Endovascular Treatment of Large Vessel Occlusion Strokes Caused by Infective Endocarditis: A Systematic Review, Meta-Analysis, and Case Presentation
Abstract
:1. Introduction
2. Materials and Methods
3. Results
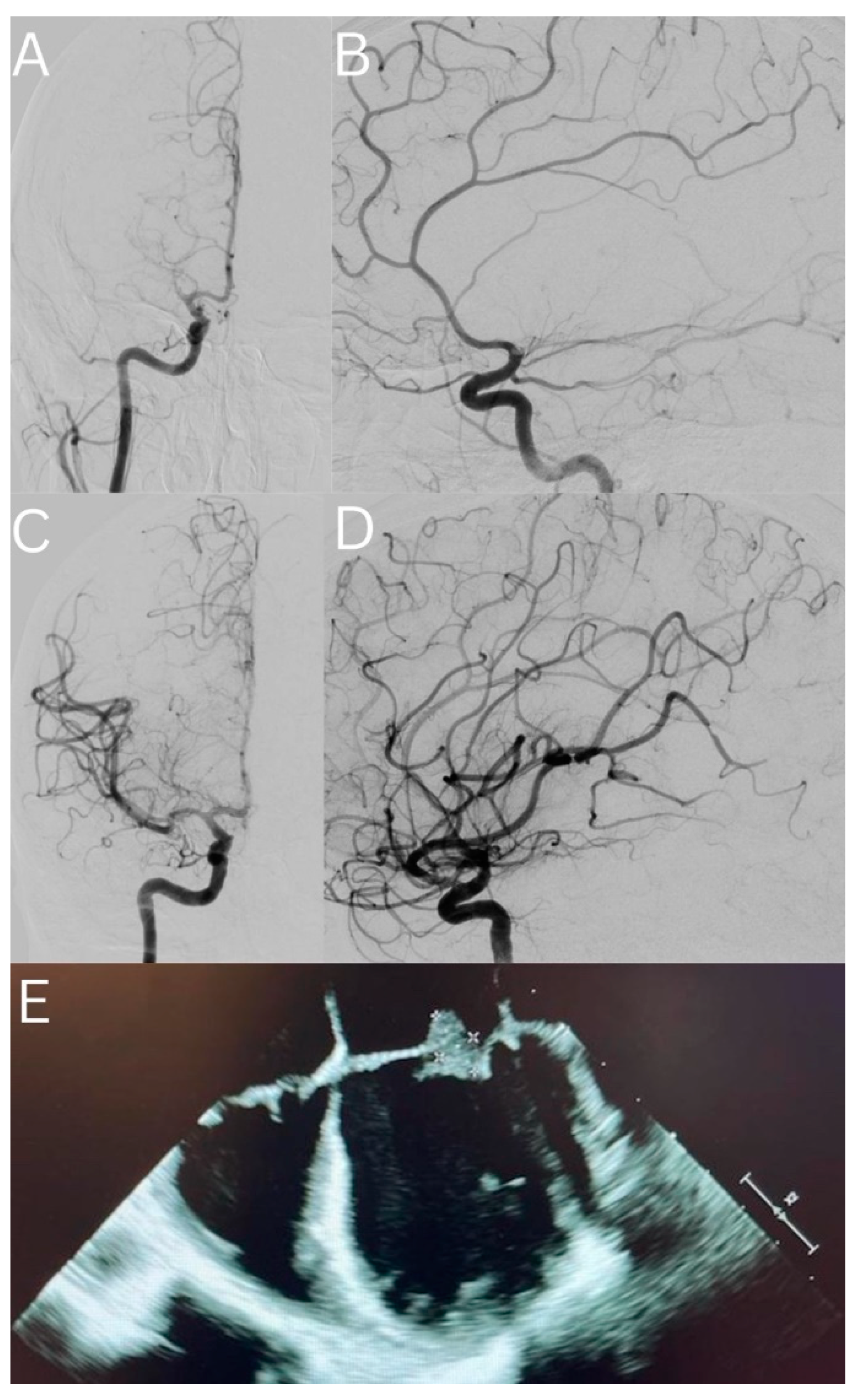
3.1. Case Presentation
3.2. Details of the Included Studies
3.3. Results of the Meta-Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baddour, L.M.; Wilson, W.R.; Bayer, A.S.; Fowler, V.G.; Tleyjeh, I.M.; Rybak, M.J.; Barsic, B.; Lockhart, P.B.; Gewitz, M.H.; Levison, M.E.; et al. Infective endocarditis in adults: Diagnosis, antimicrobial therapy, and management of complications: A scientific statement for healthcare professionals from the American Heart Association. Circulation 2015, 132, 1435–1486. [Google Scholar] [CrossRef] [PubMed]
- Thuny, F.; Avierinos, J.; Tribouilloy, C.; Giorgi, R.; Casalta, J.; Milandre, L.; Brahim, A.; Nadji, G.; Riberi, A.; Collart, F.; et al. Impact of cerebrovascular complications on mortality and neurologic outcome during infective endocarditis: A prospective multicentre study. Eur. Heart J. 2007, 28, 1155–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arboix, A.; Bechich, S.; Oliveres, M.; García-Eroles, L.; Massons, J.; Targa, C. Ischemic stroke of unusual cause: Clinical features, etiology and outcome. Eur. J. Neurol. 2001, 8, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Bonaros, N.; Czerny, M.; Pfausler, B.; Müller, S.; Bartel, T.; Thielmann, M.; Shehada, S.; Folliguet, T.; Obadia, J.; Holfeld, J.; et al. Infective endocarditis and neurologic events: Indications and timing for surgical interventions. Eur. Heart J. Suppl. 2020, 22, M19–M25. [Google Scholar] [CrossRef] [PubMed]
- Guzek, A.; Braksator, W.; Gąsior, Z.; Kuśmierczyk, M.; Różański, J.; Rybicki, Z. Infective endocarditis—Can we treat it more effectively? Kardiochir Torakochirurgia Pol. 2020, 17, 8–14. [Google Scholar] [CrossRef]
- Zipes, D.P. Braunwald’s heart disease: A textbook of cardiovascular medicine. BMH Med. J. 2018, 5, 63. [Google Scholar]
- Nogles, T.E.; Galuska, M.A. Middle Cerebral Artery Stroke Treasure Island (FL): StatPearls Publishing. 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556132/ (accessed on 10 October 2022).
- Bettencourt, S.; Ferro, J.M. Acute ischemic stroke treatment in infective endocarditis: Systematic review. J. Stroke Cerebrovasc. Dis. 2020, 29, 104598. [Google Scholar] [CrossRef]
- Asaithambi, G.; Adil, M.M.; Qureshi, A.I. Thrombolysis for ischemic stroke associated with infective endocarditis: Results from the nationwide inpatient sample. Stroke 2013, 44, 2917–2919. [Google Scholar] [CrossRef] [Green Version]
- Maheshwari, R.; Cordato, D.J.; Wardman, D.; Thomas, P.; Bhaskar, S.M.M. Clinical outcomes following reperfusion therapy in acute ischemic stroke patients with infective endocarditis: A systematic review. J. Cent. Nerv. Syst. Dis. 2022, 14, 11795735221081597. [Google Scholar] [CrossRef]
- Maheshwari, R.; Wardman, D.; Cordato, D.J.; Bhaskar, S.M.M. Acute Ischaemic Stroke in Infective Endocarditis: Pathophysiology and Clinical Outcomes in Patients Treated with Reperfusion Therapy. Immuno 2021, 1, 347–359. [Google Scholar] [CrossRef]
- D’Anna, L. Endovascular treatment of ischemic large-vessel stroke due to infective endocarditis: Case series and review of the literature. Neurol. Sci. 2020, 41, 3517–3525. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.; Mayo, P.; Trillo, S.; Gómez-Escalonilla, C.; Caniego, J.L.; Moreu, M.; Vega, J.; Rosati, S.; Simal, P.; Carrill, Á.X.; et al. Management of large vessel occlusion stroke related to infective endocarditis: Is mechanical thrombectomy a safe option? J. Stroke Cerebrovasc. Dis. 2020, 29, 105248. [Google Scholar] [CrossRef] [PubMed]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmi, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feil, K.; Küpper, C.; Tiedt, S.; Dimitriadis, K.; Herzberg, M.; Dorn, F.; Liebig, T.; Dieterich, M.; Kellert, L. Safety and efficacy of mechanical thrombectomy in infective endocarditis: A matched case-control analysis from the German Stroke Registry-Endovascular Treatment. Eur. J. Neurol. 2021, 28, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Marnat, G.; Sibon, I.; Gory, B.; Richard, S.; Olindo, S.; Consoli, A.; Bourcier, R.; Kyheng, M.; Labreuche, J.; Darganzali, C.; et al. Safety and outcomes of mechanical thrombectomy for acute stroke related to infective endocarditis: A case-control study. Int. J. Stroke 2021, 16, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, R.J.; Cho, S.; Thatikunta, P.; Deshpande, A.; Wisco, D.; Uchino, K. Acute Ischemic Stroke Therapy in Infective Endocarditis: Case Series and Systematic Review. J Stroke Cerebrovasc. Dis. 2019, 28, 2207–2212. [Google Scholar] [CrossRef]
- Sader, E.; Abdalkader, M.; Thom, N.; Nguyen, T.N.; McDonald, S.; Greer, D.; Brown, S.C.; Mohamedali, A.; Gutierrez, J.; Shi, H.; et al. Endovascular Treatment of Infective Endocarditis-Related Acute Large Vessel Occlusion Stroke. J. Stroke Cerebrovasc. Dis. 2021, 30, 105775. [Google Scholar] [CrossRef]
- Ambrosioni, J.; Urra, X.; Hernández-Meneses, M.; Almela, M.; Falces, C.; Tellez, A.; Quintana, E.; Fuster, D.; Sandoval, E.; Vidal, B.; et al. Mechanical Thrombectomy for Acute Ischemic Stroke Secondary to Infective Endocarditis. Clin. Infect. Dis. 2018, 66, 1286–1289. [Google Scholar] [CrossRef] [Green Version]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar]
- Mowla, A.; Singh, K.; Mehla, S.; Ahmed, M.K.; Shirani, P.; Kamal, H.; Krishna, C.; Sawyer, R.N., Jr.; Ching, M.; Siddiqui, A.H.; et al. Is acute reperfusion therapy safe in acute ischemic stroke patients who harbor unruptured intracranial aneurysm? Int. J. Stroke 2015, 10, 113–118. [Google Scholar] [CrossRef]
- Yaeger, K.A.; Martini, M.L.; Hardigan, T.; Ladner, T.; Hao, Q.; Paul, I. Mortality reduction after thrombectomy for acute intracranial large vessel occlusion: Meta-analysis of randomized trials. J. Neurointerv. Surg. 2020, 12, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.R.; Hanlin, E.; Glurich, I.; Mazza, J.J.; Yale, S.H. Virchow’s contribution to the understanding of thrombosis and cellular biology. Clin. Med. Res. 2010, 8, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Yellapu, V.; Ackerman, D.; Longo, S.; Stawicki, S.P. Septic Embolism in Endocarditis: Anatomic and Pathophysiologic Considerations. Advanced Concepts in Endocarditis; IntechOpen: London, UK, 2018. [Google Scholar]
- Elsaghir, H.; Al Khalili, Y. Septic Emboli. StatPearls [Internet]; StatPearls Publishing: Tampa, FL, USA, 2021. [Google Scholar]
- Ferro, J.M.; Fonseca, A.C. Chapter 7—Infective endocarditis. In Handbook of Clinical Neurology; Biller, J., Ferro, J.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 119, pp. 75–91. [Google Scholar]
- Ram, A.; Deslouches, J.; Punnapuzha, S. Mycotic Aneurysm: A Rare Etiology of a Common Presentation. Cureus 2022, 14, e27105. [Google Scholar] [CrossRef] [PubMed]


| Author | Country | Design | Age, Mean (SD) or Median (IQR) | Sex (Female) | Follow-Up | N. | Pathology | Risk Factors (N.) | Occluded Vessel Side (Left) | Vessel (N.) | IVT (N.) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Feil, 2020 [15] | Germany | Cohort/ case-control | 69 (13.3) | 24 | 24 h | 55 | HTN: 37, DM: 10, hypercholesterolemia: 16, smoker (current/former): 14, and AF: 18 | 30 | BA: 8, MCA M1: 32, MCA M2: 9, and other, including ACA, carotis-T, ICA intracranial, ICA extracranial, and VA: 11 | 8 | |
| Three months | |||||||||||
| Marnat, 2020 [16] | France | Case-control | 59.2 (17.6) | 8 | 24 h | 28 | Enterococcus faecalis (6/28), Pseudomonas aeruginosa (1/28), Staphylococcus aureus (1/28), Staphylococcus capitis (1/28), Streptococcus viridans (1/28), Streptococcus gordonii (1/28), unidentified streptococcus (1/28), Candida parapsilosis (1/28), trichosporon (1/28), unidentified Gram-negative bacillus (1/28), unidentified Gram-positive bacillus (1/28), and unidentified germs (12/28). Bacteriological–histological analysis of the thrombus allowed IE diagnosis in five cases | HTN: 9/27, DM: 3/27, hypercholesterolemia: 9/27, current smoker: 8/25, antithrombotic use: 17/27, coronary artery disease: 6/27, and previous stroke/TIA: 3/27 | MCA M1: 16/28, MCA M2: 3/28, tandem: 1/28, intracranial ICA: 5/28, VB: 2/28, and extracranial ICA/others: 1/28 | 8 | |
| Three months | |||||||||||
| Marquardt, 2019 [17] | USA | Case series | 57.5 (11.6) | 0 | 24 h | 4 | Bartonella quintaina, Streptococcus anginosus, Staphylococcus aureus, and unknown | Left: 2 | MCA M1: 4 | 1 | |
| Ramos, 2020 [13] | Spain | Case series | 64.33 (14.25) | 6 | 24 h | 12 | Streptococcus viridans: 1, methicillin-resistant Staphylococcus epidermidis: 1, methicillin-resistant Staphylococcus aureus: 3, Enterococcus faecalis: 3, Staphylococcus epidermidis: 1, Klebsiella pneumoniae: 1, and negative: 2 | AF: 6, anticoagulant: 7, and surgery: 6 | MCA M1: 9, MCA M2: 2, and intracranial ICA: 1 | 2 | |
| Three months | |||||||||||
| Sader, 2021 [18] | USA | Case series | 59 (range 29–80) | 5 | Unknown | 15 | Staphylococcus aureus: 2, Staphylococcus epidermidis: 1, Enterococcus faecalis: 4, Alpha Strep: 1, Streptococcus parasanguinis: 1, Streptococcus mitis: 1, Aspergillus fumigatus: 1, Candida tropicalis: 1, Streptococcus agalactiae: 1, Streptococcus salivarius, Staphylococcus hominis, coag-negative Staphylococcus: 1, and unknown: 1 | Left: 11, and right: 5 | MCA M1: 12, MCA M2: 1, carotid terminus: 2, and cavernous: 1 | 6 | |
| Ambrosioni, 2018 [19] | Spain | Case series | 75.5 years (interquartile range 59–79 years) | 3 | 24 h | 6 | Staphylococcus aureus: 1, Streptococcus oralis: 1, Streptococcus dysgalactie: 1, Staphylococcus epidermidis: 1, and negative culture: 2 | Sub-occlusion tandem (carotid plus M1): 1, MCA M1: 3, BA: 2 | |||
| Seven days | |||||||||||
| Three months |
| Author | Follow-Up | Baseline NIHSS, Mean (SD) | Treatment Approach for Intracranial LVO | Periprocedural Complications | Reperfusion (TICI) | NIHSS in F/U Mean (SD) | ICH in F/U (N.) | Complications during Hospital Stay (N.) | Mortality or mRS = 6 (N.) | Recurrent Stroke (N.) |
|---|---|---|---|---|---|---|---|---|---|---|
| Feil, 2020 [15] | 24 h | 15 (8.14) | Aspiration catheter solo: 11, stent retriever solo: 10, combination: 31, and additive medication during MT: 13 | Device malfunction: 2, dissection and perforation: 2, clot migration and embolization: 1, ICH: 3, vasospasm: 2, and other: 4 | mTICI 2b/3: 41 | 16 (17.65) | 14 | Malignant MCA infarction: 7, recurrent stroke: 7, ICH: 17, groin hematoma: 2, groin aneurysm: 2, and other complications: 27 | 7 | |
| Three months | 33 | |||||||||
| Marnat, 2020 [16] | 24 h | 16.5 (6.66) | Aspiration: 11/25, stent retriever: 6/25, combination: 4/25, and balloon + SR: 4/25 | 2 | mTICI 3: 12/28, mTICI 2c/3: 20/28, and mTICI 2b/3: 24/28 | −3 (6.66) * | Any ICH: 10, parenchymal hematoma: 2, and sICH: 2 | 7/28 | ||
| Three months | 7/27 | |||||||||
| Marquardt, 2019 [17] | 24 h | 17.25 (3.89) | MT solitaire and penumbra: 2, MT solitaire and wingspan stent: 1, and MT penumbra with 2 mg tPA: 1 | TICI 2A: 2, TICI 2B: 1, andTICI 3: 1 | 14 (3.31) | 2 | ICH: 2 | |||
| Ramos, 2020 [13] | 24 h | 13.08 (6.94) | 2 | TICI 0: 3, TICI 1: 2, TICI 2A: 1, TICI 2B: 1, andTICI 3: 5 | 10.75 (8.92) | 6 | 6 | 3 | ||
| Three months | ||||||||||
| Sader, 2021 [18] | Immediately after | 17.93 (5.37) | TICI 0: 1, TICI 2a: 1, TICI 2c: 1, TICI 2b: 4, and TICI 3: 6 | 12.71 (8.59) | 7 | 2 | ||||
| discharge | 9.2 (6.57) | |||||||||
| Ambrosioni, 2018 [19] | 24 h | 14.33 (10.19) | Stent retriever plus carotid stent: 1, and stent retriever: 5 | mTICI 0: 1, mTICI 2B: 1, and mTICI 3: 3 | 12.66 (15.8) | 0 | ||||
| Seven days | 2 | |||||||||
| Three months | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mowla, A.; Abdollahifard, S.; Sizdahkhani, S.; Taherifard, E.; Kheshti, F.; Khatibi, K. Endovascular Treatment of Large Vessel Occlusion Strokes Caused by Infective Endocarditis: A Systematic Review, Meta-Analysis, and Case Presentation. Life 2022, 12, 2146. https://doi.org/10.3390/life12122146
Mowla A, Abdollahifard S, Sizdahkhani S, Taherifard E, Kheshti F, Khatibi K. Endovascular Treatment of Large Vessel Occlusion Strokes Caused by Infective Endocarditis: A Systematic Review, Meta-Analysis, and Case Presentation. Life. 2022; 12(12):2146. https://doi.org/10.3390/life12122146
Chicago/Turabian StyleMowla, Ashkan, Saeed Abdollahifard, Saman Sizdahkhani, Erfan Taherifard, Fatemeh Kheshti, and Kasra Khatibi. 2022. "Endovascular Treatment of Large Vessel Occlusion Strokes Caused by Infective Endocarditis: A Systematic Review, Meta-Analysis, and Case Presentation" Life 12, no. 12: 2146. https://doi.org/10.3390/life12122146
APA StyleMowla, A., Abdollahifard, S., Sizdahkhani, S., Taherifard, E., Kheshti, F., & Khatibi, K. (2022). Endovascular Treatment of Large Vessel Occlusion Strokes Caused by Infective Endocarditis: A Systematic Review, Meta-Analysis, and Case Presentation. Life, 12(12), 2146. https://doi.org/10.3390/life12122146










