol. 2001, 49, 512–517. [Google Scholar] [CrossRef]
- Raymond, G.V.; Aubourg, P.; Paker, A.; Escolar, M.; Fischer, A.; Blanche, S.; Baruchel, A.; Dalle, J.H.; Michel, G.; Prasad, V.; et al. Survival and Functional Outcomes in Boys with Cerebral Adrenoleukodystrophy with and without Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 538–548. [Google Scholar] [CrossRef]
- Chiesa, R.; Boelens, J.J.; Duncan, C.N.; Kuehl, J.S.; Sevin, C.; Kapoor, N.; Prasad, V.K.; Lindemans, C.A.; Jones, S.A.; Amartino, H.M.; et al. Variables Affecting Outcomes After Allogeneic Hematopoietic Stem Cell Transplant for Cerebral Adrenoleukodystrophy. Blood Adv. 2021. [Google Scholar] [CrossRef]
- Vargas, C.R.; Wajner, M.; Sirtori, L.R.; Goulart, L.; Chiochetta, M.; Coelho, D.; Latini, A.; Llesuy, S.; Bello-Klein, A.; Giugliani, R.; et al. Evidence that oxidative stress is increased in patients with X-linked adrenoleukodystrophy Biochim. Biophys. Acta 2004, 1688, 26–32. [Google Scholar]
- Fourcade, S.; López-Erauskin, J.; Galino, J.; Duval, C.; Naudi, A.; Jove, M.; Kemp, S.; Villarroya, F.; Ferrer, I.; Pamplona, R.; et al. Early oxidative damage underlying neurodegeneration in X-adrenoleukodystrophy. Hum. Mol. Genet. 2008, 17, 1762–1773. [Google Scholar] [CrossRef] [PubMed]
- Deon, M.; Marchetti, D.P.; Donida, B.; Wajner, M.; Vargas, C. Oxidative Stress in Patients with X-Linked Adrenoleukodystrophy. Cell. Mol. Neurobiol. 2016, 36, 497–512. [Google Scholar] [CrossRef]
- Petrillo, S.; Piemonte, F.; Pastore, A.; Tozzi, G.; Aiello, C.; Pujol, A.; Cappa, M.; Bertini, E. Glutathione imbalance in patients with X-linked adrenoleukodystrophy. Mol. Genet. Metab. 2013, 109, 366–370. [Google Scholar] [CrossRef]
- Turk, B.R.; Theisen, B.E.; Nemeth, C.L.; Marx, J.S.; Shi, X.; Rosen, M.; Jones, R.O.; Moser, A.B.; Watkins, P.A.; Raymond, G.V.; et al. Antioxidant Capacity and Superoxide Dismutase Activity in Adrenoleukodystrophy. JAMA Neurol. 2017, 74, 519–524. [Google Scholar] [CrossRef]
- Ranea-Robles, P.; Launay, N.; Ruiz, M.; Calingasan, N.Y.; Dumont, M.; Naudí, A.; Portero-Otín, M.; Pamplona, R.; Ferrer, I.; Beal, M.F.; et al. Aberrant regulation of the GSK-3β/NRF2 axis unveils a novel therapy for adrenoleukodystrophy. EMBO Mol. Med. 2018, 10, e8604. [Google Scholar] [CrossRef] [PubMed]
- Casasnovas, C.; Ruiz, M.; Schlüter, A.; Naudí, A.; Fourcade, S.; Veciana, M.; Castañer, S.; Albertí, A.; Bargalló, N.; Johnson, M.; et al. Biomarker Identification, Safety, and Efficacy of High-Dose Antioxidants for Adrenomyeloneuropathy: A Phase II Pilot Study. Neurotherapeutics 2019, 16, 1167–1182. [Google Scholar] [CrossRef]
- Raftos, J.E.; Whillier, S.; Chapman, B.E.; Kuchel, P.W. Kinetics of uptake and deacetylation of N-acetylcysteine by human erythrocytes. Int. J. Biochem. Cell Biol. 2007, 39, 1698–1706. [Google Scholar] [CrossRef]
- Radtke, K.K.; Coles, L.D.; Mishra, U.; Orchard, P.J.; Holmay, M.; Cloyd, J.C. Interaction of N-acetylcysteine and cysteine in human plasma. J. Pharm. Sci. 2012, 101, 4653–4659. [Google Scholar] [CrossRef]
- Hart, A.M.; Terenghi, G.; Kellerth, J.O.; Wiberg, M. Sensory neuroprotection, mitochondrial preservation, and therapeutic potential of N-acetyl-cysteine after nerve injury. Neuroscience 2004, 125, 91–101. [Google Scholar] [CrossRef]
- Kartha, R.V.; Zhou, J.; Basso, L.; Schröder, H.; Orchard, P.J.; Cloyd, J. Mechanisms of Antioxidant Induction with High-Dose N-Acetylcysteine in Childhood Cerebral. Adrenoleukodystrophy CNS Drugs 2015, 29, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Tolar, P.J.; Orchard, K.J.; Bjoraker, R.S.; Ziegler, E.G.; Shapiro, L. Charnas N-acetyl-l-cysteine improves outcome of advanced cerebral adrenoleukodystrophy. Bone Marrow Transplant. 2007, 39, 211–215. [Google Scholar] [CrossRef]
- Miller, W.P.; Rothman, S.M.; Nascene, D.; Kivisto, T.; Defor, T.E.; Ziegler, R.S.; Eisengart, J.; Leiser, K.; Raymond, G.; Lund, T.C.; et al. Outcomes following allogeneic hematopoietic cell transplantation for childhood cerebral adrenoleukodystrophy: The largest single-institution cohort report. Blood 2011, 118, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Reiser, G. How the brain fights fatty acids’ toxicity. Neurochem. Int. 2021, 148, 105050. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, D.P.; Donida, B.; da Rosa, H.T.; Manini, P.R.; Moura, D.J.; Saffi, J.; Deon, M.; Mescka, C.P.; Coelho, D.M.; Jardim, L.B.; et al. Protective effect of antioxidants on DNA damage in leukocytes from X-linked adrenoleukodystrophy patients. Int. J. Dev. Neurosci. 2015, 43, 8–15. [Google Scholar] [CrossRef]
- Milne, G.L.; Dai, Q.; Roberts, L.J., 2nd. The isoprostanes—25 years later. Biochim. Biophys. Acta 2015, 1851, 433–445. [Google Scholar] [CrossRef]
- Galano, J.M.; Lee, Y.Y.; Oger, C.; Vigor, C.; Vercauteren, J.; Durand, T.; Giera, M.; Lee, J.C. Isoprostanes, neuroprostanes and phytoprostanes: An overview of 25 years of research in chemistry and biology. Prog. Lipid Res. 2017, 68, 83–108. [Google Scholar] [CrossRef]
- Barber, C.N.; Raben, D.M. Lipid Metabolism Crosstalk in the Brain: Glia and Neurons. Front. Cell. Neurosci. 2019, 13, 212. [Google Scholar] [CrossRef]
- Martinez, M. Tissue levels of polyunsaturated fatty acids during early human development. J. Pediatr. 1992, 120, S129–S138. [Google Scholar] [CrossRef]
- Martínez, M.; Mougan, I. Fatty acid composition of human brain phospholipids during normal development. J. Neurochem. 1998, 71, 2528–2533. [Google Scholar] [CrossRef] [PubMed]
- Signorini, C.; De Felice, C.; Galano, J.M.; Oger, C.; Leoncini, S.; Cortelazzo, A.; Ciccoli, L.; Durand, T.; Hayek, J.; Lee, J.C. Isoprostanoids in clinical and experimental neurological disease models. Antioxidants 2018, 7, 88. [Google Scholar] [CrossRef] [PubMed]
- Signorini, C.; De Felice, C.; Durand, T.; Galano, J.M.; Oger, C.; Leoncini, S.; Ciccoli, L.; Carone, M.; Ulivelli, M.; Manna, C.; et al. Relevance of 4-F(4t)-neuroprostane and 10-F(4t)-neuroprostane to neurological diseases. Free Radic. Biol. Med. 2018, 115, 278–287. [Google Scholar] [CrossRef]
- Signorini, C.; Cardile, V.; Pannuzzo, G.; Graziano, A.C.E.; Durand, T.; Galano, J.M.; Oger, C.; Leoncini, S.; Cortelazzo, A.; Lee, J.C.; et al. Increased isoprostanoid levels in brain from murine model of Krabbe disease—Relevance of isoprostanes, dihomo-isoprostanes and neuroprostanes to disease severity. Free Radic. Biol. Med. 2019, 139, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Galano, J.M.; Mas, E.; Barden, A.; Mori, T.A.; Signorini, C.; De Felice, C.; Barrett, A.; Opere, C.; Pinot, E.; Schwedhelm, E.; et al. Isoprostanes and neuroprostanes: Total synthesis, biological activity and biomarkers of oxidative stress in humans. Prostaglandins Other Lipid Mediat. 2013, 107, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Gladine, C.; Joumard-Cubizolles, L.; Chinetti, G.; Bayle, D.; Copin, C.; Hennuyer, N.; Staels, B.; Zanoni, G.; Porta, A.; Galano, J.M.; et al. Neuroprostanes, produced by free-radical mediated peroxidation of DHA, inhibit the inflammatory response of human macrophages. Free Radic Biol Med. 2014, 75 (Suppl. S1), S15. [Google Scholar] [CrossRef]
- Galano, J.M.; Lee, J.C.; Gladine, C.; Comte, B.; Le Guennec, J.Y.; Oger, C.; Durand, T. Non-enzymatic cyclic oxygenated metabolites of adrenic, docosahexaenoic, eicosapentaenoic and α-linolenic acids; bioactivities and potential use as biomarkers. Biochim. Biophys. Acta 2015, 1851, 446–455. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; Álvarez, L.; Baquero, M.; Ferrer, I.; García, L.; Hervás-Marín, D.; Cháfer-Pericás, C. Plasma isoprostanoids assessment as Alzheimer’s disease progression biomarkers. J. Neurochem. 2021, 157, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Signorini, C.; Leoncini, S.; Durand, T.; Galano, J.M.; Guy, A.; Bultel-Poncé, V.; Oger, C.; Lee, J.C.; Ciccoli, L.; Hayek, J.; et al. Circulating 4-F4t-Neuroprostane and 10-F4t-Neuroprostane Are Related to MECP2 Gene Mutation and Natural History in Rett Syndrome. Int. J. Mol. Sci. 2021, 22, 4240. [Google Scholar] [CrossRef] [PubMed]
- Montine, K.S.; Quinn, J.F.; Zhang, J.; Fessel, J.P.; Roberts, L.J., 2nd; Morrow, J.D.; Montine, T.J. Isoprostanes and related products of lipid peroxidation in neurodegenerative diseases. Chem. Phys. Lipids 2004, 128, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Praticò, D.; Clark, C.M.; Liun, F.; Rokach, J.; Lee, V.Y.; Trojanowski, J.Q. Increase of brain oxidative stress in mild cognitive impairment: A possible predictor of Alzheimer disease. Arch. Neurol. 2002, 59, 972–976. [Google Scholar] [CrossRef]
- Praticò, D. The neurobiology of isoprostanes and Alzheimer’s disease. Biochim. Biophys. Acta 2010, 1801, 930–933. [Google Scholar] [CrossRef]
- Miller, E.; Morel, A.; Saso, L.; Saluk, J. Isoprostanes and neuroprostanes as biomarkers of oxidative stress in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2014, 2014, 572491. [Google Scholar] [CrossRef] [PubMed]
- Greco, A.; Minghetti, L. Isoprostanes as biomarkers and mediators of oxidative injury in infant and adult central nervous system diseases. Curr. Neurovasc. Res. 2004, 1, 341–354. [Google Scholar] [CrossRef]
- Coviello, C.; Perrone, S.; Buonocore, G.; Negro, S.; Longini, M.; Dani, C.; de Vries, L.S.; Groenendaal, F.; Vijlbrief, D.C.; Benders, M.J.N.L.; et al. Isoprostanes as Biomarker for White Matter Injury in Extremely Preterm Infants. Front. Pediatr. 2021, 8, 618622. [Google Scholar] [CrossRef]
- Tonni, G.; Leoncini, S.; Signorini, C.; Ciccoli, L.; De Felice, C. Pathology of perinatal brain damage: Background and oxidative stress markers. Arch. Gynecol. Obstet. 2014, 290, 13–20. [Google Scholar] [CrossRef]
- De Felice, C.; Signorini, C.; Leoncini, S.; Pecorelli, A.; Durand, T.; Valacchi, G.; Ciccoli, L.; Hayek, J. The role of oxidative stress in Rett syndrome: An overview. Ann. N. Y. Acad. Sci. 2012, 1259, 121–135. [Google Scholar] [CrossRef]
- Signorini, C.; De Felice, C.; Leoncini, S.; Giardini, A.; D’Esposito, M.; Filosa, S.; Della Ragione, F.; Rossi, M.; Pecorelli, A.; Valacchi, G.; et al. F₄-neuroprostanes mediate neurological severity in Rett syndrome. Clin. Chim. Acta 2011, 412, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- De Felice, C.; Signorini, C.; Durand, T.; Oger, C.; Guy, A.; Bultel-Poncé, V.; Galano, J.M.; Ciccoli, L.; Leoncini, S.; D’Esposito, M.; et al. F2-dihomo-isoprostanes as potential early biomarkers of lipid oxidative damage in Rett syndrome. J. Lipid Res. 2011, 52, 2287–2297. [Google Scholar] [CrossRef] [PubMed]
- Signorini, C.; Ciccoli, L.; Leoncini, S.; Carloni, S.; Perrone, S.; Comporti, M.; Balduini, W.; Buonocore, G. Free iron, total F-isoprostanes and total F-neuroprostanes in a model of neonatal hypoxic-ischemic encephalopathy: Neuroprotective effect of melatonin. J. Pineal Res. 2009, 46, 148–154. [Google Scholar] [CrossRef] [PubMed]
- De Felice, C.; Della Ragione, F.; Signorini, C.; Leoncini, S.; Pecorelli, A.; Ciccoli, L.; Scalabrì, F.; Marracino, F.; Madonna, M.; Belmonte, G.; et al. Oxidative brain damage in Mecp2-mutant murine models of Rett syndrome. Neurobiol. Dis. 2014, 68, 66–77. [Google Scholar] [CrossRef]
- Oger, C.; Brinkmann, Y.; Bouazzaoui, S.; Durand, T.; Galano, J.M. Stereocontrolled access to isoprostanes via a bicyclo[3.3.0]octane framework. Org. Lett. 2008, 10, 5087–5090. [Google Scholar] [CrossRef]
- Oger, C.; Bultel-Poncé, V.; Guy, A.; Balas, L.; Rossi, J.C.; Durand, T.; Galano, J.M. The handy use of Brown’s catalyst for a skipped diyne deuteration: Application to the synthesis of a d4-labeled-F4t-neuroprostane. Chem. Eur. J. 2010, 16, 13976–13980. [Google Scholar] [CrossRef]
- Guy, A.; Oger, C.; Hepekauzen, J.; Signorini, C.; Durand, T.; De Felice, C.; Fürstner, A.; Galano, J.M. Oxygenated metabolites of n-3 polyunsaturated fatty acid as potential oxidative stress biomarkers: Total synthesis of 8-F3t-IsoP, 10-F4t-NeuroP, and [D4]-10-F4t-NeuroP. Chem. Eur. J. 2014, 20, 6374–6380. [Google Scholar] [CrossRef]
- Galino, J.; Ruiz, M.; Fourcade, S.; Schlüter, A.; López-Erauskin, J.; Guilera, C.; Jove, M.; Naudi, A.; García-Arumí, E.; Andreu, A.L.; et al. Oxidative damage compromises energy metabolism in the axonal degeneration mouse model of X-adrenoleukodystrophy. Antioxid. Redox Signal. 2011, 15, 2095–2107. [Google Scholar] [CrossRef]
- López-Erauskin, J.; Fourcade, S.; Galino, J.; Ruiz, M.; Schlüter, A.; Naudi, A.; Jove, M.; Portero-Otin, M.; Pamplona, R.; Ferrer, I.; et al. Antioxidants halt axonal degeneration in a mouse model of X-adrenoleukodystrophy. Ann. Neurol. 2011, 70, 84–92. [Google Scholar] [CrossRef]
- Maruyama, W.; Shaomoto-Nagai, M.; Kato, Y.; Hisaka, S.; Osawa, T.; Naoi, M. Role of lipid peroxide in the neurodegenerative disorders. In Lipid Hydroperoxide-Derived Modification of Biomolecules; Subcellular Biochemistry; Kato, Y., Ed.; Springer: Dordrecht, The Netherlands, 2014; pp. 127–136. [Google Scholar]
- Ahmed, O.S.; Galano, J.M.; Pavlickova, T.; Revol-Cavalier, J.; Vigor, C.; Lee, J.C.; Oger, C.; Durand, T. Moving forward with isoprostanes, neuroprostanes and phytoprostanes: Where are we now? Essays Biochem. 2020, 64, 463–484. [Google Scholar]
- Lacampagne, A.; Le Guennec, J.-Y.; Bultel-Ponce, V.; Galano, J.-M.; Guy, A.; Durand, T.; Oger, C.; Matecki, S.; Dridi, H.; Thireau, J.; et al. Methods and Pharmaceutical Compositions for the Treatment of Disorders or Diseases Associated with Ryanodine Receptor Dysfunction. Worldwide Applications No. WO 2015/197562 A1, 30 December 2015. Available online: https://patentimages.storage.googleapis.com/89/a7/42/fbf1fe558378d8/WO2015197562A1.pdf (accessed on 9 December 2021).
- Zhou, J.; Terluk, M.R.; Orchard, P.J.; Cloyd, J.C.; Kartha, R.V. N-Acetylcysteine Reverses the Mitochondrial Dysfunction Induced by Very Long-Chain Fatty Acids in Murine Oligodendrocyte Model of Adrenoleukodystrophy. Biomedicines 2021, 9, 1826. [Google Scholar] [CrossRef]
- Marchetti, D.P.; Steffens, L.; Jacques, C.E.; Guerreiro, G.B.; Mescka, C.P.; Deon, M.; de Coelho, D.M.; Moura, D.J.; Viario, A.G.; Poletto, F.; et al. Oxidative Imbalance, Nitrative Stress, and Inflammation in C6 Glial Cells Exposed to Hexacosanoic Acid: Protective Effect of N-acetyl-l-cysteine, Trolox, and Rosuvastatin. Cell. Mol. Neurobiol. 2018, 38, 1505–1516. [Google Scholar] [CrossRef]
- Morris, G.; Walder, K.; Puri, B.K.; Berk, M.; Maes, M. The Deleterious Effects of Oxidative and Nitrosative Stress on Palmitoylation, Membrane Lipid Rafts and Lipid-Based Cellular Signalling: New Drug Targets in Neuroimmune Disorders. Mol. Neurobiol. 2016, 53, 4638–4658. [Google Scholar] [CrossRef]
- Eichler, F.; Grodd, W.; Grant, E.; Sessa, M.; Biffi, A.; Bley, A.; Kohlschuetter, A.; Loes, D.J.; Kraegeloh-Mann, I. Metachromatic leukodystrophy: A scoring system for brain MR imaging observations. AJNR Am. J. Neuroradiol. 2009, 30, 1893–1897. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; López-Cuevas, R.; Cuevas, A.; Baquero, M.; Cháfer-Pericás, C. Lipid peroxidation biomarkers correlation with medial temporal atrophy in early Alzheimer Disease. Neurochem. Int. 2019, 129, 104519. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K.; Asch, R.H.; Lindquist, D.M.; Krikorian, R. Role of polyunsaturated fatty acids in human brain structure and function across the lifespan: An update on neuroimaging findings. Prostaglandins Leukot. Essent. Fatty Acids 2018, 136, 23–34. [Google Scholar] [CrossRef]
- O’Brien, J.S.; Sampson, E.E. Fatty acids and fatty aldehyde composition of the major brain lipids in normal gray matter, white matter, and myelin. J. Lipid Res. 1965, 6, 645–651. [Google Scholar] [CrossRef]
- Levy, B.D.; Clish, C.B.; Schmidt, B.; Gronert, K.; Serhan, C.N. Lipid mediator class switching during acute inflammation: Signals in resolution. Nat. Immunol. 2001, 2, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Chamani, S.; Bianconi, V.; Tasbandi, A.; Pirro, M.; Barreto, G.E.; Jamialahmadi, T.; Sahebkar, A. Resolution of Inflammation in Neurodegenerative Diseases: The Role of Resolvins. Mediat. Inflamm. 2020, 2020, 3267172. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Lee, C.Y. Using isoprostanes as biomarkers of oxidative stress: Some rarely considered issues. Antioxid. Redox Signal. 2010, 13, 145–156. [Google Scholar] [CrossRef]
- Milne, G.L.; Musiek, E.S.; Morrow, J.D. F2-isoprostanes as markers of oxidative stress in vivo: An overview. Biomarkers 2005, 10 (Suppl. S1), S10–S23. [Google Scholar] [CrossRef]
- De Felice, C.; Ciccoli, L.; Leoncini, S.; Signorini, C.; Rossi, M.; Vannuccini, L.; Guazzi, G.; Latini, G.; Comporti, M.; Valacchi, G.; et al. Systemic oxidative stress in classic Rett syndrome. Free Radic. Biol. Med. 2009, 47, 440–448. [Google Scholar] [CrossRef]
- Nechuta, S.; Cai, Q.; Zheng, Y.; Milne, G.L.; Cai, H.; Dai, Q.; Yang, G.; Zheng, W.; Lu, W.; Shu, X.O. Urinary biomarkers of oxidative stress and breast cancer survival. Cancer Causes Control 2014, 25, 701–707. [Google Scholar] [CrossRef] [PubMed][Green Version]
- García-Flores, L.A.; Medina, S.; Martínez-Hernández, P.; Oger, C.; Galano, J.M.; Durand, T.; Casas-Pina, T.; Ferreres, F.; Gil-Izquierdo, Á. Snapshot situation of oxidative degradation of the nervous system, kidney, and adrenal glands biomarkers-neuroprostane and dihomo-isoprostanes-urinary biomarkers from infancy to elderly adults. Redox Biol. 2017, 11, 586–591. [Google Scholar] [CrossRef]
- Sprecher, H.; VanRollins, M.; Sun, F.; Wyche, A.; Needleman, P. Dihomo-prostaglandins and -thromboxane. A prostaglandin family from adrenic acid that may be preferentially synthesized in the kidney. J. Biol. Chem. 1982, 257, 3912–3918. [Google Scholar] [CrossRef]
- VanRollins, M.; Woltjer, R.L.; Yin, H.; Morrow, J.D.; Montine, T.J. F2-dihomo-isoprostanes arise from free radical attack on adrenic acid. J. Lipid Res. 2008, 49, 995–1005. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; Aubourg, P.; Pujol, A. General aspects and neuropathology of X-linked adrenoleukodystrophy. Brain Pathol. 2010, 20, 817–830. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Galano, J.M.; Leung, H.H.; Balas, L.; Oger, C.; Durand, T.; Lee, J.C. Nonenzymatic oxygenated metabolite of docosahexaenoic acid, 4(RS)-4-F4t-neuroprostane, acts as a bioactive lipid molecule in neuronal cells. FEBS Lett. 2020, 594, 1797–1808. [Google Scholar] [CrossRef]
- Lorenzano, S.; Rost, N.S.; Khan, M.; Li, H.; Lima, F.O.; Maas, M.B.; Green, R.E.; Thankachan, T.K.; Dipietro, A.J.; Arai, K.; et al. Oxidative Stress Biomarkers of Brain Damage: Hyperacute Plasma F2-Isoprostane Predicts Infarct Growth in Stroke. Stroke 2018, 49, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.C.; Chen, T.W.; Yang, T.C.; Wei, H.J.; Hsu, J.C.; Lin, C.L. Levels of F2-isoprostanes, F4-neuroprostanes, and total nitrate/nitrite in plasma and cerebrospinal fluid of patients with traumatic brain injury. Free Radic. Res. 2015, 49, 1419–1430. [Google Scholar] [CrossRef]
- Leung, H.H.; Ng, A.L.; Durand, T.; Kawasaki, R.; Oger, C.; Balas, L.; Galano, J.M.; Wong, I.Y.; Chung-Yung Lee, J. Increase in omega-6 and decrease in omega-3 polyunsaturated fatty acid oxidation elevates the risk of exudative AMD development in adults with Chinese diet. Free Radic. Biol. Med. 2019, 145, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Duncan, I.D. Myelin repair by transplantation of myelin-forming cells in globoid cell leukodystrophy. J. Neurosci. Res. 2016, 94, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
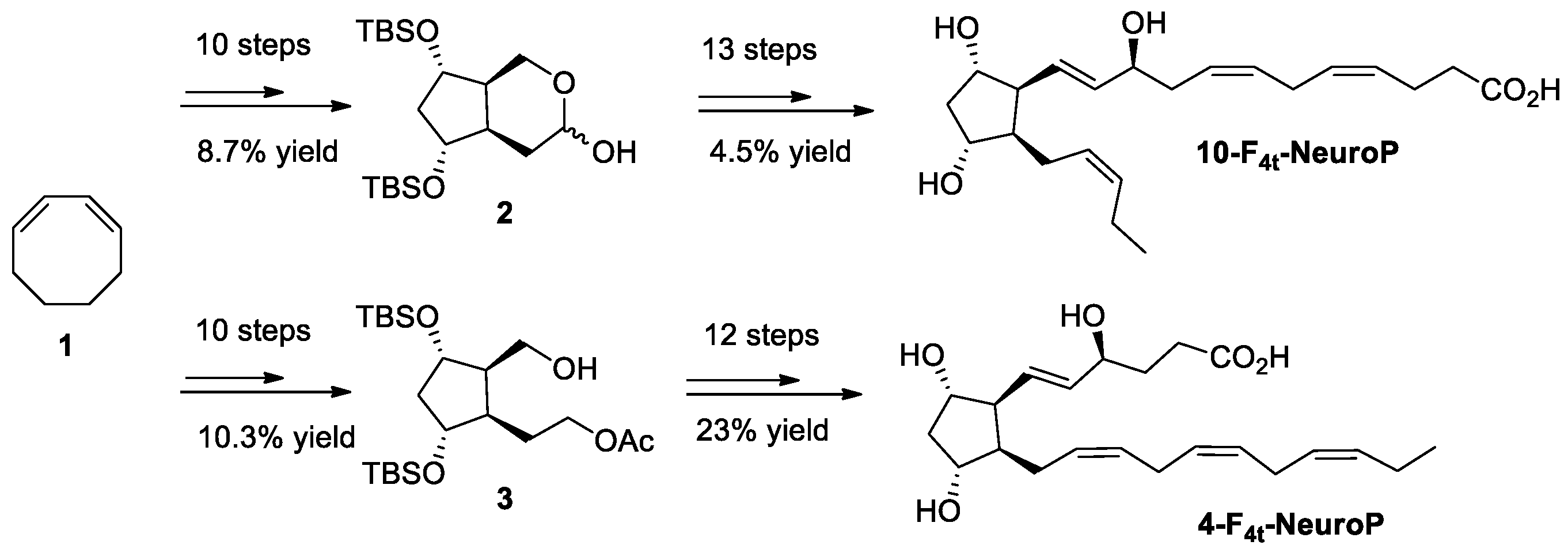
 and
and  , indicates the charge of the atoms (Br- and P+, from phophonium salt) [42].
, indicates the charge of the atoms (Br- and P+, from phophonium salt) [42].
 and
and  , indicates the charge of the atoms (Br- and P+, from phophonium salt) [42].
, indicates the charge of the atoms (Br- and P+, from phophonium salt) [42].
 and
and  , indicates the charge of the atoms (Br- and P+, from phophonium salt) [42].
, indicates the charge of the atoms (Br- and P+, from phophonium salt) [42].
 and
and  , indicates the charge of the atoms (Br- and P+, from phophonium salt) [42].
, indicates the charge of the atoms (Br- and P+, from phophonium salt) [42].

| Entire ALD Group vs Healthy Control | Analysis | Area under the ROC Curve | Standard Error | 95% C.I. | p Value |
| F2-IsoPs | 0.743 | 0.0830 | 0.598 to 0.857 | 0.0041 | |
| F2-dihomo-IsoPs | 0.9664 | 0.0330 | 0.871 to 0.997 | <0.0001 | |
| Total F4-NeuroPs | 1.000 | 0.000 | 0.927 to 1.000 | <0.0001 | |
| 10-F4t-NeuroP | 0.997 | 0.00419 | 0.921 to 1.000 | <0.0001 | |
| 4-F4t-NeuroP | 0.602 | 0.0866 | 0.452 to 0.739 | 0.023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Signorini, C.; De Felice, C.; Durand, T.; Galano, J.-M.; Oger, C.; Leoncini, S.; Hayek, J.; Lee, J.C.-Y.; Lund, T.C.; Orchard, P.J. Isoprostanoid Plasma Levels Are Relevant to Cerebral Adrenoleukodystrophy Disease. Life 2022, 12, 146. https://doi.org/10.3390/life12020146
Signorini C, De Felice C, Durand T, Galano J-M, Oger C, Leoncini S, Hayek J, Lee JC-Y, Lund TC, Orchard PJ. Isoprostanoid Plasma Levels Are Relevant to Cerebral Adrenoleukodystrophy Disease. Life. 2022; 12(2):146. https://doi.org/10.3390/life12020146
Chicago/Turabian StyleSignorini, Cinzia, Claudio De Felice, Thierry Durand, Jean-Marie Galano, Camille Oger, Silvia Leoncini, Joussef Hayek, Jetty Chung-Yung Lee, Troy C. Lund, and Paul J. Orchard. 2022. "Isoprostanoid Plasma Levels Are Relevant to Cerebral Adrenoleukodystrophy Disease" Life 12, no. 2: 146. https://doi.org/10.3390/life12020146
APA StyleSignorini, C., De Felice, C., Durand, T., Galano, J.-M., Oger, C., Leoncini, S., Hayek, J., Lee, J. C.-Y., Lund, T. C., & Orchard, P. J. (2022). Isoprostanoid Plasma Levels Are Relevant to Cerebral Adrenoleukodystrophy Disease. Life, 12(2), 146. https://doi.org/10.3390/life12020146








